Deep Sequencing to Reveal Phylo-Geographic Relationships of Juquitiba Virus in Paraguay
Abstract
:1. Introduction
2. Materials and Methods
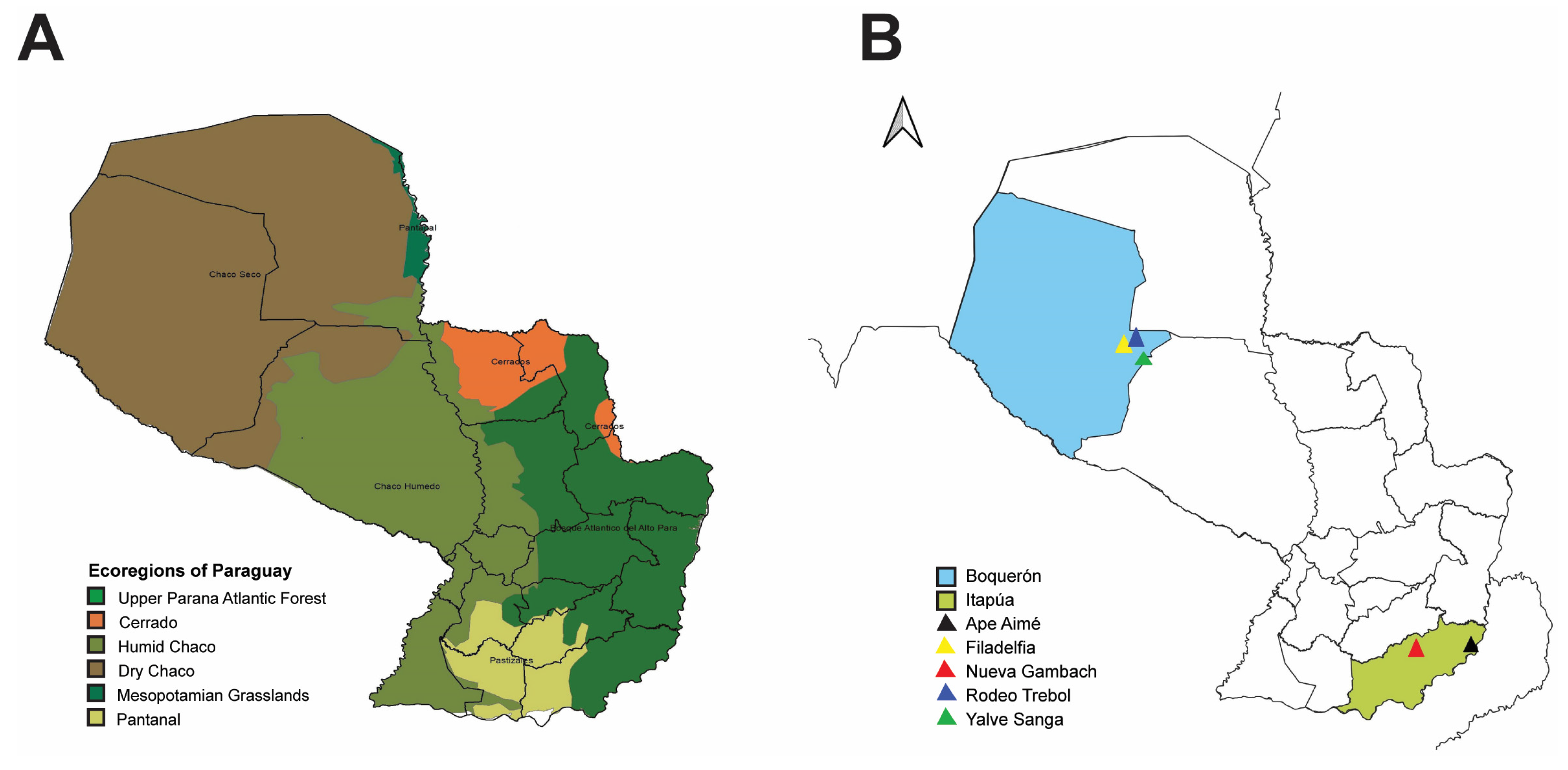
2.1. Rodent Collection
2.2. Confirmation of Rodent Species
2.3. Immunofluorescence Assay (IFA)
2.4. Primer Design and Pooling
2.5. RNA Extraction and cDNA Synthesis
2.6. Polymerase Chain Reaction (PCR)
2.7. Library Preparation and Next-Generation Sequencing (NGS)
2.8. Data and Sequence Analyses
2.9. Phylogenetic and Phylogeographic Analyses
3. Results
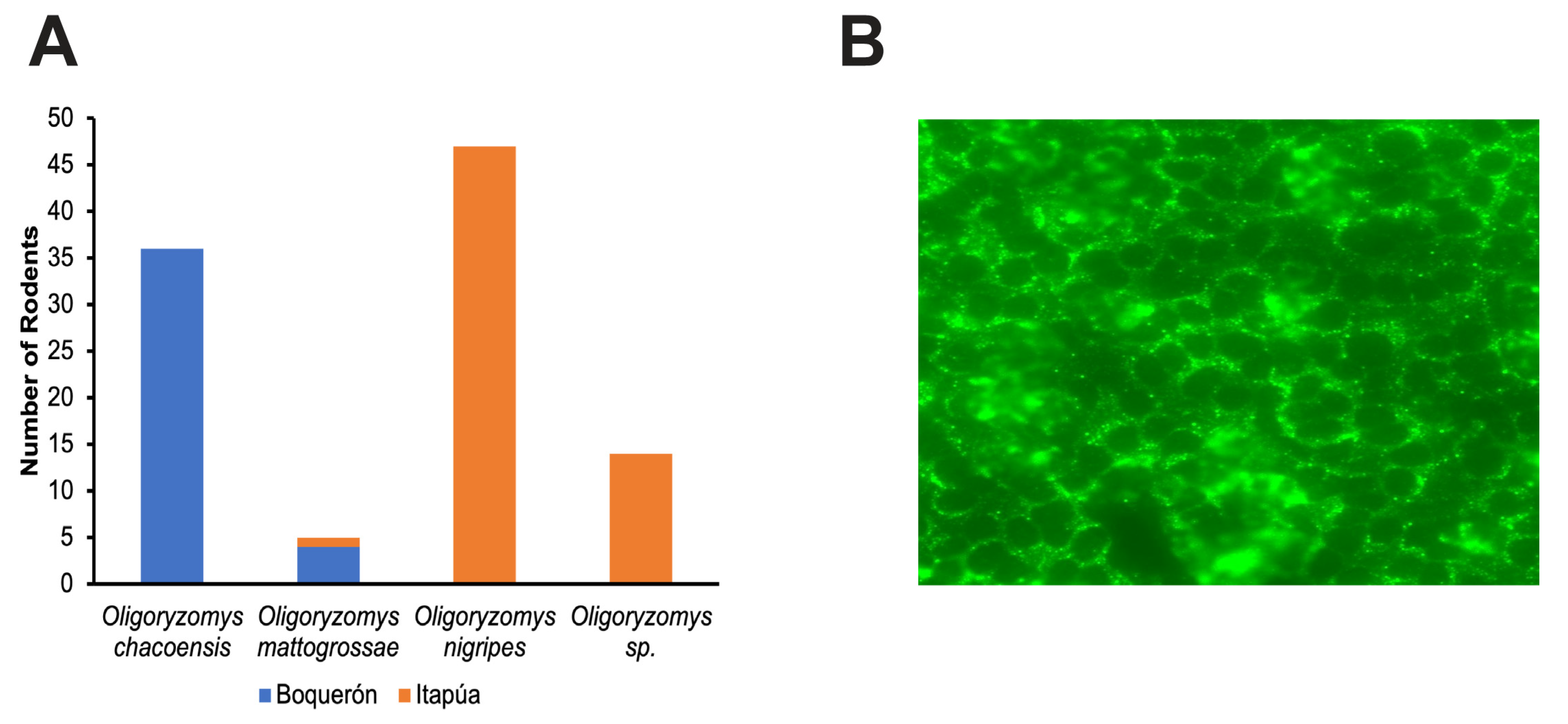
3.1. Rodent Sampling
3.2. Confirmation of Infection by IFA and NGS
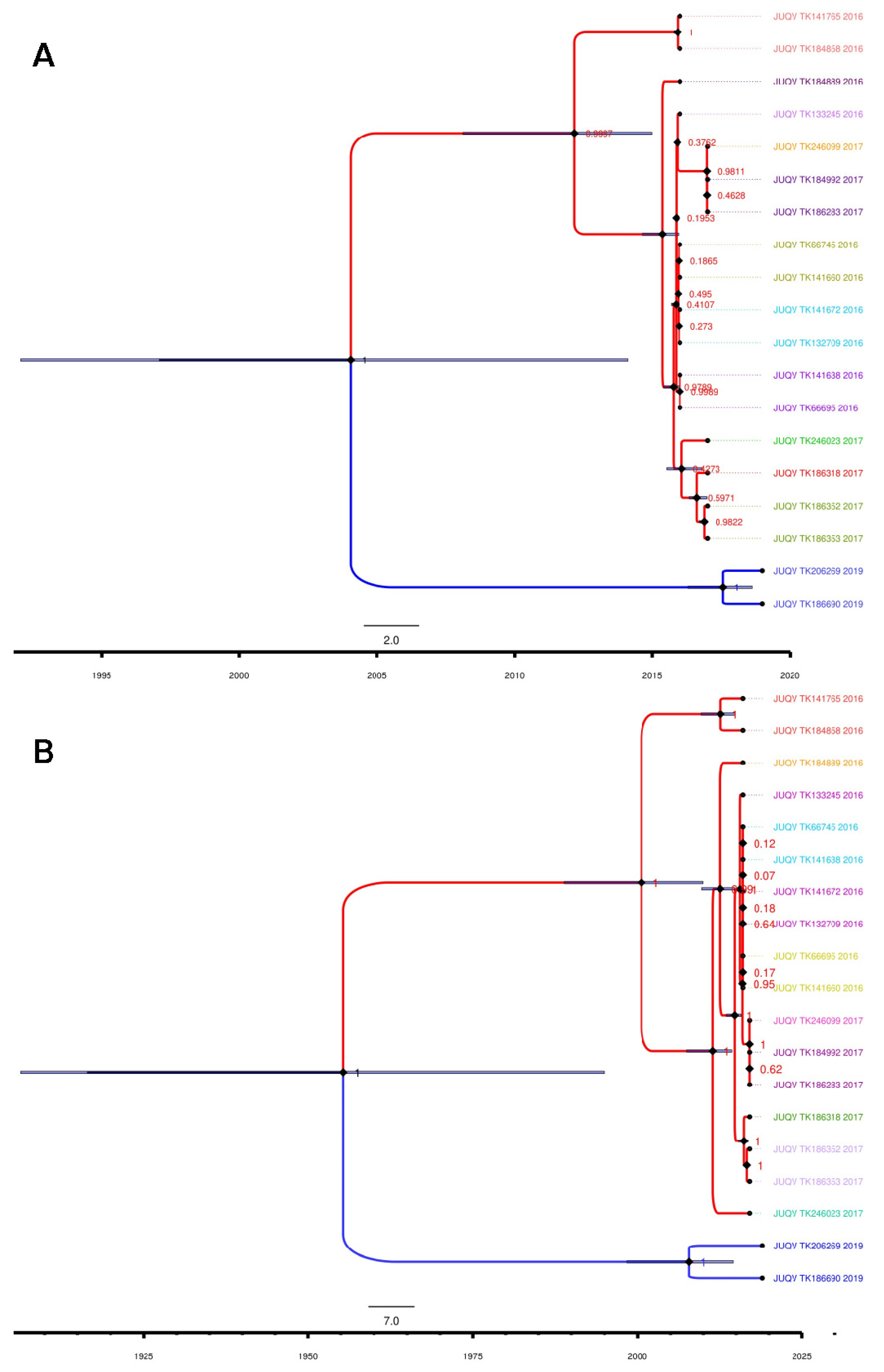
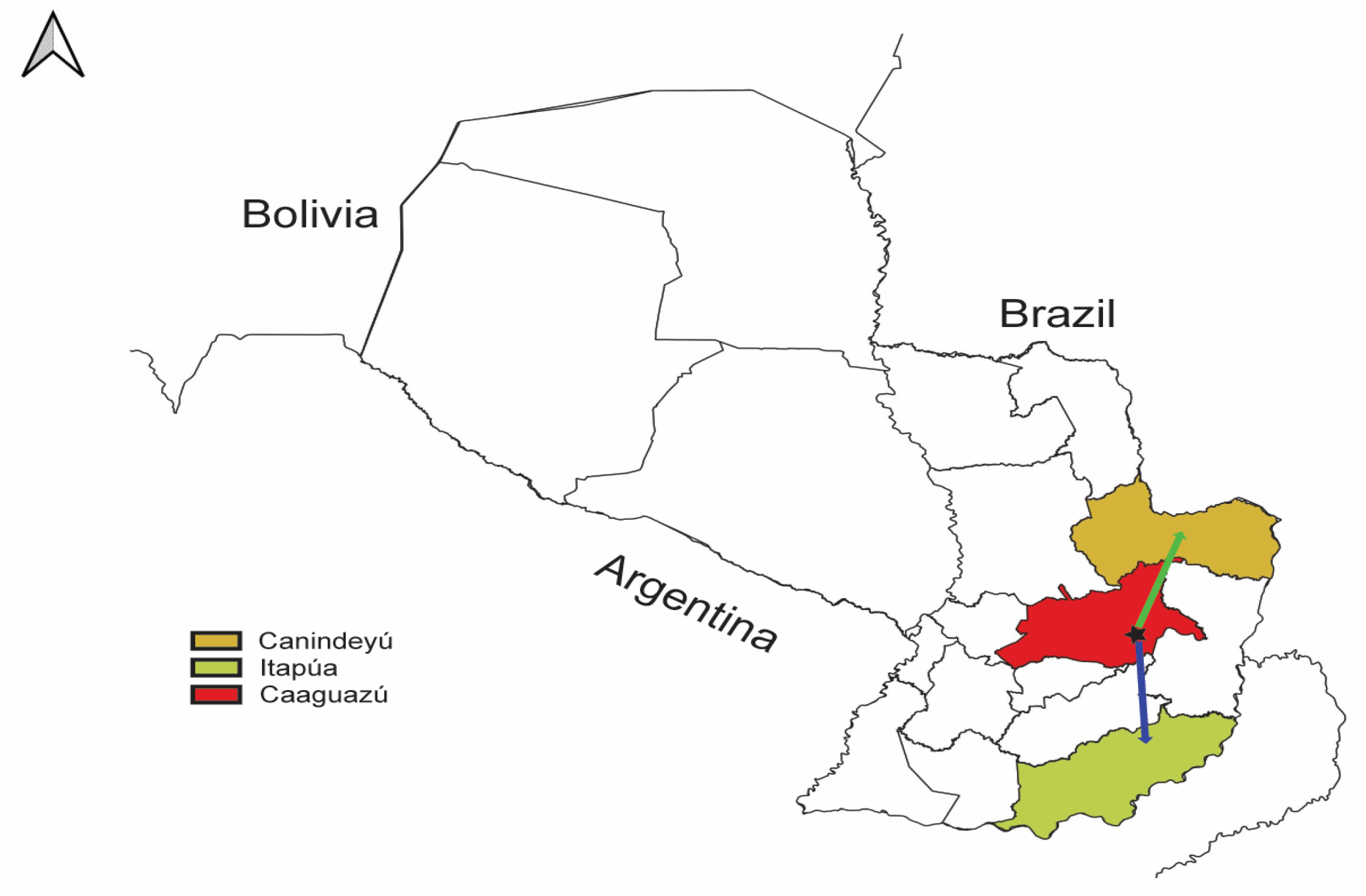
3.3. Phylogenetic and Phylogeographical Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jonsson, C.B.; Figueiredo, L.T.M.; Vapalahti, O. A Global Perspective on Hantavirus Ecology, Epidemiology, and Disease. Clin. Microbiol. Rev. 2010, 23, 412–441. [Google Scholar] [CrossRef]
- Mir, M.A. Hantaviruses. Clin. Lab. Med. 2010, 30, 67–91. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, P.W.; Johnson, K.M. Isolation of the Etiologic Agent of Korean Hemorrhagic Fever. J. Infect. Dis. 1978, 137, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Carey, D.E.; Reuben, R.; Panicker, K.N.; Shope, R.E.; Myers, R.M. Thottapalayam virus: A presumptive arbovirus isolated from a shrew in India. Indian J. Med. Res. 1971, 59, 1758–1760. [Google Scholar] [PubMed]
- Levis, S.; Morzunov, S.P.; Rowe, J.E.; Enria, D.; Pini, N.; Calderon, G.; Sabattini, M.; Jeor, S.C.S. Genetic Diversity and Epidemiology of Hantaviruses in Argentina. J. Infect. Dis. 1998, 177, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Padula, P.J.; Edelstein, A.; Miguel, S.D.L.; López, N.M.; Rossi, C.M.; Rabinovich, R.D. Hantavirus Pulmonary Syndrome Outbreak in Argentina: Molecular Evidence for Person-to-Person Transmission of Andes Virus. Virology 1998, 241, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.P.; Bellomo, C.; San Juan, J.; Pinna, D.; Forlenza, R.; Elder, M.; Padula, P.J. Person-to-Person Transmission of Andes Virus. Emerg. Infect. Dis. 2005, 11, 1848–1853. [Google Scholar] [CrossRef]
- Martínez, V.P.; Di Paola, N.; Alonso, D.O.; Pérez-Sautu, U.; Bellomo, C.M.; Iglesias, A.A.; Coelho, R.M.; López, B.; Periolo, N.; Larson, P.A.; et al. “Super-Spreaders” and Person-to-Person Transmission of Andes Virus in Argentina. N. Engl. J. Med. 2020, 383, 2230–2241. [Google Scholar] [CrossRef]
- Alonso, D.O.; Pérez-Sautu, U.; Bellomo, C.M.; Prieto, K.; Iglesias, A.; Coelho, R.; Periolo, N.; Domenech, I.; Talmon, G.; Hansen, R.; et al. Person-to-Person Transmission of Andes Virus in Hantavirus Pulmonary Syndrome, Argentina, 2014. Emerg. Infect. Dis. 2020, 26, 756–759. [Google Scholar] [CrossRef]
- Childs, J.E.; Ksiazek, T.G.; Spiropoulou, C.F.; Krebs, J.W.; Morzunov, S.; Maupin, G.O.; Gage, K.L.; Rollin, P.E.; Sarisky, J.; Enscore, R.E.; et al. Serologic and Genetic Identification of Peromyscus maniculatus as the Primary Rodent Reservoir for a New Hantavirus in the Southwestern United States. J. Infect. Dis. 1994, 169, 1271–1280. [Google Scholar] [CrossRef]
- Song, J.-W.; Baek, L.-J.; Gajdusek, D.C.; Yanagihara, R.; Gavrilovskaya, I.; Luft, B.; Mackow, E.; Hjelle, B. Isolation of pathogenic hantavirus from white-footed mouse (Peromyscus leucopus). Lancet 1994, 344, 1637. [Google Scholar] [CrossRef]
- Chu, Y.-K.; Owen, R.D.; Gonzalez, L.M.; Jonsson, C.B. The complex ecology of hantavirus in Paraguay. Am. J. Trop. Med. Hyg. 2003, 69, 263–268. [Google Scholar] [CrossRef]
- Vasconcelos, M.; Lima, V.P.; Iversson, L.B.; Rosa, M.D.; Da Rosa, A.P.; Da Rosa, E.S.; E Pereira, L.; Nassar, E.; Katz, G.; Matida, L.H.; et al. Hantavirus Pulmonary Syndrome in the rural area of juquitiba, são paulo metropolitan area, brazil. Rev. Inst. Med. Trop. São Paulo 1997, 39, 237–238. [Google Scholar] [CrossRef]
- Silva, M.V.D.; Vasconcelos, M.J.; Hidalgo, N.T.R.; Veiga, A.P.R.; Canzian, M.; Marotto, P.C.F.; Lima, V.C.P.D. Hantavirus Pulmonary Syndrome: Report of the first three cases in São Paulo, Brazil. Rev. Inst. Med. Trop. São Paulo 1997, 39, 231–234. [Google Scholar] [CrossRef]
- Suzuki, A.; Bisordi, I.; Levis, S.; Garcia, J.; Pereira, L.E.; Souza, R.P.; Sugahara, T.K.N.; Pini, N.; Enria, D.; Souza, L.T.M. Identifying Rodent Hantavirus Reservoirs, Brazil. Emerg. Infect. Dis. 2004, 10, 2127–2134. [Google Scholar] [CrossRef]
- Johnson, A.M.; Bowen, M.D.; Ksiazek, T.G.; Williams, R.J.; Bryan, R.T.; Mills, J.N.; Peters, C.J.; Nichol, S.T. Laguna Negra Virus Associated with HPS in Western Paraguay and Bolivia. Virology 1997, 238, 115–127. [Google Scholar] [CrossRef]
- Fulhorst, C.F.; Cajimat, M.N.B.; Utrera, A.; Milazzo, M.L.; Duno, G.M. Maporal virus, a hantavirus associated with the fulvous pygmy rice rat (Oligoryzomys fulvescens) in western Venezuela. Virus Res. 2004, 104, 139–144. [Google Scholar] [CrossRef]
- Torrez-Martinez, N. Bayou Virus-Associated Hantavirus Pulmonary Syndrome in Eastern Texas: Identification of the Rice Rat, Oryzomys palustris, as Reservoir Host. Emerg. Infect. Dis. 1998, 4, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Gaviria, M.; Rollin, P.E.; Hlady, W.G.; Ksiazek, T.G.; Armstrong, L.R.; Greenman, R.; Ravkov, E.; Kolber, M.; Anapol, H.; et al. Hantavirus pulmonary syndrome in Florida: Association with the newly identified Black Creek Canal virus. Am. J. Med. 1996, 100, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.H.; Hanson, J.D.; Cajimat, M.N.; Milazzo, M.L.; Fulhorst, C.F. Geographical Range of Rio Mamoré Virus (Family Bunyaviridae, Genus Hantavirus) in Association with the Small-Eared Pygmy Rice Rat (Oligoryzomys microtis). Vector-Borne Zoonotic Dis. 2010, 10, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.J.; Quiroz, E.; Gracia, F.; Sanchez, A.J.; Ksiazek, T.G.; Kitsutani, P.T.; Ruedas, L.A.; Tinnin, D.S.; Caceres, L.; Garcia, A.; et al. Hantavirus Pulmonary Syndrome in Panama: Identification of Novel Hantaviruses and Their Likely Reservoirs. Virology 2000, 277, 14–19. [Google Scholar] [CrossRef]
- Rollin, P.E.; Ksiazek, T.G.; Elliott, L.H.; Ravkov, E.V.; Martin, M.L.; Morzunov, S.; Livingstone, W.; Monroe, M.; Glass, G.; Ruo, S.; et al. Isolation of black creek canal virus, a new hantavirus fromSigmodon hispidus in Florida. J. Med. Virol. 1995, 46, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Monroe, M.C.; Morzunov, S.P.; Johnson, A.M.; Bowen, M.D.; Artsob, H.; Yates, T.; Peters, C.J.; Rollin, P.E.; Ksiazek, T.G.; Nichol, S.T. Genetic Diversity and Distribution of Peromyscus-Borne Hantaviruses in North America. Emerg. Infect. Dis. 1999, 5, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Padula, P.; Martinez, V.P.; Bellomo, C.; Maidana, S.; San Juan, J.; Tagliaferri, P.; Bargardi, S.; Vazquez, C.; Colucci, N.; Estévez, J.; et al. Pathogenic Hantaviruses, Northeastern Argentina and Eastern Paraguay. Emerg. Infect. Dis. 2007, 13, 1211–1214. [Google Scholar] [CrossRef] [PubMed]
- Delfraro, A.; Tomé, L.; D’Elía, G.; Clara, M.; Achával, F.; Russi, J.C.; Rodonz, J.R.A. Juquitiba-like Hantavirus from 2 Nonrelated Rodent Species, Uruguay. Emerg. Infect. Dis. 2008, 14, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, J.; Duré, A.I.L.; Negrão, R.; Ometto, T.; Thomazelli, L.M.; Durigon, E.L. Co-circulation in a single biome of the Juquitiba and Araraquara hantavirus detected in human sera in a sub-tropical region of Brazil: Co-Circulation of Human Hantavirus in Brazil. J. Med. Virol. 2015, 87, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Torres-Pérez, F.; Palma, R.E.; Boric-Bargetto, D.; Vial, C.; Ferrés, M.; Vial, P.A.; Martínez-Valdebenito, C.; Pavletic, C.; Parra, A.; Marquet, P.A.; et al. A 19 Year Analysis of Small Mammals Associated with Human Hantavirus Cases in Chile. Viruses 2019, 11, 848. [Google Scholar] [CrossRef]
- Riquelme, R.; Rioseco, M.L.; Bastidas, L.; Trincado, D.; Riquelme, M.; Loyola, H.; Valdivieso, F. Hantavirus Pulmonary Syndrome, Southern Chile, 1995–2012. Emerg. Infect. Dis. 2015, 21, 562–568. [Google Scholar] [CrossRef]
- Montgomery, J.M.; Blair, P.J.; Carroll, D.S.; Mills, J.N.; Gianella, A.; Iihoshi, N.; Briggiler, A.M.; Felices, V.; Salazar, M.; Olson, J.G.; et al. Hantavirus Pulmonary Syndrome in Santa Cruz, Bolivia: Outbreak Investigation and Antibody Prevalence Study. PLoS Negl. Trop. Dis. 2012, 6, e1840. [Google Scholar] [CrossRef]
- Saavedra Velasco, M.; Oyarce Calderón, A.; Vargas Herrera, N.; Pichardo Rodriguez, R.; Moreno Arteaga, C.M. Hantavirus in the peruvian jungle: A systematic review of series and cases reported. Rev. Fac. Med. Humana 2021, 21, 829–836. [Google Scholar] [CrossRef]
- Castillo Oré, R.M.; Forshey, B.M.; Huaman, A.; Villaran, M.V.; Long, K.C.; Kochel, T.J.; Guevara, C.; Montgomery, J.M.; Alvarez, C.A.; Vilcarromero, S.; et al. Serologic Evidence for Human Hantavirus Infection in Peru. Vector-Borne Zoonotic Dis. 2012, 12, 683–689. [Google Scholar] [CrossRef]
- Chu, Y.-K.; Goodin, D.; Owen, R.D.; Koch, D.; Jonsson, C.B. Sympatry of 2 Hantavirus Strains, Paraguay, 2003–2007. Emerg. Infect. Dis. 2009, 15, 1977–1980. [Google Scholar] [CrossRef]
- Guterres, A.; de Oliveira, R.C.; Fernandes, J.; Maia, R.M.; Teixeira, B.R.; Oliveira, F.C.G.; Bonvicino, C.R.; D’Andrea, P.S.; Schrago, C.G.; de Lemos, E.R.S. Co-circulation of Araraquara and Juquitiba Hantavirus in Brazilian Cerrado. Microb. Ecol. 2018, 75, 783–789. [Google Scholar] [CrossRef]
- Spruill-Harrell, B.; Pérez-Umphrey, A.; Valdivieso-Torres, L.; Cao, X.; Owen, R.D.; Jonsson, C.B. Impact of Predator Exclusion and Habitat on Seroprevalence of New World Orthohantavirus Harbored by Two Sympatric Rodents within the Interior Atlantic Forest. Viruses 2021, 13, 1963. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.C.; Júnior, J.B.; Pereira, L.S.; Meneguete, P.S.; Teixeira, B.R.; Guterres, A.; Bonvicino, C.R.; Dias, C.M.G.; De Jesus Oliveira Júnior, R.; Fernandes, J.; et al. A Fatal Hantavirus Pulmonary Syndrome Misdiagnosed as Dengue: An Investigation into the First Reported Case in Rio de Janeiro State, Brazil. Am. J. Trop. Med. Hyg. 2017, 97, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.C.; Sant’Ana, M.M.; Guterres, A.; Fernandes, J.; Hillesheim, N.L.F.K.; Lucini, C.; Gomes, R.; Lamas, C.; Bochner, R.; Zeccer, S.; et al. Hantavirus pulmonary syndrome in a highly endemic area of Brazil. Epidemiol. Infect. 2016, 144, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Borba, L.D.; Delfraro, A.; Raboni, S.M.; Duarte Dos Santos, C.N. First evidence of asymptomatic infection related to the Araucaria (Juquitiba-like) hantavirus. Case Rep. 2013, 2013, bcr2013009910. [Google Scholar] [CrossRef]
- Raboni, S.M.; Probst, C.M.; Bordignon, J.; Zeferino, A.; Dos Santos, C.N.D. Hantaviruses in Central South America: Phylogenetic analysis of the S segment from HPS cases in Paraná, Brazil. J. Med. Virol. 2005, 76, 553–562. [Google Scholar] [CrossRef]
- Raboni, S.M.; Rubio, G.; DE Borba, L.; Zeferino, A.; Skraba, I.; Goldenberg, S.; Dos Santos, C.N.D. Clinical survey of hantavirus in southern Brazil and the development of specific molecular diagnosis tools. Am. J. Trop. Med. Hyg. 2005, 72, 800–804. [Google Scholar] [CrossRef]
- Figueiredo, L.T.M.; Moreli, M.L.; De Sousa, R.L.M.; Borges, A.A.; De Figueiredo, G.G.; Machado, A.M.; Bisordi, I.; Nagasse-Sugahara, T.K.; Suzuki, A.; Pereira, L.E.; et al. Hantavirus Pulmonary Syndrome, Central Plateau, Southeastern, and Southern Brazil. Emerg. Infect. Dis. 2009, 15, 561–567. [Google Scholar] [CrossRef]
- Dinerstein, E. (Ed.) A Conservation Assessment of the Terrestrial Ecoregions of Latin America and the Caribbean; World Bank: Washington, DC, USA, 1995; ISBN 978-0-8213-3295-5. [Google Scholar]
- Owen, R.D.; Sánchez, H.; Atkinson, K.; McMahon, L.; Jonsson, C.B. New and noteworthy records of rodents (Mammalia, Rodentia, Cricetidae and Echimyidae) from Paraguay. Check List. 2018, 14, 721–730. [Google Scholar] [CrossRef]
- de la Sancha, N.U.; D’Elía, G. Additions to the Paraguayan mammal fauna: The first records of two marsupials (Didelphimorphia, Didelphidae) with comments on the alpha taxonomy of Cryptonanus and Philander. Mammalia 2015, 79, 343–356. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st-Century Forest Cover Change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Piquer-Rodríguez, M.; Fehlenberg, V.; Gavier Pizarro, G.; Kuemmerle, T. Land-Use Competition in the South American Chaco. In Land Use Competition; Niewöhner, J., Bruns, A., Hostert, P., Krueger, T., Nielsen, J.Ø., Haberl, H., Lauk, C., Lutz, J., Müller, D., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 215–229. ISBN 978-3-319-33626-8. [Google Scholar]
- Camp, J.V.; Spruill-Harrell, B.; Owen, R.D.; Solà-Riera, C.; Williams, E.P.; Eastwood, G.; Sawyer, A.M.; Jonsson, C.B. Mixed Effects of Habitat Degradation and Resources on Hantaviruses in Sympatric Wild Rodent Reservoirs within a Neotropical Forest. Viruses 2021, 13, 85. [Google Scholar] [CrossRef]
- Eastwood, G.; Camp, J.V.; Chu, Y.K.; Sawyer, A.M.; Owen, R.D.; Cao, X.; Taylor, M.K.; Valdivieso-Torres, L.; Sage, R.D.; Yu, A.; et al. Habitat, species richness and hantaviruses of sigmodontine rodents within the Interior Atlantic Forest, Paraguay. PLoS ONE 2018, 13, e0201307. [Google Scholar] [CrossRef]
- Clay, R.; Del Castillo, H.; de Egea, J. Paraguay: Contextos eco-regionales, geográficos y socioeconómicos. In Áreas de Importancia para la Conservación de las aves en Paraguay; Guyra Paraguay/BirdLife International: Asunción, Paraguay, 2008; p. 466. ISBN 978-99953-848-2-1. [Google Scholar]
- Sikes, R.S.; the Animal Care and Use Committee of the American Society of Mammalogists. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef]
- D’Elia, G.; Pardiñas, U.F.J. Subfamily Sigmodontinae. In Mammals in South America; The University of Chicago Press: Chicago, IL, USA, 2015; Volume 2, pp. 63–687. ISBN 13: 978-0-226-16957-6. [Google Scholar]
- Taylor, M.K.; Williams, E.P.; Wongsurawat, T.; Jenjaroenpun, P.; Nookaew, I.; Jonsson, C.B. Amplicon-Based, Next-Generation Sequencing Approaches to Characterize Single Nucleotide Polymorphisms of Orthohantavirus Species. Front. Cell. Infect. Microbiol. 2020, 10, 565591. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package 2022. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 4 January 2023).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. J. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Lemey, P.; Rambaut, A.; Drummond, A.J.; Suchard, M.A. Bayesian Phylogeography Finds Its Roots. PLoS Comput. Biol. 2009, 5, e1000520. [Google Scholar] [CrossRef] [PubMed]
- Dellicour, S.; Gill, M.S.; Faria, N.R.; Rambaut, A.; Pybus, O.G.; Suchard, M.A.; Lemey, P. Relax, Keep Walking—A Practical Guide to Continuous Phylogeographic Inference with BEAST. Mol. Biol. Evol. 2021, 38, 3486–3493. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Bouckaert, R.R.; Drummond, A.J. bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol. Biol. 2017, 17, 42. [Google Scholar] [CrossRef]
- Kingman, J.F.C. The coalescent. Stoch. Process. Their Appl. 1982, 13, 235–248. [Google Scholar] [CrossRef]
- Ramsden, C.; Melo, F.L.; Figueiredo, L.M.; Holmes, E.C.; Zanotto, P.M.A.; the VGDN Consortium. High Rates of Molecular Evolution in Hantaviruses. Mol. Biol. Evol. 2008, 25, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, R.R. DensiTree: Making sense of sets of phylogenetic trees. Bioinformatics 2010, 26, 1372–1373. [Google Scholar] [CrossRef]
- Nahata, K.D.; Bielejec, F.; Monetta, J.; Dellicour, S.; Rambaut, A.; Suchard, M.A.; Baele, G.; Lemey, P. SPREAD 4: Online visualisation of pathogen phylogeographic reconstructions. Virus Evol. 2022, 8, veac088. [Google Scholar] [CrossRef]
- Dellicour, S.; Rose, R.; Faria, N.R.; Lemey, P.; Pybus, O.G. SERAPHIM: Studying environmental rasters and phylogenetically informed movements. Bioinformatics 2016, 32, 3204–3206. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Pybus, O.G.; Suchard, M.A.; Lemey, P.; Bernardin, F.J.; Rambaut, A.; Crawford, F.W.; Gray, R.R.; Arinaminpathy, N.; Stramer, S.L.; Busch, M.P.; et al. Unifying the spatial epidemiology and molecular evolution of emerging epidemics. Proc. Natl. Acad. Sci. USA 2012, 109, 15066–15071. [Google Scholar] [CrossRef]
- Trovão, N.S.; Suchard, M.A.; Baele, G.; Gilbert, M.; Lemey, P. Bayesian Inference Reveals Host-Specific Contributions to the Epidemic Expansion of Influenza A H5N1. Mol. Biol. Evol. 2015, 32, msv185. [Google Scholar] [CrossRef]
- Holmes, E.C.; Zhang, Y.-Z. The evolution and emergence of hantaviruses. Curr. Opin. Virol. 2015, 10, 27–33. [Google Scholar] [CrossRef]
- Guo, W.-P.; Lin, X.-D.; Wang, W.; Tian, J.-H.; Cong, M.-L.; Zhang, H.-L.; Wang, M.-R.; Zhou, R.-H.; Wang, J.-B.; Li, M.-H.; et al. Phylogeny and Origins of Hantaviruses Harbored by Bats, Insectivores, and Rodents. PLOS Pathog. 2013, 9, e1003159. [Google Scholar] [CrossRef]
- Yanagihara, R.; Gu, S.H.; Arai, S.; Kang, H.J.; Song, J.-W. Hantaviruses: Rediscovery and new beginnings. Virus Res. 2014, 187, 6–14. [Google Scholar] [CrossRef]
- Ramsden, C.; Holmes, E.C.; Charleston, M.A. Hantavirus Evolution in Relation to Its Rodent and Insectivore Hosts: No Evidence for Codivergence. Mol. Biol. Evol. 2008, 26, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Plyusnin, A.; Sironen, T. Evolution of hantaviruses: Co-speciation with reservoir hosts for more than 100MYR. Virus Res. 2014, 187, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Song, J.-W.; Sumibcay, L.; Bennett, S.N.; Nerurkar, V.R.; Parmenter, C.; Cook, J.A.; Yates, T.L.; Yanagihara, R. Hantavirus in Northern Short-tailed Shrew, United States. Emerg. Infect. Dis. 2007, 13, 1420–1423. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Bennett, S.N.; Sumibcay, L.; Cook, J.A.; Song, J.-W.; Hope, A.; Parmenter, C.; Nerurkar, V.R.; Yates, T.L.; Yanagihara, R. Phylogenetically distinct hantaviruses in the masked shrew (Sorex cinereus) and dusky shrew (Sorex monticolus) in the United States. Am. J. Trop. Med. Hyg. 2008, 78, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Klempa, B.; Fichet-Calvet, E.; Lecompte, E.; Auste, B.; Aniskin, V.; Meisel, H.; Barrière, P.; Koivogui, L.; ter Meulen, J.; Krüger, D.H. Novel Hantavirus Sequences in Shrew, Guinea. Emerg. Infect. Dis. 2007, 13, 520–522. [Google Scholar] [CrossRef]
- Song, J.-W.; Kang, H.J.; Song, K.-J.; Truong, T.T.; Bennett, S.N.; Arai, S.; Truong, N.U.; Yanagihara, R. Newfound Hantavirus in Chinese Mole Shrew, Vietnam. Emerg. Infect. Dis. 2007, 13, 1784–1787. [Google Scholar] [CrossRef]
- de Araujo, J.; Thomazelli, L.M.; Henriques, D.A.; Lautenschalager, D.; Ometto, T.; Dutra, L.M.; Aires, C.C.; Favorito, S.; Durigon, E.L. Detection of hantavirus in bats from remaining rain forest in São Paulo, Brazil. BMC Res. Notes 2012, 5, 690. [Google Scholar] [CrossRef]
- Gu, S.; Lim, B.; Kadjo, B.; Arai, S.; Kim, J.-A.; Nicolas, V.; Lalis, A.; Denys, C.; Cook, J.; Dominguez, S.; et al. Molecular Phylogeny of Hantaviruses Harbored by Insectivorous Bats in Côte d’Ivoire and Vietnam. Viruses 2014, 6, 1897–1910. [Google Scholar] [CrossRef]
- Melo, M.N.; Maia, F.G.M.; Vieira, T.M.; Jonsson, C.B.; Goodin, D.; Gonçalves, C.B.; Barroso, P.D.; Sabino-Santos, G.; de Lara Muylaert, R.; Salazar-Bravo, J.; et al. Evidence of Hantavirus Infection Among Bats in Brazil. Am. J. Trop. Med. Hyg. 2015, 93, 404–406. [Google Scholar] [CrossRef]
- Sabino-Santos, G., Jr.; Maia, F.G.M.; Martins, R.B.; Gagliardi, T.B.; de Souza, W.M.; Muylaert, R.L.; Luna, L.K.; Melo, D.M.; Cardoso, R.; Barbosa, N.; et al. Natural infection of Neotropical bats with hantavirus in Brazil. Sci. Rep. 2018, 8, 9018. [Google Scholar] [CrossRef]
- Raboni, S.M.; Delfraro, A.; de Borba, L.; Teixeira, B.R.; Stella, V.; de Araujo, M.R.; Carstensen, S.; Rubio, G.; Maron, A.; Lemos, E.R.S.; et al. Hantavirus infection prevalence in wild rodents and human anti-hantavirus serological profiles from different geographic areas of South Brazil. Am. J. Trop. Med. Hyg. 2012, 87, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Guterres, A.; De Oliveira, R.; Fernandes, J.; Strecht, L.; Casado, F.; De Oliveira, F.C.G.; D’Andrea, P.; Bonvicino, C.; Schrago, C.; De Lemos, E.R. Characterization of Juquitiba Virus in Oligoryzomys fornesi from Brazilian Cerrado. Viruses 2014, 6, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.C.; Gentile, R.; Guterres, A.; Fernandes, J.; Teixeira, B.R.; Vaz, V.; Valdez, F.P.; Vicente, L.H.B.; Da Costa-Neto, S.F.; Bonvicino, C.; et al. Ecological study of hantavirus infection in wild rodents in an endemic area in Brazil. Acta Tropica 2014, 131, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Milholland, M.T.; Castro-Arellano, I.; Suzán, G.; Garcia-Peña, G.E.; Lee, T.E.; Rohde, R.E.; Alonso Aguirre, A.; Mills, J.N. Global Diversity and Distribution of Hantaviruses and Their Hosts. EcoHealth 2018, 15, 163–208. [Google Scholar] [CrossRef]
- Chu, Y.K.; Milligan, B.; Owen, R.D.; Goodin, D.G.; Jonsson, C.B. Phylogenetic and geographical relationships of hantavirus strains in eastern and western Paraguay. Am. J. Trop. Med. Hyg. 2006, 75, 1127–1134. [Google Scholar] [CrossRef]
- Vaheri, A.; Strandin, T.; Hepojoki, J.; Sironen, T.; Henttonen, H.; Mäkelä, S.; Mustonen, J. Uncovering the mysteries of hantavirus infections. Nat. Rev. Microbiol. 2013, 11, 539–550. [Google Scholar] [CrossRef]
- Meier, K.; Thorkelsson, S.R.; Quemin, E.R.J.; Rosenthal, M. Hantavirus Replication Cycle—An Updated Structural Virology Perspective. Viruses 2021, 13, 1561. [Google Scholar] [CrossRef]
- Jääskeläinen, K.M.; Kaukinen, P.; Minskaya, E.S.; Plyusnina, A.; Vapalahti, O.; Elliott, R.M.; Weber, F.; Vaheri, A.; Plyusnin, A. Tula and Puumala hantavirus NSs ORFs are functional and the products inhibit activation of the interferon-beta promoter. J. Med. Virol. 2007, 79, 1527–1536. [Google Scholar] [CrossRef]
- Cirkovic, V.; Dellicour, S.; Stamenkovic, G.; Siljic, M.; Gligic, A.; Stanojevic, M. Phylogeographic analysis of Tula hantavirus highlights a single introduction to central Europe. Virus Evol. 2022, 8, veac112. [Google Scholar] [CrossRef]
- Dellicour, S.; Rose, R.; Pybus, O.G. Explaining the geographic spread of emerging epidemics: A framework for comparing viral phylogenies and environmental landscape data. BMC Bioinform. 2016, 17, 82. [Google Scholar] [CrossRef]
- Kalkauskas, A.; Perron, U.; Sun, Y.; Goldman, N.; Baele, G.; Guindon, S.; De Maio, N. Sampling bias and model choice in continuous phylogeography: Getting lost on a random walk. PLOS Comput. Biol. 2021, 17, e1008561. [Google Scholar] [CrossRef] [PubMed]
- Dellicour, S.; Vrancken, B.; Trovão, N.S.; Fargette, D.; Lemey, P. On the importance of negative controls in viral landscape phylogeography. Virus Evol. 2018, 4, vey023. [Google Scholar] [CrossRef] [PubMed]
- De Maio, N.; Wu, C.-H.; O’Reilly, K.M.; Wilson, D. New Routes to Phylogeography: A Bayesian Structured Coalescent Approximation. PLoS Genet. 2015, 11, e1005421. [Google Scholar] [CrossRef]
- Souza, W.M.; Bello, G.; Amarilla, A.A.; Alfonso, H.L.; Aquino, V.H.; Figueiredo, L.T.M. Phylogeography and evolutionary history of rodent-borne hantaviruses. Infect. Genet. Evol. 2014, 21, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Guterres, A.; De Oliveira, R.C.; Fernandes, J.; D’Andrea, P.S.; Bonvicino, C.R.; Bragagnolo, C.; Guimarães, G.D.; Almada, G.L.; Machado, R.R.; Lavocat, M.; et al. Phylogenetic analysis of the S segment from Juquitiba hantavirus: Identification of two distinct lineages in Oligoryzomys nigripes. Infect. Genet. Evol. 2013, 18, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Black, W.C.; Doty, J.B.; Hughes, M.T.; Beaty, B.J.; Calisher, C.H. Temporal and geographic evidence for evolution of Sin Nombre virus using molecular analyses of viral RNA from Colorado, New Mexico and Montana. Virol. J. 2009, 6, 102. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Chen, Y.; Li, M.; Ma, J.; Wang, B.; Luo, J.; He, H. Genetic Characterization and Molecular Evolution of Urban Seoul Virus in Southern China. Viruses 2019, 11, 1137. [Google Scholar] [CrossRef]
- Drummond, A.J.; Bouckaert, R.R. Bayesian Evolutionary Analysis with BEAST, 1st ed.; Cambridge University Press: Cambridge, UK, 2015; ISBN 978-1-107-01965-2. [Google Scholar]
- Frost, S.D.W.; Volz, E.M. Viral phylodynamics and the search for an ‘effective number of infections’. Phil. Trans. R. Soc. B 2010, 365, 1879–1890. [Google Scholar] [CrossRef]
- Owen, R.D. Ecology of small terrestrial mammals in an isolated cerrado patch, eastern paraguay: Communities, species, and effects of enso, precipitation, and fire. Mastozool. Neotrop. 2013, 20, 97–112. [Google Scholar]
- Puttker, T.; Sommer, S.; Meyer-Lucht, Y. Movement distances of five rodent and two marsupial species in forest fragments of the coastal Atlantic Rainforest, Brazil. Ecotropica 2006, 12, 131–139. [Google Scholar]
- Pires, A.S.; Koeler Lira, P.; Fernandez, F.A.S.; Schittini, G.M.; Oliveira, L.C. Frequency of movements of small mammals among Atlantic Coastal Forest fragments in Brazil. Biol. Conserv. 2002, 108, 229–237. [Google Scholar] [CrossRef]
- Guterres, A.; De Oliveira, R.C.; Fernandes, J.; De Lemos, E.R.S. The mystery of the phylogeographic structural pattern in rodent-borne hantaviruses. Mol. Phylogenet. Evol. 2019, 136, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Seo, T.-K.; Thorne, J.L.; Hasegawa, M.; Kishino, H. A viral sampling design for testing the molecular clock and for estimating evolutionary rates and divergence times. Bioinformatics 2002, 18, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.W.; Lanfear, R.; Bromham, L.; Phillips, M.J.; Soubrier, J.; Rodrigo, A.G.; Cooper, A. Time-dependent rates of molecular evolution: Time-dependent rates of molecular evolution. Mol. Ecol. 2011, 20, 3087–3101. [Google Scholar] [CrossRef]
| Lines | A | B | C | D | E | F | G | Total |
|---|---|---|---|---|---|---|---|---|
| Species | (N = 66) | (N = 68) | (N = 68) | (N = 50) | (N = 67) | (N = 36) | (N = 3) | (N = 358) |
| Family: Cricetidae | ||||||||
| Akodon toba | 23 (34.8%) | 22 (32.4%) | 30 (44.1%) | 11 (22.0%) | 32 (47.8%) | 2 (5.6%) | 0 (0%) | 120 (33.5%) |
| Andalgalomys pearsoni | 0 (0%) | 1 (1.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.3%) |
| Calomys callosus | 2 (3.0%) | 5 (7.4%) | 2 (2.9%) | 0 (0%) | 3 (4.5%) | 0 (0%) | 0 (0%) | 12 (3.4%) |
| Calomys laucha | 5 (7.6%) | 14 (20.6%) | 11 (16.2%) | 7 (14.0%) | 2 (3.0%) | 2 (5.6%) | 2 (66.7%) | 43 (12.0%) |
| Graomys chacoensis | 8 (12.1%) | 3 (4.4%) | 2 (2.9%) | 4 (8.0%) | 6 (9.0%) | 2 (5.6%) | 0 (0%) | 25 (7.0%) |
| Holochilus chacarius | 0 (0%) | 0 (0%) | 1 (1.5%) | 0 (0%) | 0 (0%) | 6 (16.7%) | 0 (0%) | 7 (2.0%) |
| Necromys lasiurus | 15 (22.7%) | 12 (17.6%) | 15 (22.1%) | 21 (42.0%) | 11 (16.4%) | 21 (58.3%) | 1 (33.3%) | 96 (26.8%) |
| Pseudoryzomys simplex | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.3%) |
| Oligoryzomys chacoensis | 11 (16.7%) | 5 (7.4%) | 5 (7.4%) | 3 (6.0%) | 10 (14.9%) | 2 (5.6%) | 0 (0%) | 36 (10.1%) |
| Oligoryzomys mattogrossae | 0 (0%) | 3 (4.4%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2.8%) | 0 (0%) | 4 (1.1%) |
| Family: Didelphidae | ||||||||
| Gracilinanus agilis | 1 (1.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.3%) |
| Monodelphis kunsi | 1 (1.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.3%) |
| Thylamys pusillus | 0 (0%) | 2 (2.9%) | 2 (2.9%) | 3 (6.0%) | 2 (3.0%) | 0 (0%) | 0 (0%) | 9 (2.5%) |
| Family: Caviidae | ||||||||
| Galea musteloides | 0 (0%) | 1 (1.5%) | 0 (0%) | 0 (0%) | 1 (1.5%) | 0 (0%) | 0 (0%) | 2 (0.6%) |
| Lines | A | B | C | D | E | F | Total |
|---|---|---|---|---|---|---|---|
| Species | (N = 37) | (N = 39) | (N = 50) | (N = 41) | (N = 31) | (N = 56) | (N = 254) |
| Family: Cricetidae | |||||||
| Akodon azarae | 12 (32.4%) | 21 (53.8%) | 12 (24.0%) | 4 (9.8%) | 7 (22.6%) | 10 (17.9%) | 66 (26.0%) |
| Akodon montensis | 16 (43.2%) | 5 (12.8%) | 22 (44.0%) | 18 (43.9%) | 5 (16.1%) | 30 (53.6%) | 96 (37.8%) |
| Akodon sp. | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2.4%) | 0 (0%) | 0 (0%) | 1 (0.4%) |
| Euryoryzomys sp. | 0 (0%) | 0 (0%) | 1 (2.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.4%) |
| Mus musculus | 1 (2.7%) | 2 (5.1%) | 0 (0%) | 1 (2.4%) | 9 (29.0%) | 1 (1.8%) | 14 (5.5%) |
| Oligoryzomys flavescens | 1 (2.7%) | 2 (5.1%) | 0 (0%) | 2 (4.9%) | 0 (0%) | 2 (3.6%) | 7 (2.8%) |
| Oligoryzomys mattogrossae | 0 (0%) | 2 (5.1%) | 1 (2.0%) | 2 (4.9%) | 1 (3.2%) | 0 (0%) | 6 (2.4%) |
| Oligoryzomys nigripes | 6 (16.2%) | 5 (12.8%) | 12 (24.0%) | 7 (17.1%) | 9 (29.0%) | 10 (17.9%) | 49 (19.3%) |
| Oligoryzomys | 0 (0%) | 1 (2.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.4%) |
| Sooretamys angouya | 0 (0%) | 1 (2.6%) | 1 (2.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (0.8%) |
| Thaptomys nigrita | 0 (0%) | 0 (0%) | 0 (0%) | 6 (14.6%) | 0 (0%) | 3 (5.4%) | 9 (3.5%) |
| Family: Didelphidae | |||||||
| Monodelphis dimidiata | 1 (2.7%) | 0 (0%) | 1 (2.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (0.8%) |
| Location | Parameter | A | B | C | D | E | F | G | Overall |
|---|---|---|---|---|---|---|---|---|---|
| Boquerón | Shannon diversity (H) | 1.69 | 1.88 | 1.55 | 1.59 | 1.56 | 1.35 | 0.63 | 1.82 |
| Shannon equitability (EH) | 0.81 | 0.79 | 0.75 | 0.82 | 0.71 | 0.70 | 0.91 | 0.69 | |
| Itapúa | Shannon diversity (H) | 1.32 | 1.50 | 1.36 | 1.65 | 1.50 | 1.30 | N/A | 1.64 |
| Shannon equitability (EH) | 0.73 | 0.72 | 0.70 | 0.79 | 0.75 | 0.72 | N/A | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nnamani, E.I.; Spruill-Harrell, B.; Williams, E.P.; Taylor, M.K.; Owen, R.D.; Jonsson, C.B. Deep Sequencing to Reveal Phylo-Geographic Relationships of Juquitiba Virus in Paraguay. Viruses 2023, 15, 1798. https://doi.org/10.3390/v15091798
Nnamani EI, Spruill-Harrell B, Williams EP, Taylor MK, Owen RD, Jonsson CB. Deep Sequencing to Reveal Phylo-Geographic Relationships of Juquitiba Virus in Paraguay. Viruses. 2023; 15(9):1798. https://doi.org/10.3390/v15091798
Chicago/Turabian StyleNnamani, Evans Ifebuche, Briana Spruill-Harrell, Evan Peter Williams, Mariah K. Taylor, Robert D. Owen, and Colleen B. Jonsson. 2023. "Deep Sequencing to Reveal Phylo-Geographic Relationships of Juquitiba Virus in Paraguay" Viruses 15, no. 9: 1798. https://doi.org/10.3390/v15091798






