A Novel Variant of Avian Reovirus Is Pathogenic to Vaccinated Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Vaccine
2.2. Cells and Animals
2.3. Clinical Samples and Virus Isolation
2.4. Immunofluorescence Assay
2.5. Viral RNA Extraction, Reverse Transcription-Polymerase Chain Reaction (RT-PCR), and Sequencing
2.6. Sequence Analysis
2.7. Pathogenicity Evaluation of the Isolated Strain
2.8. Cross-Neutralization Assay
2.9. Immune Protection Assay
3. Results
3.1. SD19/11103 Isolation and Identification
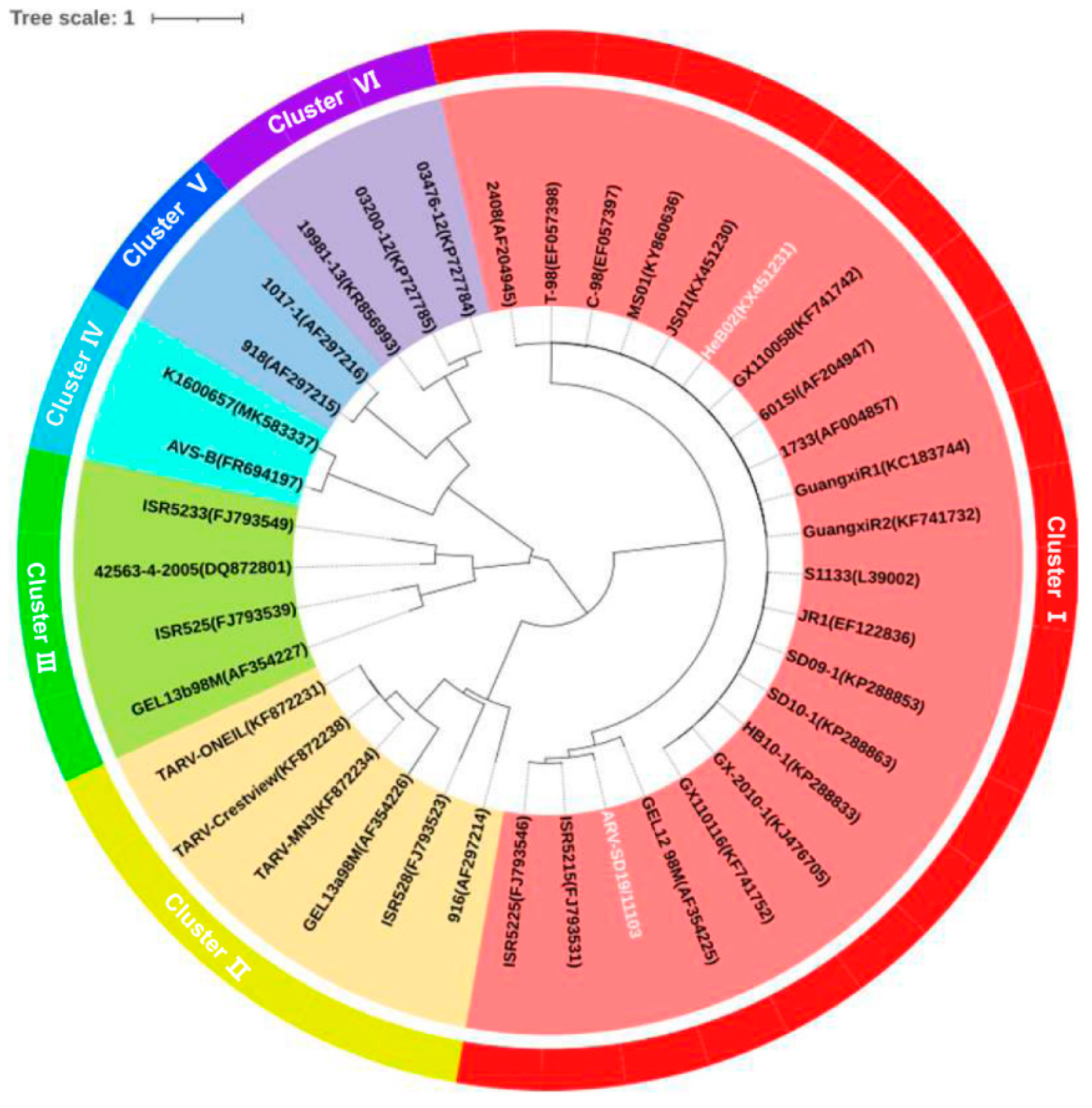
3.2. SD19/11103 Evolutionarily Belongs to the Variant ARV
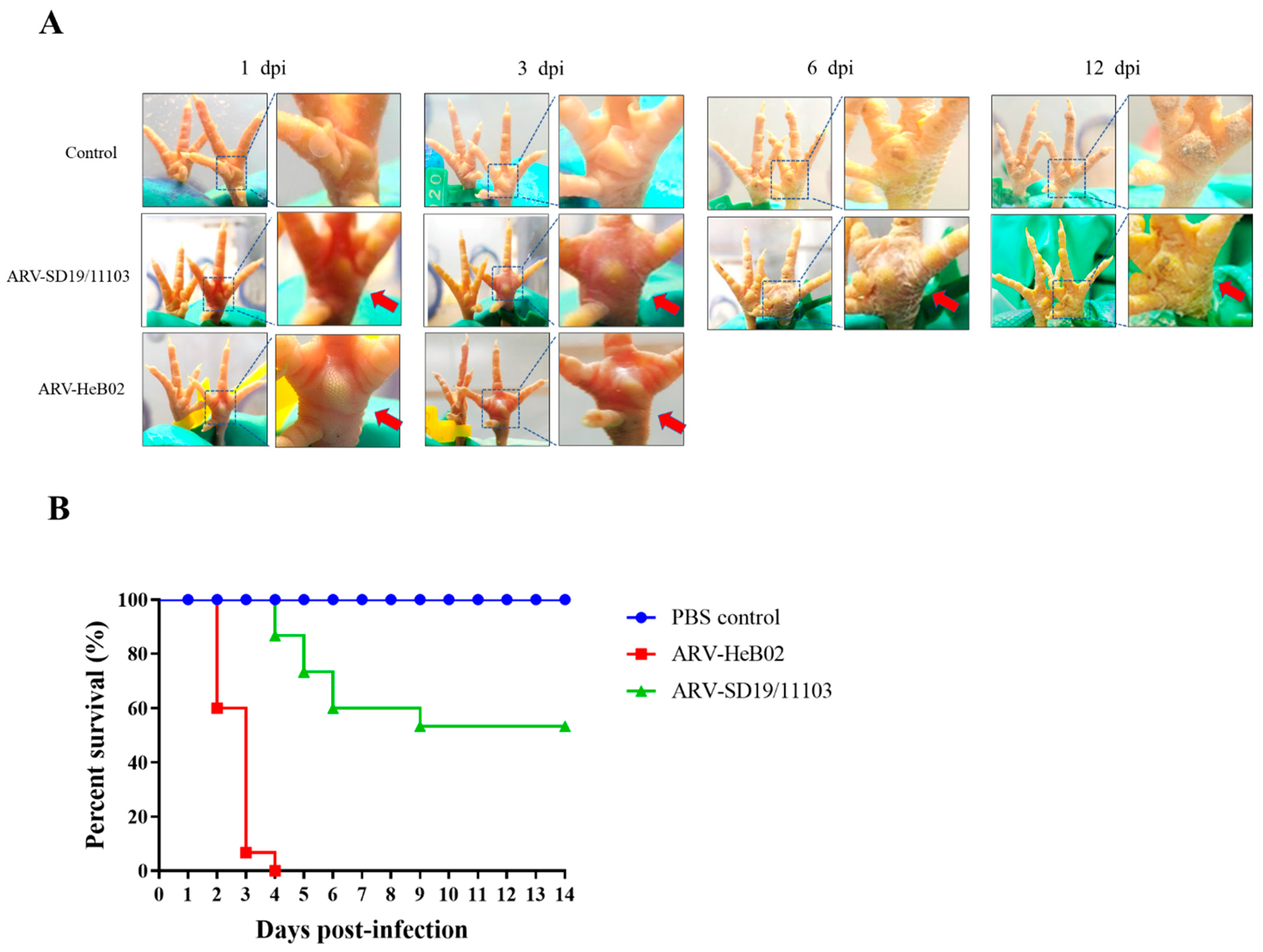
3.3. SD19/11103 Is Pathogenic to SPF Chickens
3.4. SD19/11103 Showed Obvious Antigenicity Different Compared with Genotype Cluster I ARV
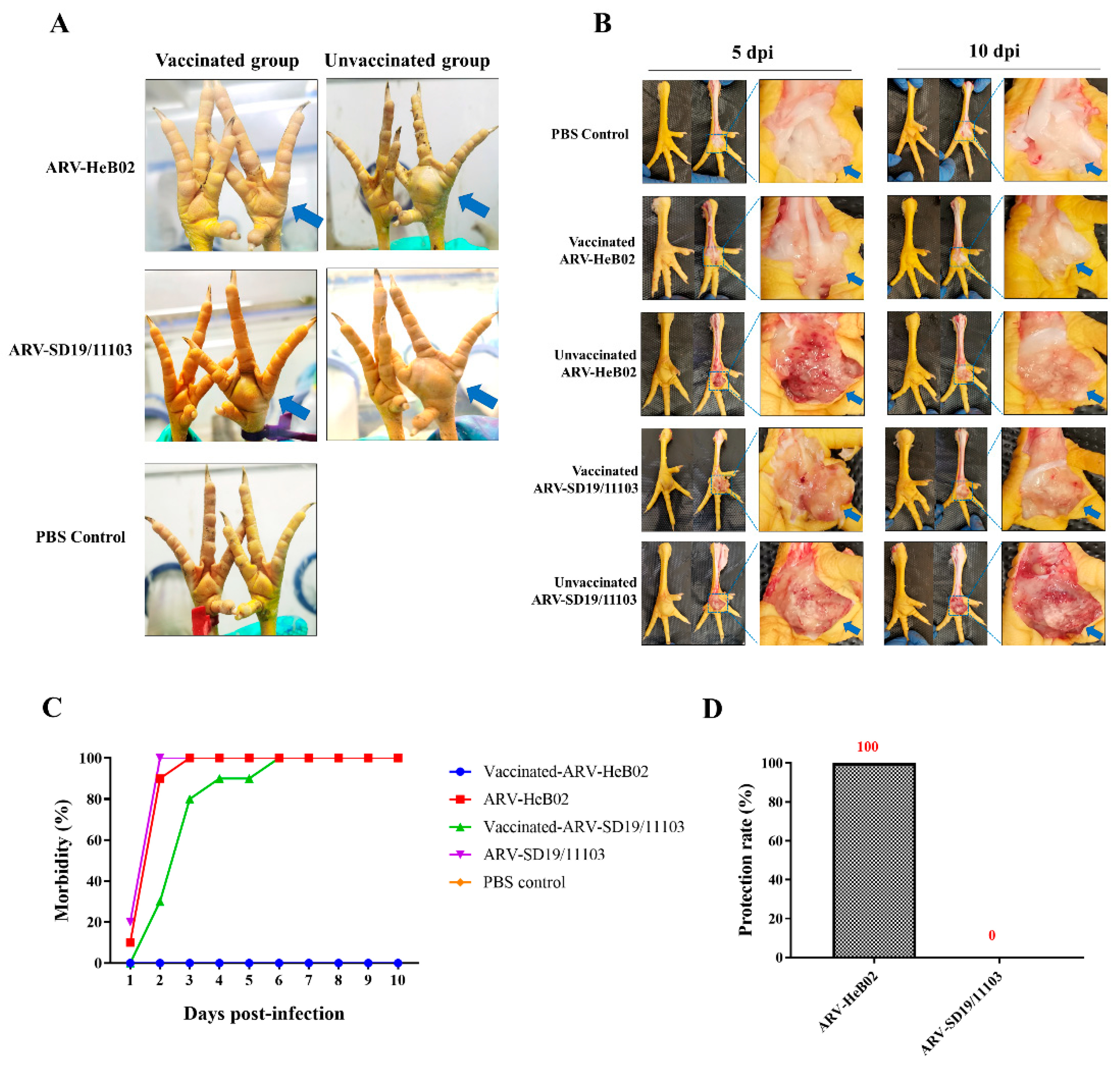
3.5. SD19/11103 Induced Severe Lesions in Immunized Chickens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spandidos, D.A.; Graham, A.F. Physical and chemical characterization of an avian reovirus. J. Virol. 1976, 19, 968–976. [Google Scholar] [CrossRef]
- Fahey, J.E.; Crawley, J.F. Studies on Chronic Respiratory Disease of Chickens II. Isolation of a Virus. Can. J. Comp. Med. Vet. Sci. 1954, 18, 13–21. [Google Scholar]
- Dandar, E.; Balint, A.; Kecskemeti, S.; Szentpali-Gavaller, K.; Kisfali, P.; Melegh, B.; Farkas, S.L.; Banyai, K. Detection and characterization of a divergent avian reovirus strain from a broiler chicken with central nervous system disease. Arch. Virol. 2013, 158, 2583–2588. [Google Scholar] [CrossRef]
- Lu, H.; Tang, Y.; Dunn, P.A.; Wallner-Pendleton, E.A.; Lin, L.; Knoll, E.A. Isolation and molecular characterization of newly emerging avian reovirus variants and novel strains in Pennsylvania, USA, 2011–2014. Sci. Rep. 2015, 5, 14727. [Google Scholar] [CrossRef]
- Nunez, L.F.N.; Santander Parra, S.H.; Mettifogo, E.; Catroxo, M.H.B.; Astolfi-Ferreira, C.S.; Piantino Ferreira, A.J. Isolation of chicken astrovirus from specific pathogen-free chicken embryonated eggs. Poult. Sci. 2015, 94, 947–954. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, H. Genomic characterization of a broiler reovirus field strain detected in Pennsylvania. Infect. Genet. Evol. 2015, 31, 177–182. [Google Scholar] [CrossRef]
- Zhong, L.; Gao, L.; Liu, Y.; Li, K.; Wang, M.; Qi, X.; Gao, Y.; Wang, X. Genetic and pathogenic characterisation of 11 avian reovirus isolates from northern China suggests continued evolution of virulence. Sci. Rep. 2016, 6, 35271. [Google Scholar] [CrossRef] [PubMed]
- Meanger, J.; Wickramasinghe, R.; Enriquez, C.E.; Robertson, M.D.; Wilcox, G.E. Type-specific antigenicity of avian reoviruses. Avian Pathol. 1995, 24, 121–134. [Google Scholar] [CrossRef]
- Benavente, J.; Martinez-Costas, J. Avian reovirus: Structure and biology. Virus Res. 2007, 123, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Dandar, E.; Huhtamo, E.; Farkas, S.L.; Oldal, M.; Jakab, F.; Vapalahti, O.; Banyai, K. Complete genome analysis identifies Tvarminne avian virus as a candidate new species within the genus Orthoreovirus. J. Gen. Virol. 2014, 95, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Palya, V.; Glavits, R.; Dobos-Kovacs, M.; Ivanics, E.; Nagy, E.; Banyai, K.; Reuter, G.; Szucs, G.; Dan, A.; Benko, M. Reovirus identified as cause of disease in young geese. Avian Pathol. 2003, 32, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Xie, Z.; Xie, L.; Liu, J.; Pang, Y.; Deng, X.; Xie, Z.; Fan, Q.; Luo, S.; Feng, J.; et al. Sequencing and phylogenetic analysis of an avian reovirus genome. Virus Genes 2014, 48, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.; Balk, F.; Born, L.; van Roozelaar, D.; Heijmans, J.; Gielkens, A.; ter Huurne, A. Classification of Dutch and German avian reoviruses by sequencing the sigma C protein. Vet. Res. 2003, 34, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Palomino-Tapia, V.; Mitevski, D.; Inglis, T.; van der Meer, F.; Abdul-Careem, M.F. Molecular characterization of emerging avian reovirus variants isolated from viral arthritis cases in Western Canada 2012–2017 based on partial sigma (sigma)C gene. Virology 2018, 522, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tamura, K.; Nei, M. MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 2004, 5, 150–163. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Krisztian, B.; Eszter, D.; Dorsey, K.M.; Tamas, M.; Vilmos, P. The genomic constellation of a novel avian orthoreovirus strain associated with runting-stunting syndrome in broilers. Virus Genes 2011, 42, 82–89. [Google Scholar]
- Niu, X.; Wang, Y.; Li, M.; Zhang, X.; Wu, Y. Transcriptome analysis of avian reovirusmediated changes in gene expression of normal chicken fibroblast DF-1 cells. BMC Genom. 2017, 18, 911. [Google Scholar] [CrossRef]
- Lublin, A.; Goldenberg, D.; Rosenbluth, E.; Heller, E.D.; Pitcovski, J. Wide-range protection against avian reovirus conferred by vaccination with representatives of four defined genotypes. Vaccine 2011, 29, 8683–8688. [Google Scholar] [CrossRef]
- Shmulevitz, M.; Yameen, Z.; Dawe, S.; Shou, J.; O’Hara, D.; Holmes, I.; Duncan, R. Sequential Partially Overlapping Gene Arrangement in the Tricistronic S1 Genome Segments of Avian Reovirus and Nelson Bay Reovirus: Implications for Translation Initiation. J. Virol. 2002, 76, 609–618. [Google Scholar] [CrossRef]
- Ayalew, L.E.; Gupta, A.; Fricke, J.; Ahmed, K.A.; Popowich, S.; Lockerbie, B.; Tikoo, S.K.; Ojkic, D.; Gomis, S. Phenotypic, genotypic and antigenic characterization of emerging avian reoviruses isolated from clinical cases of arthritis in broilers in Saskatchewan, Canada. Sci. Rep. 2017, 7, 3565. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Lee, L.H.; Hsu, H.W.; Kuo, L.C.; Liao, M.H. Molecular evolution of avian reovirus: Evidence for genetic diversity and reassortment of the S-class genome segments and multiple cocirculating lineages. Virology 2003, 314, 336–349. [Google Scholar] [CrossRef]
- Sellers, H.S. Current limitations in control of viral arthritis and tenosynovitis caused by avian reoviruses in commercial poultry. Vet. Microbiol. 2017, 206, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, R.; Meanger, J.; Enriquez, C.E.; Wilcox, G.E. Avian reovirus proteins associated with neutralization of virus infectivity. Virology 1993, 194, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Liu, H.J.; Chang, C.D.; Chang, C.I.; Hsu, J.L.; Liao, M.H.; Lee, J.W.; Shih, W.L. Avian reovirus S1133-induced DNA damage signaling and subsequent apoptosis in cultured cells and in chickens. Arch. Virol. 2011, 156, 1917–1929. [Google Scholar] [CrossRef]
- Shih, W.L.; Hsu, H.W.; Liao, M.H.; Lee, L.H.; Liu, H.J. Avian reovirus sigma C protein induces apoptosis in cultured cells. Virology 2004, 321, 65–74. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, W.; Li, X.; Cao, H.; Wang, Y.; Zheng, S.J. Critical role of eukaryotic elongation factor 1 alpha 1 (EEF1A1) in avian reovirus sigma-C-induced apoptosis and inhibition of viral growth. Arch. Virol. 2015, 160, 1449–1461. [Google Scholar] [CrossRef]
- Cavanagh, D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007, 38, 281–297. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Liu, H.-J.; Lai, M.-J.; Yu, F.-L.; Hsu, H.-Y.; Lee, J.-W.; Shih, W.-L. Avian Reovirus activates a novel proapoptotic signal by linking Src to p53. Apoptosis 2006, 11, 2179–2193. [Google Scholar]
- Goldenberg, D.; Pasmanik-Chor, M.; Pirak, M.; Kass, N.; Lublin, A.; Yeheskel, A.; Heller, D.; Pitcovski, J. Genetic and antigenic characterization of sigma C protein from avian reovirus. Avian Pathol. 2010, 39, 189–199. [Google Scholar] [CrossRef]


| Sera | Virus | |
|---|---|---|
| ARV-HeB02 | ARV-SD19/11103 | |
| ARV-HeB02 | 212 | 23 |
| ARV-SD19/11103 | - | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Luo, D.; Gao, J.; Li, K.; Liu, C.; Qi, X.; Cui, H.; Zhang, Y.; Wang, S.; Wang, X.; et al. A Novel Variant of Avian Reovirus Is Pathogenic to Vaccinated Chickens. Viruses 2023, 15, 1800. https://doi.org/10.3390/v15091800
Liu R, Luo D, Gao J, Li K, Liu C, Qi X, Cui H, Zhang Y, Wang S, Wang X, et al. A Novel Variant of Avian Reovirus Is Pathogenic to Vaccinated Chickens. Viruses. 2023; 15(9):1800. https://doi.org/10.3390/v15091800
Chicago/Turabian StyleLiu, Rui, Dan Luo, Jinhui Gao, Kai Li, Changjun Liu, Xiaole Qi, Hongyu Cui, Yanping Zhang, Suyan Wang, Xiaomei Wang, and et al. 2023. "A Novel Variant of Avian Reovirus Is Pathogenic to Vaccinated Chickens" Viruses 15, no. 9: 1800. https://doi.org/10.3390/v15091800
APA StyleLiu, R., Luo, D., Gao, J., Li, K., Liu, C., Qi, X., Cui, H., Zhang, Y., Wang, S., Wang, X., Gao, Y., & Gao, L. (2023). A Novel Variant of Avian Reovirus Is Pathogenic to Vaccinated Chickens. Viruses, 15(9), 1800. https://doi.org/10.3390/v15091800








