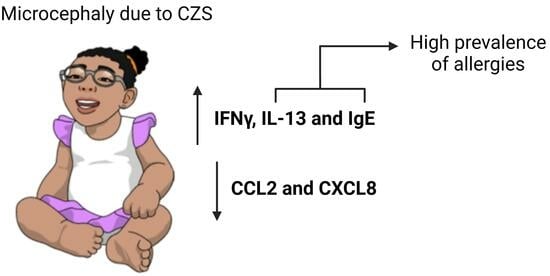
Low CCL2 and CXCL8 Production and High Prevalence of Allergies in Children with Microcephaly Due to Congenital Zika Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Blood Sample Collection and Processing
2.3. Total RNA Extraction, Complementary DNA (cDNA) Synthesis, and qPCR
2.4. Cytometric Bead Array (CBA) for Quantification of Serum Chemokines
2.5. Serum IgE Measurement
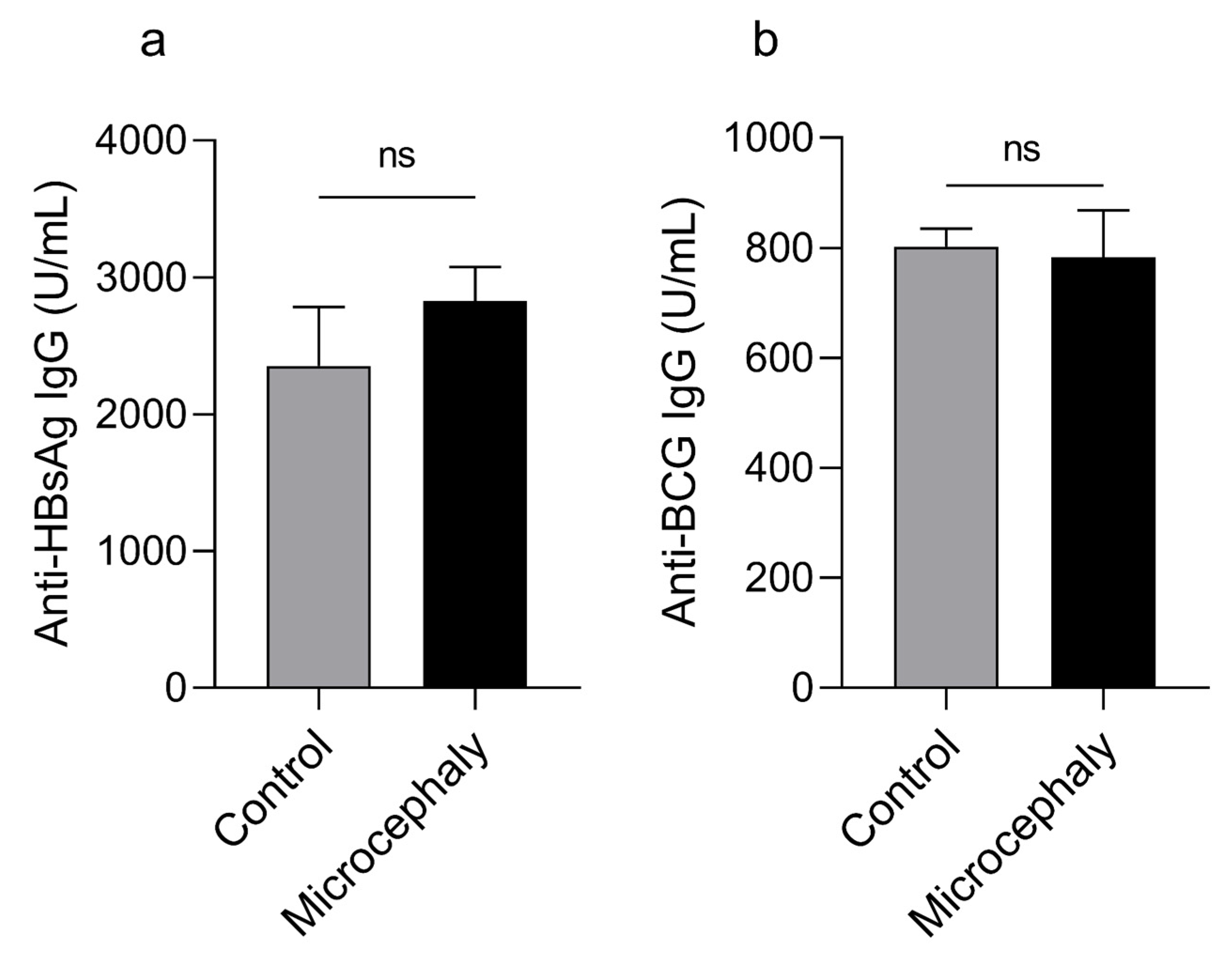
2.6. Serum Anti-HBsAg and Anti-BCG Measurements
2.7. Statistical Analysis
3. Results
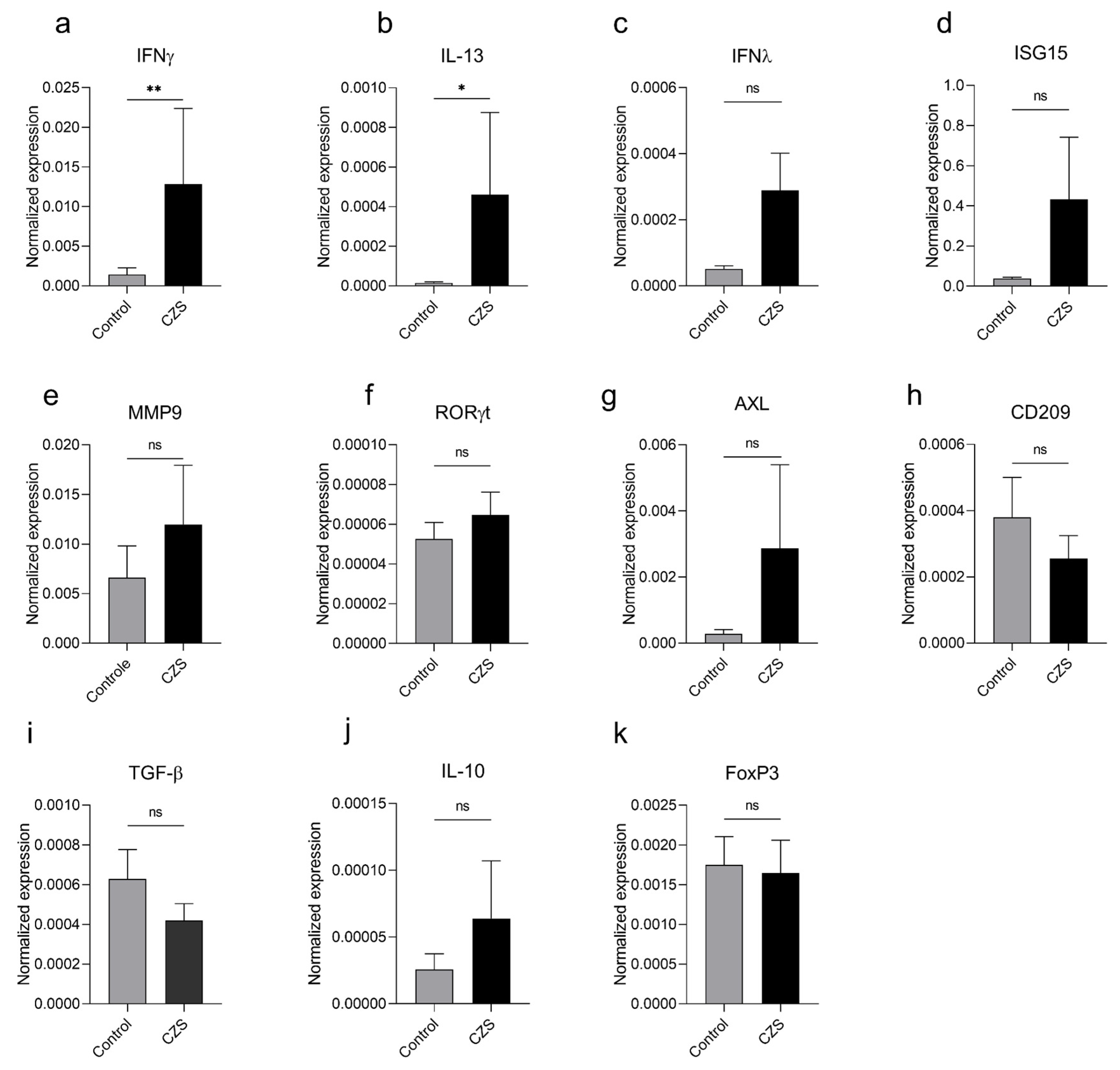
3.1. Children with Microcephaly Show Increased Expression of Antagonistic Cytokines IFNγ and IL-13
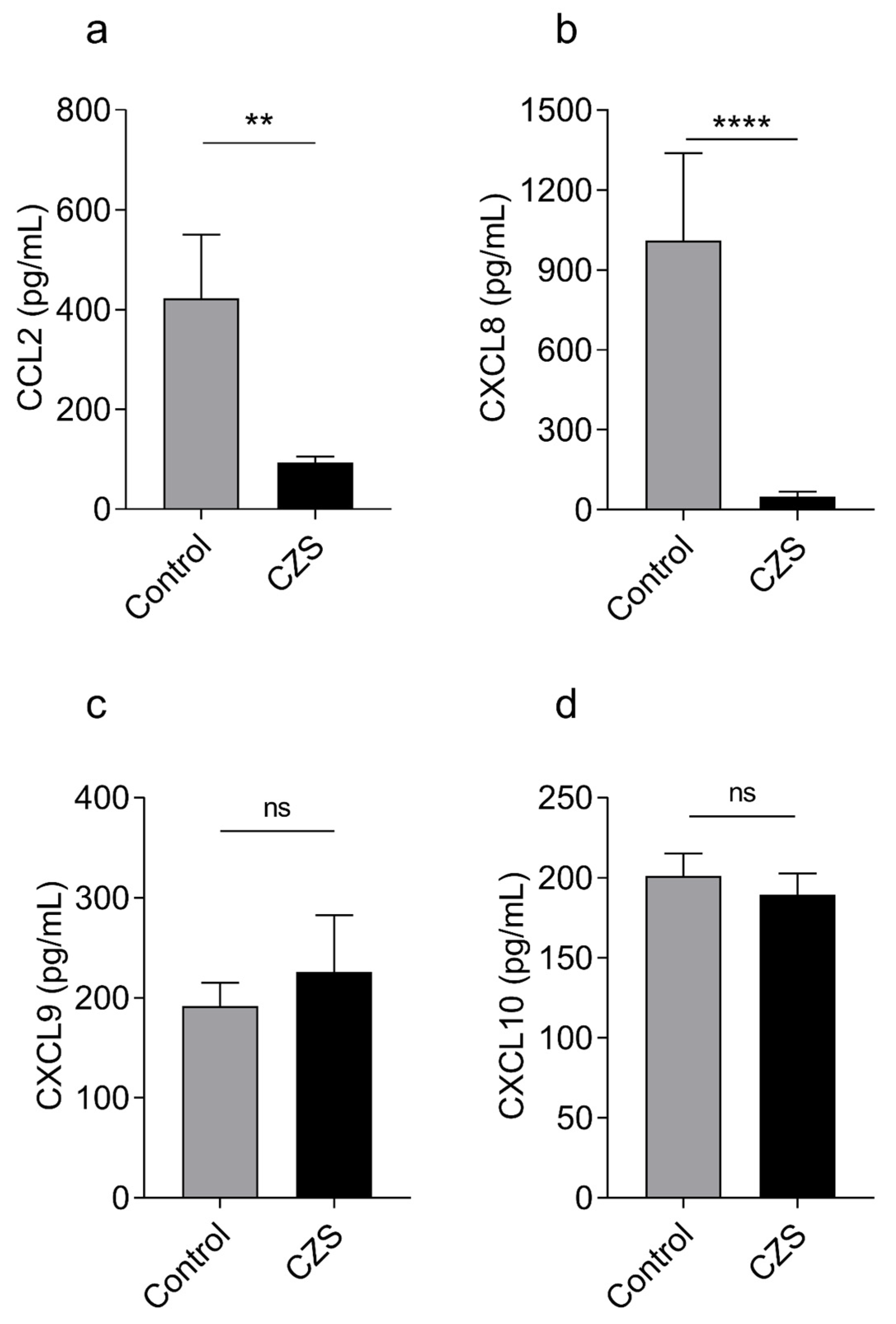
3.2. Children with Microcephaly Show Marked Decreased Serum Levels of CCL2 and CXCL8
3.3. Children with CZS Have an Increased Frequency of Allergies and Elevated Levels of Serum IgE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Devakumar, D.; Bamford, A.; Ferreira, M.U.; Broad, J.; Rosch, R.E.; Groce, N.; Breuer, J.; Cardoso, M.A.; Copp, A.J.; Alexandre, P.; et al. Infectious causes of microcephaly: Epidemiology, pathogenesis, diagnosis, and management. Lancet Infect. Dis. 2018, 18, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Barcellos, C.; Brasil, P.; Cruz, O.G.; Honório, N.A.; Kuper, H.; Carvalho, M.S. The Zika Virus Epidemic in Brazil: From Discovery to Future Implications. Int. J. Environ. Res. Public Health 2018, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Di Cavalcanti, D.; Alves, L.V.; Furtado, G.J.; Santos, C.C.; Feitosa, F.G.; Ribeiro, M.C.; Menge, P.; Lira, I.M.; Alves, J.G. Echocardiographic findings in infants with presumed congenital Zika syndrome: Retrospective case series study. PLoS ONE 2017, 12, e0175065. [Google Scholar] [CrossRef]
- Orofino, D.H.; Passos, S.R.; de Oliveira, R.V.; Farias, C.V.B.; Leite, M.D.F.M.; Pone, S.M.; Pone, M.V.D.S.; Teixeira Mendes, H.A.; Moreira, M.E.L.; Nielsen-Saines, K. Cardiac findings in infants with in utero exposure to Zika virus—A cross sectional study. PLoS Negl. Trop. Dis. 2018, 12, e0006362. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.A.; Souza-Santos, R.; Carvalho, L.M.A.; Barros, W.B.; Neves, L.M.; Brasil, P.; Wakimoto, M.D. Congenital Zika syndrome: A systematic review. PLoS ONE 2020, 15, e0242367. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, A.C.A.; Bezerra, W.P.; de Souza, R.L.L.; Pereira, L.C.; Nascimento, L.M.D.; Branco, A.C.C.C.; Simas, L.E.C.; de Almeida, V.A.; Palmeira, P.H.d.S.; Bezerra, C.M.; et al. Immunological imbalance in microcephalic children with congenital Zika virus syndrome. Med. Microbiol. Immunol. 2022, 211, 219–235. [Google Scholar] [CrossRef]
- Souza, A.S.R.; De Souza, A.I.; Faquin, S.D.L.L.; Neto, O.G.D.S.; Honorato, E.; Mattos, A.G.L.; Holanda, S.C.; Figueiroa, J.N.; Schettini, J. Altered intrauterine ultrasound, fetal head circumference growth and neonatal outcomes among suspected cases of congenital Zika syndrome in Brazil. Rev. Bras. Saúde Materno Infant. 2016, 16, S7–S15. [Google Scholar] [CrossRef]
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379–390. [Google Scholar] [CrossRef]
- Vinhaes, C.L.; Arriaga, M.B.; de Almeida, B.L.; Oliveira, J.V.; Santos, C.S.; Calcagno, J.I.; Carvalho, T.X.; Giovanetti, M.; Alcantara, L.C.J.; de Siqueira, I.C.; et al. Newborns with Zika Virus-Associated Microcephaly Exhibit Marked Systemic Inflammatory Imbalance. J. Infect. Dis. 2020, 222, 670–680. [Google Scholar] [CrossRef]
- Nascimento-Carvalho, G.C.; Nascimento-Carvalho, E.C.; Ramos, C.L.; Vilas-Boas, A.-L.; Moreno-Carvalho, O.A.; Vinhaes, C.L.; Barreto-Duarte, B.; Queiroz, A.T.L.; Andrade, B.B.; Nascimento-Carvalho, C.M. Zika-exposed microcephalic neonates exhibit higher degree of inflammatory imbalance in cerebrospinal fluid. Sci. Rep. 2021, 11, 8474. [Google Scholar] [CrossRef]
- Kam, Y.-W.; Leite, J.A.; Lum, F.-M.; Tan, J.J.L.; Lee, B.; Judice, C.C.; Teixeira, D.A.d.T.; Andreata-Santos, R.; Vinolo, M.A.; Angerami, R.; et al. Specific Biomarkers Associated with Neurological Complications and Congenital Central Nervous System Abnormalities from Zika Virus–Infected Patients in Brazil. J. Infect. Dis. 2017, 216, 172–181. [Google Scholar] [CrossRef]
- Ha, H.; Debnath, B.; Neamati, N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 2017, 7, 1543–1588. [Google Scholar] [CrossRef]
- Lin, Z.; Shi, J.L.; Chen, M.; Zheng, Z.M.; Li, M.Q.; Shao, J. CCL2: An important cytokine in normal and pathological pregnancies: A review. Front. Immunol. 2023, 13, 1053457. [Google Scholar] [CrossRef] [PubMed]
- Saghafian-Hedengren, S.; Sundström, Y.; Sohlberg, E.; Nilsson, C.; Linde, A.; Troye-Blomberg, M.; Berg, L.; Sverremark-Ekström, E. Herpesvirus Seropositivity in Childhood Associates with Decreased Monocyte-Induced NK Cell IFN-γ Production. J. Immunol. 2009, 182, 2511–2517. [Google Scholar] [CrossRef]
- Secretaria de Vigilância em Saúde Vírus Zika no Brasil: A Resposta do SUS, 1st ed.; Ministério da Saúde: Rio de Janeiro, Brazil, 2017.
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; Da Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef]
- Ellwood, P.; Asher, M.I.; Beasley, R.; Clayton, T.O.; Stewart, A.W.; ISAAC Steering Committee. The international study of asthma and allergies in childhood (ISAAC): Phase three rationale and methods. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2005, 9, 10–16. [Google Scholar]
- Asher, M.; Weiland, S.K. The International Study of Asthma and Allergies in Childhood (ISAAC). ISAAC Steering Committee. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 1998, 28 (Suppl. 5), 52–66, discussion 90–91. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Vanheule, V.; Janssens, R.; Struyf, S.; Proost, P. Overview of the Mechanisms that May Contribute to the Non-Redundant Activities of Interferon-Inducible CXC Chemokine Receptor 3 Ligands. Front. Immunol. 2018, 8, 1970. [Google Scholar] [CrossRef]
- Wen, Z.; Song, H.; Ming, G.-L. How does Zika virus cause microcephaly? Genes Dev. 2017, 31, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, I.S.; de Carvalho, A.T.; Cunha, D.P.; da Fonseca, D.L.M.; El Khawanky, N.; Freire, P.P.; Cabral-Miranda, G.; Schimke, L.F.; Camara, N.O.S.; Ochs, H.D.; et al. The clinical spectrum and immunopathological mechanisms underlying ZIKV-induced neurological manifestations. PLoS Negl. Trop. Dis. 2021, 15, e0009575. [Google Scholar] [CrossRef] [PubMed]
- Christian, K.M.; Song, H.; Ming, G.-L. Pathophysiology and Mechanisms of Zika Virus Infection in the Nervous System. Annu. Rev. Neurosci. 2019, 42, 249–269. [Google Scholar] [CrossRef]
- Agrelli, A.; de Moura, R.R.; Crovella, S.; Brandão, L.A.C. ZIKA virus entry mechanisms in human cells. Infect. Genet. Evol. 2019, 69, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Diamond, M.S. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe 2017, 21, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.C.; de Mendonça, L.R.; Rezende, A.M.; Carrera, R.M.; Aníbal-Silva, C.E.; Demers, M.; D’Aiuto, L.; Wood, J.; Chowdari, K.V.; Griffiths, M.; et al. The Transcriptional and Protein Profile from Human Infected Neuroprogenitor Cells Is Strongly Correlated to Zika Virus Microcephaly Cytokines Phenotype Evidencing a Persistent Inflammation in the CNS. Front. Immunol. 2019, 10, 1928. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.S.S.; de Sousa, J.R.; Araujo, M.T.F.; Filho, A.J.M.; de Alcantara, B.N.; Araujo, F.M.C.; Queiroz, M.G.L.; Cruz, A.C.R.; Vasconcelos, B.H.B.; Chiang, J.O.; et al. In situ immune response and mechanisms of cell damage in central nervous system of fatal cases microcephaly by Zika virus. Sci. Rep. 2018, 8, 1. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Wang, T.; Dai, H.; Wan, N.; Moore, Y.; Dai, Z. The role for monocyte chemoattractant protein-1 in the generation and function of memory CD8+ T cells. J. Immunol. 2008, 180, 2886–2893. [Google Scholar] [CrossRef]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, S. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front. Immunol. 2018, 9, 1930. [Google Scholar] [CrossRef]
- Nio, Y.; Yamauchi, T.; Iwabu, M.; Okada-Iwabu, M.; Funata, M.; Yamaguchi, M.; Ueki, K.; Kadowaki, T. Monocyte chemoattractant protein-1 (MCP-1) deficiency enhances alternatively activated M2 macrophages and ameliorates insulin resistance and fatty liver in lipoatrophic diabetic A-ZIP transgenic mice. Diabetologia 2012, 55, 3350–3358. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.C.; Sciavolino, P.J.; Lee, T.H.; Vilcek, J. Downregulation of interleukin 8 gene expression in human fibroblasts: Unique mechanism of transcriptional inhibition by interferon. Proc. Natl. Acad. Sci. USA 1992, 89, 9049–9053. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.M.; Heller, N.M. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine 2015, 75, 38–50. [Google Scholar] [CrossRef]
- Gowthaman, U.; Chen, J.S.; Zhang, B.; Flynn, W.F.; Lu, Y.; Song, W.; Joseph, J.; Gertie, J.A.; Xu, L.; Collet, M.A.; et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 2019, 365, eaaw6433. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, U.; Chen, J.S.; Eisenbarth, S.C. Regulation of IgE by T follicular helper cells. J. Leukoc. Biol. 2020, 107, 409–418. [Google Scholar] [CrossRef]



| Patients | Sample Size | Mean Age # (±SD) | Gender |
|---|---|---|---|
| Control group | 17 | 41.6 (±23.22) | 53% Female 47% Male |
| CZS group | 19 | 49.5 (±11.33) | 47% Female 53% Male |
| Primer | Direct (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| β-actin | GAGAGGCATCCTCACCCTGAAGTA | CACACGCAGCTCATTGTAGAAGGT |
| GAPDH | GAAGGTGAAGGTCGGAGT | GAAGATGGTGATGGGATTTC |
| IFNγ | TGTCGCCAGCAGCTAAAACA | TGCAGGCAGGACAACCATTA |
| IFNλ | CGCCTTGGAAGAGTCACTCA | GAAGCCTCAGGTCCCAATTC |
| IL-13 | GCAATGGCAGCATGGTATGG | CTGCACAGTACATGCCAGCT |
| MMP9 | AAGGATGGGAAGTACTGGCG | GCTCCTCAAAGACCGAGTCC |
| RORγt | TGAGAAGGACAGGGAGCCAA | CCACAGATTTTGCAAGGGATCA |
| AXL | CTGGGGAAGACTCTGGGAGA | CATCGTCTTCACAGCCACCT |
| CD209 | TGCTGAGGAGCAGAACTTCC | TACTGCTTGAAGCTGGGCAA |
| TGFβ | GGAAATTGAGGGCTTTCGCC | AGTGAACCCGTTGATGTCCA |
| FoxP3 | CAGCACATTCCCAGAGTTCCTC | GCGTGTGAACCAGTGGTAGATC |
| IL-10 | TAGAGTCGCCACCCTGATGT | ACATCAAGGCGCATGTGAAC |
| ISG15 | TGGCGGGCAACGAATT | GGGTGATCTGCGCCTTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezerra, W.P.; Salmeron, A.C.A.; Branco, A.C.C.C.; Morais, I.C.; de Farias Sales, V.S.; Machado, P.R.L.; Souto, J.T.; de Araújo, J.M.G.; Guedes, P.M.d.M.; Sato, M.N.; et al. Low CCL2 and CXCL8 Production and High Prevalence of Allergies in Children with Microcephaly Due to Congenital Zika Syndrome. Viruses 2023, 15, 1832. https://doi.org/10.3390/v15091832
Bezerra WP, Salmeron ACA, Branco ACCC, Morais IC, de Farias Sales VS, Machado PRL, Souto JT, de Araújo JMG, Guedes PMdM, Sato MN, et al. Low CCL2 and CXCL8 Production and High Prevalence of Allergies in Children with Microcephaly Due to Congenital Zika Syndrome. Viruses. 2023; 15(9):1832. https://doi.org/10.3390/v15091832
Chicago/Turabian StyleBezerra, Wallace Pitanga, Amanda Costa Ayres Salmeron, Anna Cláudia Calvielli Castelo Branco, Ingryd Camara Morais, Valéria Soraya de Farias Sales, Paula Renata Lima Machado, Janeusa Trindade Souto, Josélio Maria Galvão de Araújo, Paulo Marcos da Matta Guedes, Maria Notomi Sato, and et al. 2023. "Low CCL2 and CXCL8 Production and High Prevalence of Allergies in Children with Microcephaly Due to Congenital Zika Syndrome" Viruses 15, no. 9: 1832. https://doi.org/10.3390/v15091832
APA StyleBezerra, W. P., Salmeron, A. C. A., Branco, A. C. C. C., Morais, I. C., de Farias Sales, V. S., Machado, P. R. L., Souto, J. T., de Araújo, J. M. G., Guedes, P. M. d. M., Sato, M. N., & Nascimento, M. S. L. (2023). Low CCL2 and CXCL8 Production and High Prevalence of Allergies in Children with Microcephaly Due to Congenital Zika Syndrome. Viruses, 15(9), 1832. https://doi.org/10.3390/v15091832