Carambolaside W Inhibited H1N1 Influenza Virus-Induced Oxidative Stress through STAT-3/BCL-XL Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Materials
2.3. Plant Material
2.4. TCID50 of the Influenza Virus
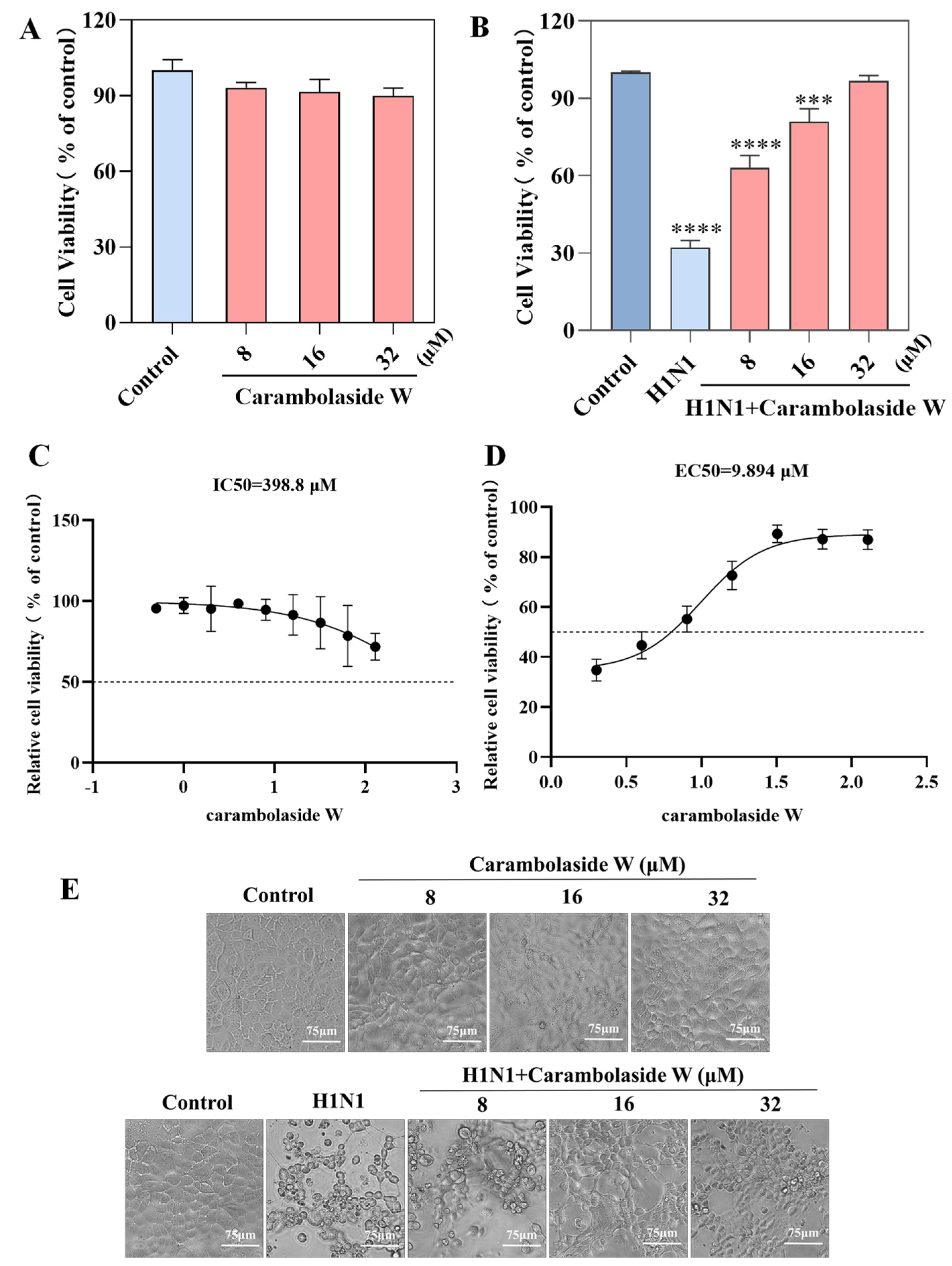
2.5. Detection of Drug Toxicity and Drug Therapeutic Capacity
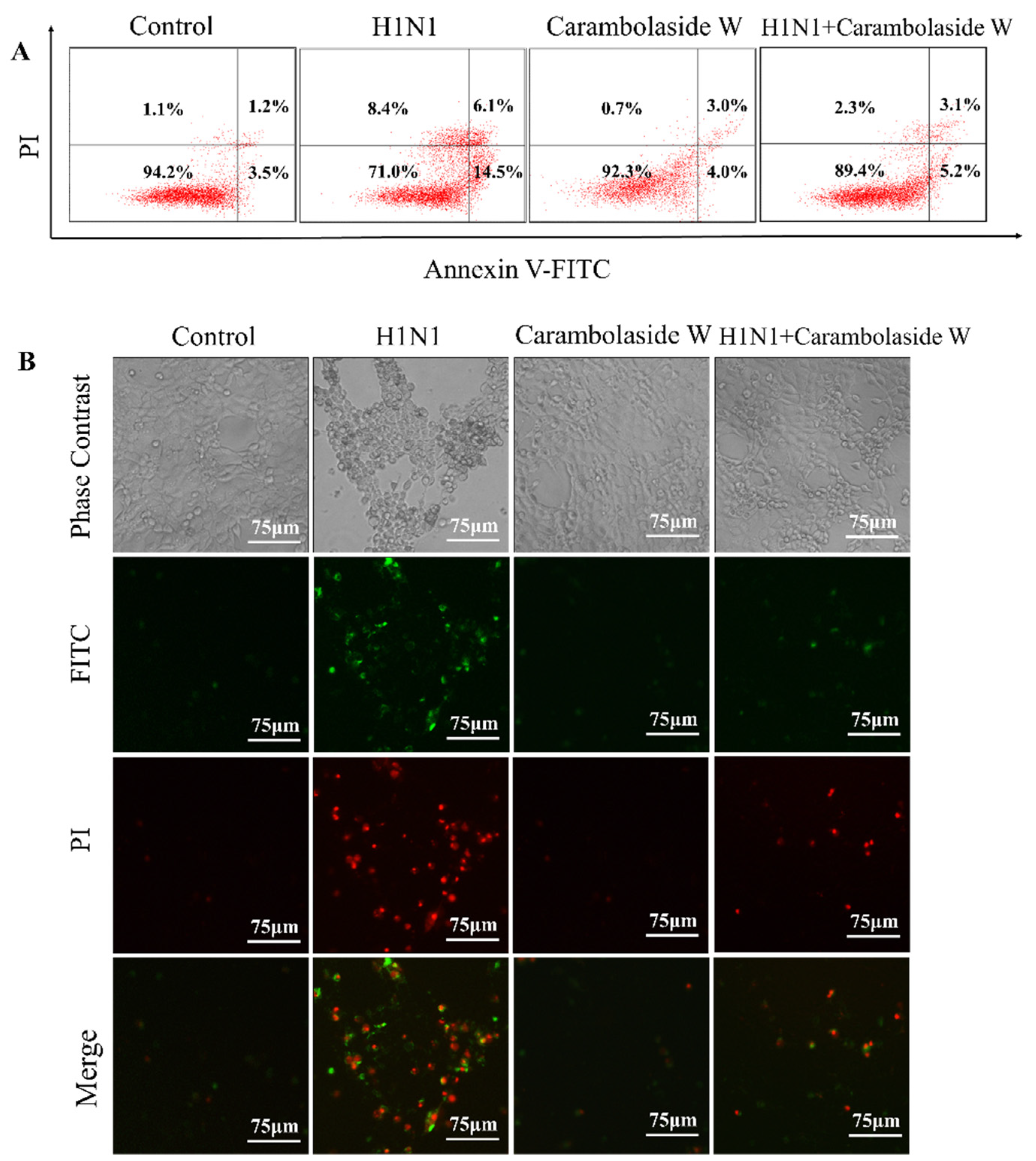
2.6. Detection of Apoptosis
2.7. Cell Cycle Experiment
2.8. Alteration of Mitochondrial Membrane Potential
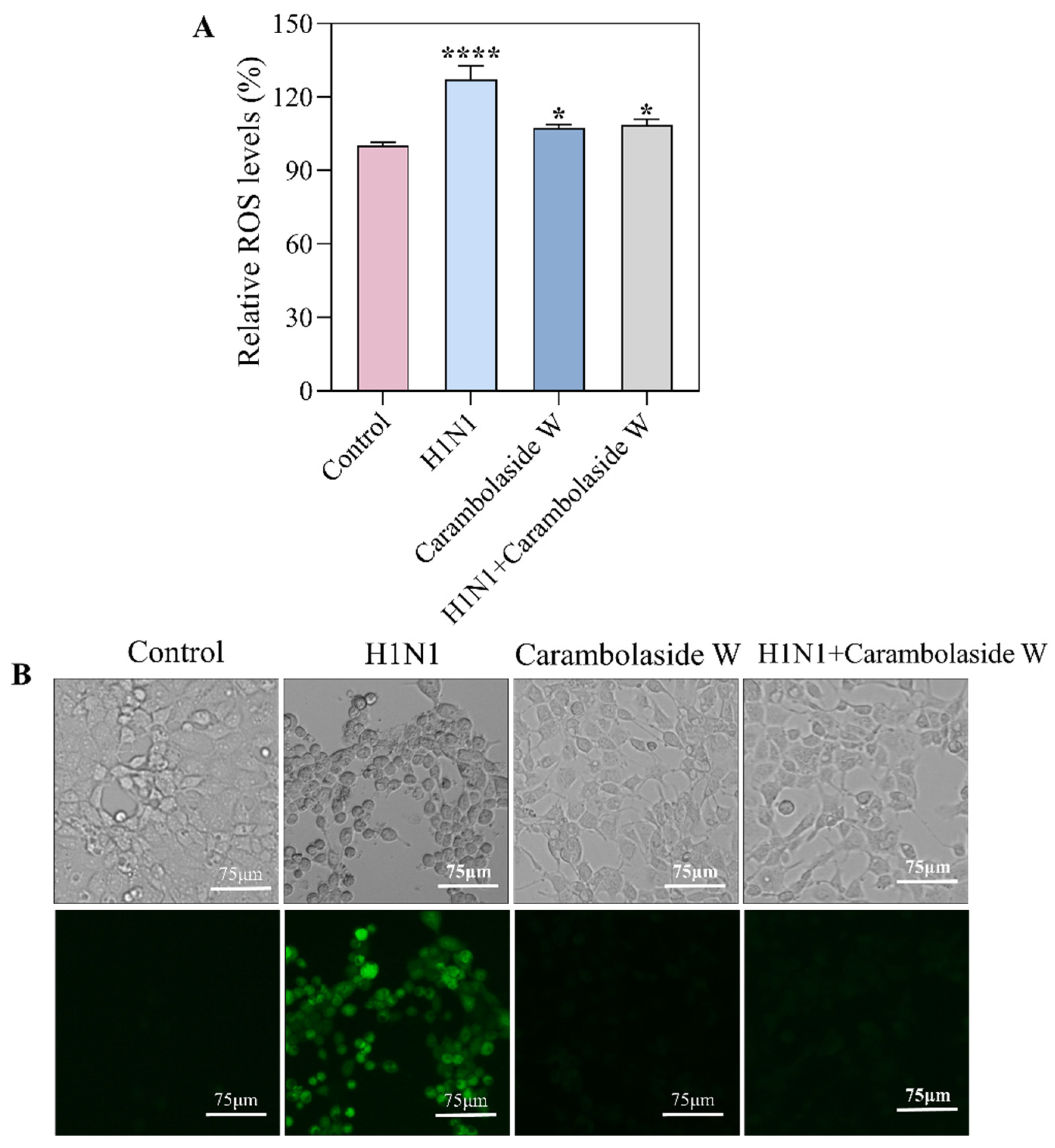
2.9. Reactive Oxygen Detection
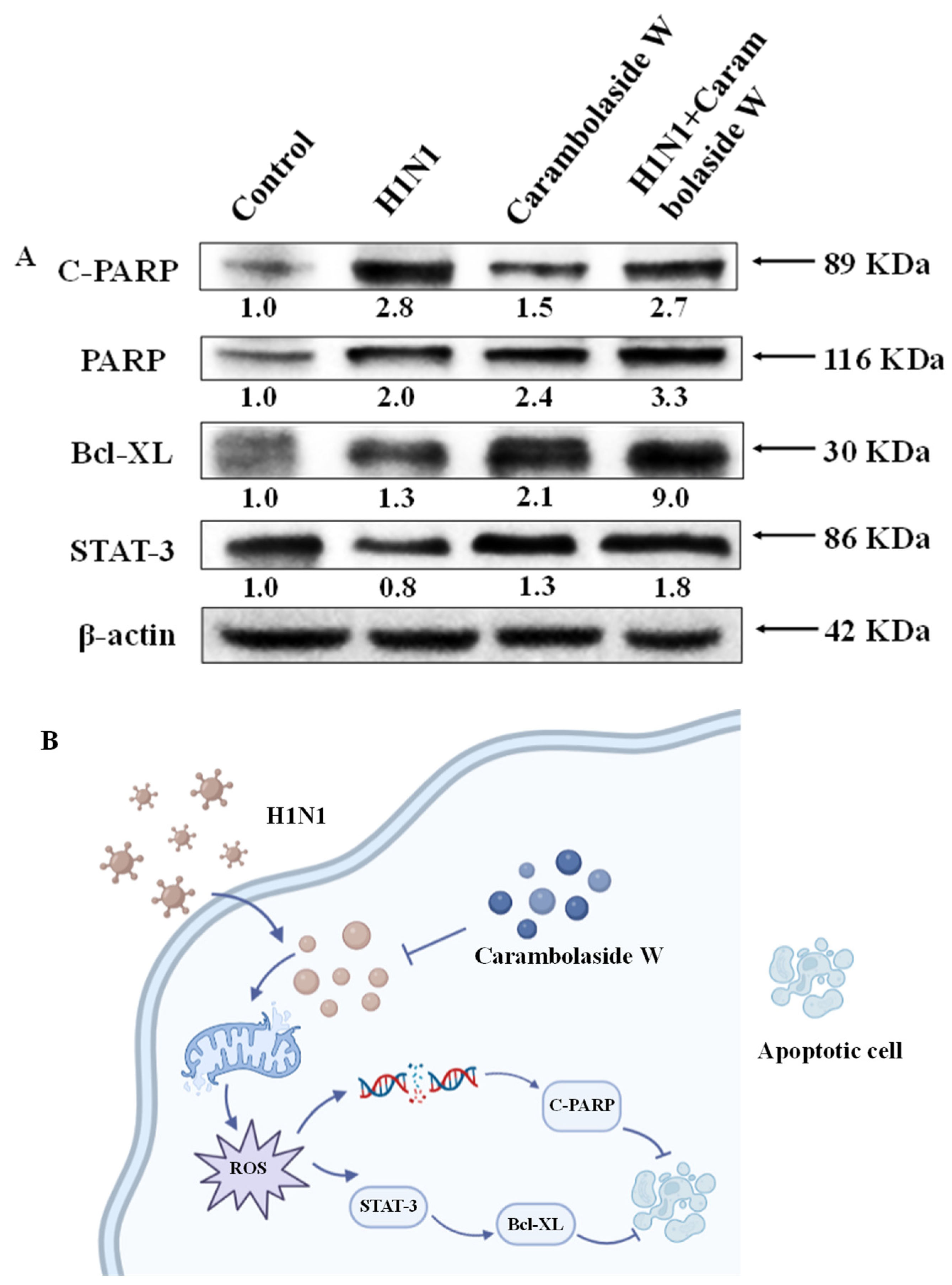
2.10. Detection of Western Blot
2.11. Statistical Analysis
3. Results
3.1. Structural Elucidation of Carambolaside W
3.2. Antiviral Ability of Carambolaside W
3.3. Detection of Apoptosis Status after H1N1 Infection
3.4. Effects of Carambolaside W on Cell Cycle
3.5. Effects of H1N1 Infection of MDCK on Mitochondria
3.6. Changes of Reactive Oxygen Species Produced by Carambolaside W
3.7. Effect of Carambolaside W on Protein Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, M.; Zhao, X.; Hua, S.; Du, X.; Peng, Y.; Li, X.; Lan, Y.; Wang, D.; Wu, A.; Shu, Y.; et al. Antigenic Patterns and Evolution of the Human Influenza A (H1N1) Virus. Sci. Rep. 2015, 5, srep14171. [Google Scholar] [CrossRef]
- Park, J.E.; Ryu, Y. Transmissibility and severity of influenza virus by subtype. Infect. Genet. Evol. 2018, 65, 288–292. [Google Scholar] [CrossRef]
- Tonelli, M.; Cichero, E. Fight Against H1N1 Influenza A Virus: Recent Insights Towards the Development of Druggable Compounds. Curr. Med. Chem. 2016, 23, 1802–1817. [Google Scholar] [CrossRef]
- Hutchinson, E.C. Influenza Virus. Trends Microbiol. 2018, 26, 809–810. [Google Scholar] [CrossRef]
- Du, R.; Cui, Q.; Chen, Z.; Zhao, X.; Lin, X.; Rong, L. Revisiting influenza A virus life cycle from a perspective of genome balance. Virol. Sin. 2023, 38, 1–8. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Hui, D.S.; Zambon, M.; Wentworth, D.E.; Monto, A.S. Influenza. Lancet 2022, 400, 693–706. [Google Scholar] [CrossRef]
- Schloer, S.; Goretzko, J.; Pleschka, S.; Ludwig, S.; Rescher, U. Combinatory Treatment with Oseltamivir and Itraconazole Targeting Both Virus and Host Factors in Influenza A Virus Infection. Viruses 2020, 12, 703. [Google Scholar] [CrossRef]
- Te Velthuis, A.J.W.; Zubkova, T.G.; Shaw, M.; Mehle, A.; Boltz, D.; Gmeinwieser, N.; Stammer, H.; Milde, J.; Müller, L.; Margitich, V. Enisamium Reduces Influenza Virus Shedding and Improves Patient Recovery by Inhibiting Viral RNA Polymerase Activity. Antimicrob. Agents Chemother. 2021, 65, 10–1128. [Google Scholar] [CrossRef]
- Tanner, A.R.; Dorey, R.B.; Brendish, N.J.; Clark, T.W. Influenza vaccination: Protecting the most vulnerable. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2021, 30, 200258. [Google Scholar] [CrossRef]
- Lampejo, T. Influenza and antiviral resistance: An overview. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1201–1208. [Google Scholar] [CrossRef]
- Takashita, E. Influenza Polymerase Inhibitors: Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.M.; Kim, J.; Tenson, T.; Min, J.Y.; Kainov, D.E. Influenza Virus Infection, Interferon Response, Viral Counter-Response, and Apoptosis. Viruses 2017, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Muthu, N.; Lee, S.Y.; Phua, K.K.; Bhore, S.J. Nutritional, Medicinal and Toxicological Attributes of Star-Fruits (Averrhoa carambola L.): A Review. Bioinformation 2016, 12, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Lakmal, K.; Yasawardene, P.; Jayarajah, U.; Seneviratne, S.L. Nutritional and medicinal properties of Star fruit (Averrhoa carambola): A review. Food Sci. Nutr. 2021, 9, 1810–1823. [Google Scholar] [CrossRef]
- Bag, A.; Bhattacharyya, S.K.; Chattopadhyay, R.R. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 2013, 3, 244–252. [Google Scholar] [CrossRef]
- Thomas, R.; Jebin, N.; Saha, R.; Sarma, D.K. Antioxidant and antimicrobial effects of kordoi (Averrhoa carambola) fruit juice and bamboo (Bambusa polymorpha) shoot extract in pork nuggets. Food Chem. 2016, 190, 41–49. [Google Scholar] [CrossRef]
- Abduh, M.S.; Saghir, S.A.M.; Al Hroob, A.M.; Bin-Ammar, A.; Al-Tarawni, A.H.; Murugaiyah, V.; Mahmoud, A.M. Averrhoa carambola leaves prevent dyslipidemia and oxidative stress in a rat model of poloxamer-407-induced acute hyperlipidemia. Front. Pharmacol. 2023, 14, 1134812. [Google Scholar] [CrossRef]
- Cabrini, D.A.; Moresco, H.H.; Imazu, P.; da Silva, C.D.; Pietrovski, E.F.; Mendes, D.A.; da Silveira Prudente, A.; Pizzolatti, M.G.; Brighente, I.M.; Otuki, M.F. Analysis of the Potential Topical Anti-Inflammatory Activity of Averrhoa carambola L. in Mice. Evid.-Based Complement. Altern. Med. Ecam. 2011, 2011, 908059. [Google Scholar] [CrossRef]
- Nowak, D.; Gośliński, M.; Przygoński, K.; Wojtowicz, E. Averrhoa carambola L., Cyphomandra betacea, Myrciaria dubia as a Source of Bioactive Compounds of Antioxidant Properties. Foods 2023, 12, 753. [Google Scholar] [CrossRef]
- Silva, K.B.; Pinheiro, C.T.S.; Soares, C.R.M.; Souza, M.A.; Matos-Rocha, T.J.; Fonseca, S.A.; Pavão, J.; Costa, J.G.; Pires, L.L.S.; Santos, A.F. Phytochemical characterization, antioxidant potential and antimicrobial activity of Averrhoa carambola L. (Oxalidaceae) against multiresistant pathogens. Braz. J. Biol. 2021, 81, 509–515. [Google Scholar] [CrossRef]
- Biondo, C.; Lentini, G.; Beninati, C.; Teti, G. The dual role of innate immunity during influenza. Biomed. J. 2019, 42, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.J.; Thomas, P.G. New fronts emerge in the influenza cytokine storm. Semin. Immunopathol. 2017, 39, 541–550. [Google Scholar] [CrossRef]
- Siddika, A.; Zahan, T.; Khatun, L.; Habib, M.R.; Aziz, M.A.; Tareq, A.R.M.; Rahman, M.H.; Karim, M.R. In vivo the antioxidative extract of Averrhoa carambola Linn. leaves induced apoptosis in Ehrilch ascites carcinoma by modulating p53 expression. Food Sci. Biotechnol. 2020, 29, 1251–1260. [Google Scholar] [PubMed]
- Chew, K.L.; Soh, P.; Octavia, S.; Teo, J. Identification of Mycobacterium abscessus to subspecies level with Bruker MALDI Biotyper. Pathology 2022, 54, 795–797. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Xie, H.; Jiang, Y.; Wei, X. Flavonoids isolated from the fresh sweet fruit of Averrhoa carambola, commonly known as star fruit. Phytochemistry 2018, 153, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Chen, D.; Zheng, R.; Liu, X.; Zhao, M.; Zhu, B.; Li, Y. Duvira Antarctic polysaccharide inhibited H1N1 influenza virus-induced apoptosis through ROS mediated ERK and STAT-3 signaling pathway. Mol. Biol. Rep. 2022, 49, 6225–6233. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, Z.; Wang, Z.; Guo, J.; Wen, J. Reactive oxygen species associated immunoregulation post influenza virus infection. Front. Immunol. 2022, 13, 927593. [Google Scholar] [CrossRef]
- Han, J.; Perez, J.; Schafer, A.; Cheng, H.; Peet, N.; Rong, L.; Manicassamy, B. Influenza Virus: Small Molecule Therapeutics and Mechanisms of Antiviral Resistance. Curr. Med. Chem. 2018, 25, 5115–5127. [Google Scholar] [CrossRef]
- Mehrbod, P.; Ande, S.R.; Alizadeh, J.; Rahimizadeh, S.; Shariati, A.; Malek, H.; Hashemi, M.; Glover, K.K.M.; Sher, A.A.; Coombs, K.M.; et al. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence 2019, 10, 376–413. [Google Scholar] [CrossRef]
- Liu, X.; Chen, D.; Su, J.; Zheng, R.; Ning, Z.; Zhao, M.; Zhu, B.; Li, Y. Selenium nanoparticles inhibited H1N1 influenza virus-induced apoptosis by ROS-mediated signaling pathways. RSC Adv. 2022, 12, 3862–3870. [Google Scholar] [CrossRef]
- Mifsud, E.J.; Kuba, M.; Barr, I.G. Innate Immune Responses to Influenza Virus Infections in the Upper Respiratory Tract. Viruses 2021, 13, 2090. [Google Scholar] [CrossRef] [PubMed]
- Nagai, E.; Iwai, M.; Koketsu, R.; Okuno, Y.; Suzuki, Y.; Morimoto, R.; Sumitani, H.; Ohshima, A.; Enomoto, T.; Isegawa, Y. Anti-Influenza Virus Activity of Adlay Tea Components. Plant Foods Hum. Nutr. 2019, 74, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, Y.; Xu, T.; Guo, M.; Wang, C.; Zhao, M.; Chen, H.; Kuang, J.; Li, W.; Zhang, Y.; et al. Inhibition of Enterovirus 71 by Selenium Nanoparticles Loaded with siRNA through Bax Signaling Pathways. ACS Omega 2020, 5, 12495–12500. [Google Scholar] [CrossRef]
- Davy, C.; Doorbar, J. G2/M cell cycle arrest in the life cycle of viruses. Virology 2007, 368, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Chan, D.C. Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 2016, 212, 379–387. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; Li, C.; Hu, H.; Li, W.; Wang, L.; Hu, J.; Liao, C.; Liang, H.; He, Z.; et al. Chrysin Ameliorates Influenza Virus Infection in the Upper Airways by Repressing Virus-Induced Cell Cycle Arrest and Mitochondria-Dependent Apoptosis. Front. Immunol. 2022, 13, 872958. [Google Scholar] [CrossRef]
- To, J.; Torres, J. Viroporins in the Influenza Virus. Cells 2019, 8, 654. [Google Scholar] [CrossRef]
- Rosa, N.; Ivanova, H.; Wagner, L.E., 2nd; Kale, J.; La Rovere, R.; Welkenhuyzen, K.; Louros, N.; Karamanou, S.; Shabardina, V.; Lemmens, I.; et al. Bcl-xL acts as an inhibitor of IP(3)R channels, thereby antagonizing Ca(2+)-driven apoptosis. Cell Death Differ. 2022, 29, 788–805. [Google Scholar] [CrossRef]
- Wyżewski, Z.; Świtlik, W.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P. The Role of Bcl-xL Protein in Viral Infections. Int. J. Mol. Sci. 2021, 22, 1956. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines Approved by the Guidelines Review Committee. In Pandemic Influenza Preparedness and Response: A WHO Guidance Document; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Mettelman, R.C.; Thomas, P.G. Human Susceptibility to Influenza Infection and Severe Disease. Cold Spring Harb. Perspect. Med. 2021, 11, a038711. [Google Scholar] [CrossRef]
- Plans-Rubió, P. Prevention and control of influenza in persons with chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2007, 2, 41–53. [Google Scholar] [CrossRef]
- Sarker, A.; Gu, Z.; Mao, L.; Ge, Y.; Hou, D.; Fang, J.; Wei, Z.; Wang, Z. Influenza-existing drugs and treatment prospects. Eur. J. Med. Chem. 2022, 232, 114189. [Google Scholar] [CrossRef]
- Boltz, D.A.; Aldridge, J.R., Jr.; Webster, R.G.; Govorkova, E.A. Drugs in development for influenza. Drugs 2010, 70, 1349–1362. [Google Scholar] [CrossRef]
- Kim, H.; Webster, R.G.; Webby, R.J. Influenza Virus: Dealing with a Drifting and Shifting Pathogen. Viral Immunol. 2018, 31, 174–183. [Google Scholar] [CrossRef]
- Herold, S.; Ludwig, S.; Pleschka, S.; Wolff, T. Apoptosis signaling in influenza virus propagation, innate host defense, and lung injury. J. Leukoc. Biol. 2012, 92, 75–82. [Google Scholar] [CrossRef]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef]
- Kotha, A.; Sekharam, M.; Cilenti, L.; Siddiquee, K.; Khaled, A.; Zervos, A.S.; Carter, B.; Turkson, J.; Jove, R. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol. Cancer Ther. 2006, 5, 621–629. [Google Scholar] [CrossRef]
- Honma, M.; Minami-Hori, M.; Takahashi, H.; Iizuka, H. Podoplanin expression in wound and hyperproliferative psoriatic epidermis: Regulation by TGF-β and STAT-3 activating cytokines, IFN-γ, IL-6, and IL-22. J. Dermatol. Sci. 2012, 65, 134–140. [Google Scholar] [CrossRef]




| Carambolaside W | |||||
|---|---|---|---|---|---|
| H/C | δH (J in Hz) | δC | H/C | δH (J in Hz) | δC |
| 1 | 133.9 | 5‴ | 3.37, m | 77.5 | |
| 2 | 6.90/7.00, br s | 130.4 | 6‴ | 3.87, dd (12.1, 4.0) | 62.5 |
| 3 | 6.63/6.67, d (6.9) | 116.3 | 3.68, dd (12.1, 3.5) | ||
| 4 | 156.5 | 1⁗ | 127.2 | ||
| 5 | 6.63/6.67, d (6.9) | 116.3 | 2⁗ | 7.37, d (8.4) | 131.2 |
| 6 | 6.90/7.00, br s | 130.4 | 3⁗ | 6.82, d (8.4) | 116.8 |
| 7 | 2.74/2.64, br s (2H) | 30.2 | 4⁗ | 161.1 | |
| 8 | 3.36/3.09, br s (2H) | 47.4 | 5⁗ | 6.82, d (8.4) | 116.8 |
| 9 | 206.1 | 6⁗ | 7.37, d (8.4) | 131.2 | |
| 1′ | 105.3 | 7⁗ | 7.44, d (15.9) | 146.3 | |
| 2′ | 165.0 | 8⁗ | 6.08, d (15.9) | 115.2 | |
| 3′ | 105.3 | 9⁗ | 168.2 | ||
| 4′ | 164.7 | A1 | 5.74, br s | 107.0 | |
| 5′ | 6.18 (s) | 96.2 | A2 | 4.18, m | 92.9 |
| 6′ | 161.7 | A3 | 4.12, dd (7.8, 4.8) | 76.2 | |
| 1″ | 5.13, d (10.2) | 73.8 | A4 | 3.97, m | 84.4 |
| 2″ | 5.69, br s | 71.7 | A5 | 3.64, m | 61.9 |
| 3″ | 3.97, m | 82.2 | 3.79, br d (11.1) | ||
| 4″ | 4.02, br s | 73.1 | F1 | 3.96, m | 105.1 |
| 5″ | 3.87, m | 76.2 | F2 | 3.39, m | 72.7 |
| 6″ | 1.33, d (6.3, 3H) | 17.2 | F3 | 3.25, m | 72.1 |
| 1‴ | 4.45, d (7.7) | 105.5 | F4 | 3.42, m | 74.8 |
| 2‴ | 3.29, m | 76.2 | F5 | 2.89, br s | 71.7 |
| 3‴ | 3.31, m | 77.9 | F6 | 0.80, br d (6.2) | 16.9 |
| 4‴ | 3.35, m | 71.3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Lai, J.; Li, J.; Liu, X.; Chen, H.; Li, C.; Zhu, B.; Jia, X.; Li, Y. Carambolaside W Inhibited H1N1 Influenza Virus-Induced Oxidative Stress through STAT-3/BCL-XL Signaling Pathway. Viruses 2023, 15, 1858. https://doi.org/10.3390/v15091858
Su J, Lai J, Li J, Liu X, Chen H, Li C, Zhu B, Jia X, Li Y. Carambolaside W Inhibited H1N1 Influenza Virus-Induced Oxidative Stress through STAT-3/BCL-XL Signaling Pathway. Viruses. 2023; 15(9):1858. https://doi.org/10.3390/v15091858
Chicago/Turabian StyleSu, Jingyao, Jia Lai, Jiali Li, Xia Liu, Haitian Chen, Chuqing Li, Bing Zhu, Xuchao Jia, and Yinghua Li. 2023. "Carambolaside W Inhibited H1N1 Influenza Virus-Induced Oxidative Stress through STAT-3/BCL-XL Signaling Pathway" Viruses 15, no. 9: 1858. https://doi.org/10.3390/v15091858





