Global Burden of Lumpy Skin Disease, Outbreaks, and Future Challenges
Abstract
:1. Background
2. Virus, Pathology and Transmission
2.1. Lumpy Skin Disease Virus
2.2. Viral Structure, Nature, and Genome Characteristics
2.3. Viral Replication and Resistance
2.4. Host–Pathogen Interaction
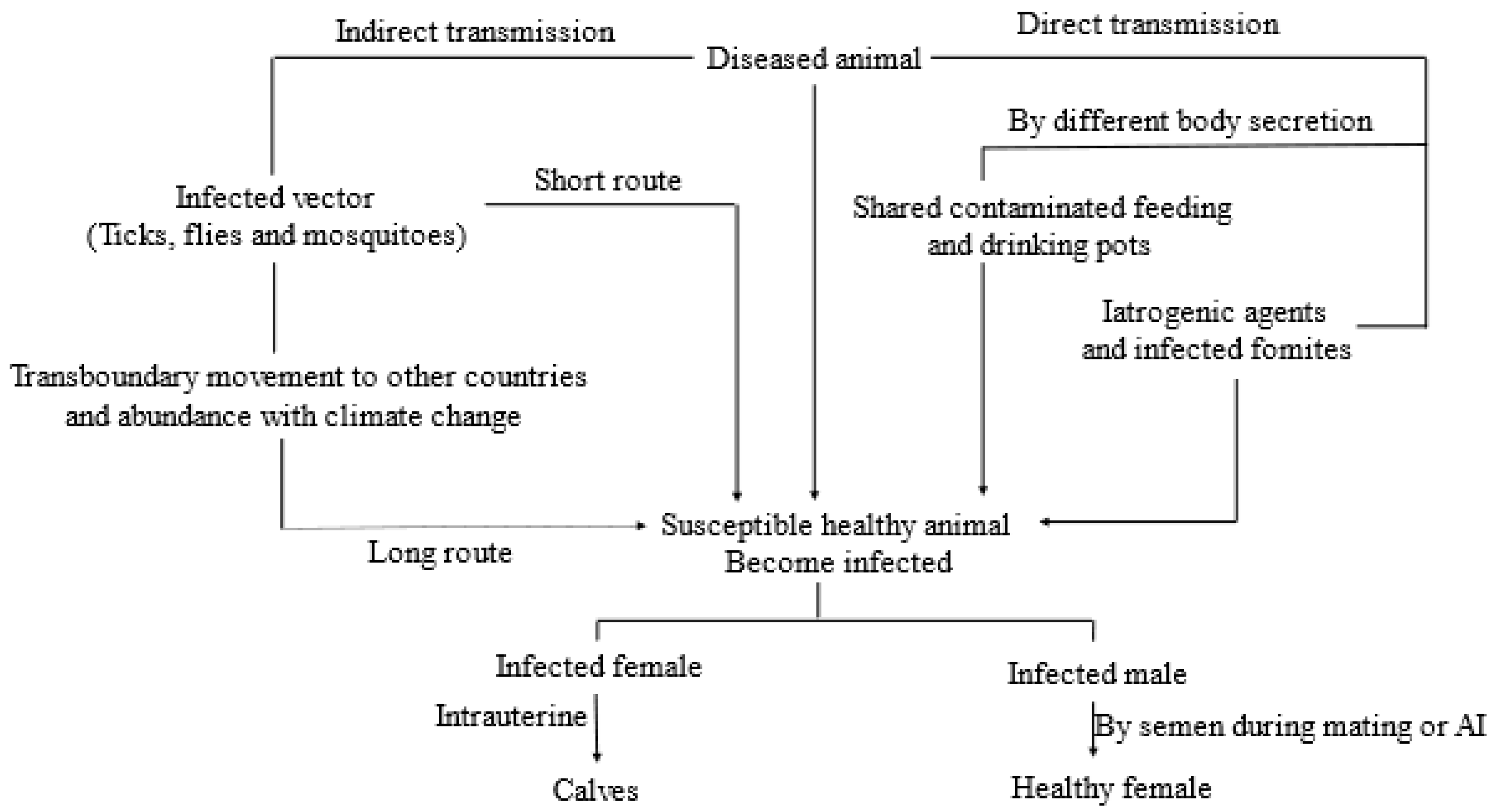
2.5. Viral Transmission
2.6. Pathogenesis and Effects on Host Body
2.7. Clinical Manifestation
2.8. Hematological Assessment
2.9. Biochemical Assessment
2.10. Pathological Assessment
3. Historical Outbreaks and Re-Emergence
4. Diagnosis and Management
4.1. Presumptive Diagnosis
4.2. Confirmatory Diagnosis
4.3. Differential Diagnosis
4.4. Treatment Strategies
5. Vaccines and Vaccine Controversies
6. Economic Impact
7. Transboundary Biosecurity Threat
8. Biosecurity Policies
9. Incursion Threat and Global Chaos
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemmons, E.A.; Alfson, K.J.; Dutton, J.W. Transboundary Animal Diseases, an Overview of 17 Diseases with Potential for Global Spread and Serious Consequences. Animals 2021, 11, 2039. [Google Scholar] [CrossRef]
- Das, M.; Chowdhury, M.S.R.; Akter, S.; Mondal, A.K.; Uddin, M.J.; Rahman, M.M.; Rahman, M.M. An Updated Review on Lumpy Skin Disease: Perspective of Southeast Asian Countries. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 322–333. [Google Scholar] [CrossRef]
- Yilmaz, H. Lumpy Skin Disease: Global and Turkish Perspectives. Approaches Poult. Dairy Vet. Sci. 2017, 1, 11–15. [Google Scholar] [CrossRef]
- Organização Mundial de Saúde. World Health Statistics 2022 (Monitoring Health of the SDGs); World Health Organization: Geneva, Switzerland, 2022; ISBN 9789240051140. [Google Scholar]
- Pandey, N.; Hopker, A.; Prajapati, G.; Rahangdale, N.; Gore, K.; Sargison, N. Observations on Presumptive Lumpy Skin Disease in Native Cattle and Asian Water Buffaloes around the Tiger Reserves of the Central Indian Highlands. New Zealand Vet. J. 2022, 70, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Amenu, A. Review on Epidemiological Aspects and Economic Impact of Lumpy Skin Disease. J. Dairy Vet. Sci. 2018, 7, 555716. [Google Scholar] [CrossRef]
- Al-Salihi, K.A.; Hassan, I.Q. Lumpy Skin Disease in Iraq: Study of the Disease Emergence. Transbound. Emerg. Dis. 2015, 62, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Davies, F.G. Special Review Series Lumpy Skin Disease, an African Capripox Virus Disease of Cattle. Br. Vet. J. 1991, 147, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Mulatu, E.; Feyisa, A. Review: Lumpy Skin Disease. J. Vet. Sci. Technol. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Khan, Y.R.; Ali, A.; Hussain, K.; Ijaz, M.; Rabbani, A.H.; Khan, R.L.; Abbas, S.N.; Aziz, M.U.; Ghaffar, A.; Sajid, H.A. A Review: Surveillance of Lumpy Skin Disease (LSD) a Growing Problem in Asia. Microb. Pathog. 2021, 158, 105050. [Google Scholar] [CrossRef]
- Saltykov, Y.V.; Kolosova, A.A.; Feodorova, V.A. Update of Lumpy Skin Disease: Emergence in Asian Part of Eurasia. Acta Vet. 2022, 72, 287–299. [Google Scholar] [CrossRef]
- National Lumpy Skin Disease Action Plan 2022. Available online: https://www.agriculture.gov.au/sites/default/files/documents/lsd-national-action-plan.pdf (accessed on 27 July 2023).
- Moudgil, G.; Chadha, J.; Khullar, L.; Chhibber, S.; Harjai, K. Lumpy Skin Disease: A Comprehensive Review on Virus Biology, Pathogenesis, and Sudden Global Emergence. Preprints 2023, 2023020074. [Google Scholar] [CrossRef]
- OIE WOAH. OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Lumpy Skin Disease. 2010. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/A_summry.htm (accessed on 27 July 2023).
- Tamire, M. Current Status of Lumpy Skin Disease and Its Economic Impacts in Ethiopia. J. Vaccine Res. 2022, 1, 103. [Google Scholar]
- Degu, T. Epidemiological Status and Economic Impact of Lumpy Skin Disease-Review. Int. J. Recent Biotechnol. 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Abera, Z.; Degefu, H.; Gari, G.; Ayana, Z. Review on Epidemiology and Economic Importance of Lumpy Skin Disease. Int. J. Basic Appl. Virol. 2015, 4, 8–21. [Google Scholar]
- Tuppurainen, E.; Dietze, K.; Wolff, J.; Bergmann, H.; Beltran-alcrudo, D.; Fahrion, A.; Lamien, C.E.; Busch, F.; Sauter-louis, C.; Conraths, F.J.; et al. Review: Vaccines and Vaccination against Lumpy Skin Disease. Vaccines 2021, 9, 1136. [Google Scholar] [CrossRef]
- Roth, J.A. Animal Disease Information and Prevention Materials Developed by the Center for Food Security and Public Health. Iowa State Univ. Anim. Ind. Rep. 2007, 4. Available online: https://www.iastatedigitalpress.com/air/article/id/6062/ (accessed on 27 July 2023).
- Mutua, E.N.; Bett, B.K.; Bukachi, S.A.; Estambale, B.A.; Nyamongo, I.K. From Policy to Practice: An Assessment of Biosecurity Practices in Cattle, Sheep and Goats Production, Marketing and Slaughter in Baringo County, Kenya. PLoS ONE 2022, 17, e0266449. [Google Scholar] [CrossRef]
- Charlier, J.; Barkema, H.W.; Becher, P.; De Benedictis, P.; Hansson, I.; Hennig-Pauka, I.; La Ragione, R.; Larsen, L.E.; Madoroba, E.; Maes, D.; et al. Disease Control Tools to Secure Animal and Public Health in a Densely Populated World. Lancet Planet. Health 2022, 6, e812–e824. [Google Scholar] [CrossRef]
- Tulman, E.R.; Afonso, C.L.; Lu, Z.; Zsak, L.; Kutish, G.F.; Rock, D.L. Genome of Lumpy Skin Disease Virus. J. Virol. 2001, 75, 7122–7130. [Google Scholar] [CrossRef]
- Parvin, R.; Chowdhury, E.H.; Islam, M.T.; Begum, J.A.; Nooruzzaman, M.; Globig, A.; Dietze, K.; Hoffmann, B.; Tuppurainen, E. Clinical Epidemiology, Pathology, and Molecular Investigation of Lumpy Skin Disease Outbreaks in Bangladesh during 2020–2021 Indicate the Re-Emergence of an Old African Strain. Viruses 2022, 14, 2529. [Google Scholar] [CrossRef]
- Bakar, L.; Hussein, A.; Tamam, S.; Madbouly, H. Isolation of Lumpy Skin Disease Virus Isolated from SPPV Vaccinated Cattle. J. Vet. Med. Res. 2021, 28, 38–44. [Google Scholar] [CrossRef]
- Lojkić, I.; Šimić, I.; Krešić, N.; Bedeković, T. Complete Genome Sequence of a Lumpy Skin Disease Virus Strain Isolated from the Skin of a Vaccinated Animal. Genome Announc. 2018, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Tuppurainen, E.S.M.; Babiuk, S.; Klement, E. Lumpy Skin Disease; Springer: Cham, Switzerland, 2018; pp. 1–109. [Google Scholar] [CrossRef]
- Tulman, E.R.; Afonso, C.L.; Lu, Z.; Zsak, L.; Sur, J.-H.; Sandybaev, N.T.; Kerembekova, U.Z.; Zaitsev, V.L.; Kutish, G.F.; Rock, D.L. The Genomes of Sheeppox and Goatpox Viruses. J. Virol. 2002, 76, 6054–6061. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.A. Lumpy Skin Disease-A Review. Trop. Anim. Health Prod. 1988, 20, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, C.; Lamien, C.E.; Fakhfakh, E.; Chadeyras, A.; Aba-Adulugba, E.; Libeau, G.; Tuppurainen, E.; Wallace, D.B.; Adam, T.; Silber, R.; et al. Capripoxvirus G-Protein-Coupled Chemokine Receptor: A Host-Range Gene Suitable for Virus Animal Origin Discrimination. J. Gen. Virol. 2009, 90, 1967–1977. [Google Scholar] [CrossRef]
- Bhatt, L.; Bhoyar, R.C.; Jolly, B.; Israni, R.; Vignesh, H.; Scaria, V.; Sivasubbu, S. The Genome Sequence of Lumpy Skin Disease Virus from an Outbreak in India Suggests a Distinct Lineage of the Virus. Arch. Virol. 2023, 168, 81. [Google Scholar] [CrossRef]
- Söllner, J.H.; Mettenleiter, T.C.; Petersen, B. Genome Editing Strategies to Protect Livestock from Viral Infections. Viruses 2021, 13, 1996. [Google Scholar] [CrossRef]
- Ma, J.; Yuan, Y.; Shao, J.; Sun, M.; He, W.; Chen, J.; Liu, Q. Genomic characterization of lumpy skin disease virus in southern China. Transbound. Emerg. Dis. 2021, 69, 2788–2799. [Google Scholar] [CrossRef]
- Grant, K.; Jenkins, C.; Arnold, C.; Green, J.; Zambon, M. Implementing Pathogen Genomics–A case study. Public Health England. 2018. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/731057/implementing_pathogen_genomics_a_case_study.pdf (accessed on 27 July 2023).
- Singh, J.; Kumar, M.; Sharma, A.; Pandey, G.; Chae, K.; Lee, S. We Are IntechOpen, the World’ s Leading Publisher of Open Access Books Built by Scientists, for Scientists TOP 1%. Intech 2016, 11, 13. [Google Scholar]
- Zewdie, G.A. Review on: Lumpy Skin Disease: Enhance Awareness on the Epidemiological Situation and Diagnosis; Prevention and Control Measures in Ethiopia. Virol. Immunol. J. 2021, 5, 1–11. [Google Scholar] [CrossRef]
- Biosecurity New Zealand. Technical Advice, Risk of Lumpy Skin Disease via Import of Cattle and Buffalo Meat and Meat Products for Human and Animal Consumption. Import Risk Analysis | NZ Government. 2022. Available online: https://www.mpi.govt.nz/dmsdocument/51352-Technical-Advice-Risk-of-lumpy-skin-disease-via-import-of-cattle-and-buffalo-meat-and-meat-products-for-human-and-animal-consumption (accessed on 27 July 2023).
- World Organisation for Animal Health. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE: Paris, France, 2017; Available online: http://www.oie.int/en/international-standard-setting/terrestrial-manual/access-online/ (accessed on 27 July 2023).
- Azeem, S.; Sharma, B.; Shabir, S.; Akbar, H.; Venter, E. Lumpy Skin Disease Is Expanding Its Geographic Range: A Challenge for Asian Livestock Management and Food Security. Vet. J. 2022, 279, 105785. [Google Scholar] [CrossRef] [PubMed]
- Al-Salihi, K. Lumpy Skin Disease: Review of Literature. Mirror Res. Vet. Sci. Anim. 2014, 3, 6–23. [Google Scholar]
- Kumar, P.; Kumari, R.R.; Devi, S.; Tripathi, M.K.; Singh, J.; Kumar, R.; Kumar, M. Emergence and Transboundary Spread of Lumpy Skin Disease in South Asia. Indian J. Anim. Sci. 2021, 91, 507–517. [Google Scholar] [CrossRef]
- USDA. Lumpy Skin Disease Standard Operating Procedures. The Foreign Animal Disease Preparedness and Response Plan (FAD PReP). 2016; pp. 1–10. Available online: https://www.aphis.usda.gov/animal_health/emergency_management/downloads/sop/lsdv_fadprep_ee.pdf (accessed on 27 July 2023).
- Pal, M.; Paulos Gutama, K. Can Lumpy Skin Disease Be Considered a Zoonosis? Am. J. Infect. Dis. Microbiol. 2023, 11, 13–17. [Google Scholar] [CrossRef]
- Fagbo, S.; Coetzer, J.A.W.; Venter, E.H.; Venter, E. Seroprevalence of Rift Valley Fever and Lumpy Skin Disease in African Buffalo (Syncerus Caffer) in the Kruger National Park and Hluhluwe-IMfolozi Park, South Africa. J. South Afr. Vet. Assoc. 2014, 85, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tuppurainen, E.S.M.; Oura, C.A.L. Review: Lumpy Skin Disease: An Emerging Threat to Europe, the Middle East and Asia. Transbound. Emerg. Dis. 2012, 59, 40–48. [Google Scholar] [CrossRef]
- OIE (WOAH). Technical Meeting on Lumpy Skin Disease (LSD). LSD Situation in Viet Nam. 21 December 2020. Available online: https://rr-asia.woah.org/wp-content/uploads/2021/01/4-201221_lsd_vietnam_update_oie_meeting.pdf (accessed on 27 July 2023).
- Elhaig, M.M.; Selim, A.; Mahmoud, M. Lumpy Skin Disease in Cattle: Frequency of Occurrence in a Dairy Farm and a Preliminary Assessment of Its Possible Impact on Egyptian Buffaloes. Onderstepoort J. Vet. Res. 2017, 84, 1–6. [Google Scholar] [CrossRef]
- Sudhakar, S.B.; Mishra, N.; Kalaiyarasu, S.; Jhade, S.K.; Hemadri, D.; Sood, R.; Bal, G.C.; Nayak, M.K.; Pradhan, S.K.; Singh, V.P. Lumpy Skin Disease (LSD) Outbreaks in Cattle in Odisha State, India in August 2019: Epidemiological Features and Molecular Studies. Transbound. Emerg. Dis. 2020, 67, 2408–2422. [Google Scholar] [CrossRef]
- Babiuk, S.; Bowden, T.R.; Boyle, D.B.; Wallace, D.B.; Kitching, R.P. Capripoxviruses: An Emerging Worldwide Threat to Sheep, Goats and Cattle. Transbound. Emerg. Dis. 2008, 55, 263–272. [Google Scholar] [CrossRef]
- Farah Gumbe, A.A. Review on Lumpy Skin Disease and Its Economic Impacts in Ethiopia. J. Dairy Vet. Anim. Res. 2018, 7, 39–46. [Google Scholar] [CrossRef]
- Tageldin, M.H.; Wallace, D.B.; Gerdes, G.H.; Putterill, J.F.; Greyling, R.R.; Phosiwa, M.N.; Al Busaidy, R.M.; Al Ismaaily, S.I. Lumpy Skin Disease of Cattle: An Emerging Problem in the Sultanate of Oman. Trop. Anim. Health Prod. 2014, 46, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Gari, G.; Waret-Szkuta, A.; Grosbois, V.; Jacquiet, P.; Roger, F. Risk Factors Associated with Observed Clinical Lumpy Skin Disease in Ethiopia. Epidemiol. Infect. 2010, 138, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.E. Lumpy Skin Disease Virus; Springer: Berlin/Heidelberg, Germany, 1968; pp. 111–131. [Google Scholar] [CrossRef]
- Sudhakar, S.B.; Mishra, N.; Kalaiyarasu, S.; Ahirwar, K.; Chatterji, S.; Parihar, O.; Singh, V.P.; Sanyal, A. Lumpy Skin Disease Virus Infection in Free-Ranging Indian Gazelles (Gazella Bennettii). Emerg. Infect. Dis. 2023, 29, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Gibbs, E.; Horzinek, M.S.M. Veterinary Virology, 3rd ed.; Academic Press: San Diego, CA, USA, 1999; ISBN 9780080552033. [Google Scholar]
- Lefèvre, P.; Blancou, J.; Chermette, R.; Uilenberg, G. Infectious and Parasitic Diseases of Livestock (2 Volume Set); CABI: Wallingford, UK, 2010; ISBN 13: 9782743008727. [Google Scholar]
- Zhang, J.H.; Huang, Y.G. The Immune System: A New Look at Pain. Chin. Med. J. 2006, 119, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Boulter, E.A.; Appleyard, G. Differences between Extracellular and Intracellular Forms of Poxvirus and Their Implications. Prog. Med. Virol. Fortschritte Med. Virusforsch. Prog. Virol. Medicale 1973, 16, 86–108. [Google Scholar]
- Coetzer, J.A.W.; Tustin, R.C. Infectious Diseases of Livestock, 2nd ed.; Oxford University Press: Oxford, UK, 2004; ISBN 0195761715. [Google Scholar]
- Milovanović, M.; Dietze, K.; Milicévić, V.; Radojičić, S.; Valčić, M.; Moritz, T.; Hoffmann, B. Humoral Immune Response to Repeated Lumpy Skin Disease Virus Vaccination and Performance of Serological Tests. BMC Vet. Res. 2019, 15, 1–9. [Google Scholar] [CrossRef]
- Tuppurainen, E.S.M.; Venter, E.H.; Coetzer, J.A.W. The Detection of Lumpy Skin Disease Virus in Samples of Experimentally Infected Cattle Using Different Diagnostic Techniques. Onderstepoort J. Vet. Res. 2005, 72, 153–164. [Google Scholar] [CrossRef]
- Ratyotha, K.; Prakobwong, S.; Piratae, S. Lumpy Skin Disease: A Newly Emerging Disease in Southeast Asia. Vet. World 2022, 15, 2764–2771. [Google Scholar] [CrossRef] [PubMed]
- Sprygin, A.; Artyuchova, E.; Babin, Y.; Prutnikov, P.; Kostrova, E.; Byadovskaya, O.; Kononov, A. Epidemiological Characterization of Lumpy Skin Disease Outbreaks in Russia in 2016. Transbound. Emerg. Dis. 2018, 65, 1514–1521. [Google Scholar] [CrossRef]
- Chihota, C.M.; Rennie, L.F.; Kitching, R.P.; Mellor, P.S. Mechanical Transmission of Lumpy Skin Disease Virus by Aedes Aegypti (Diptera: Culicidae). Epidemiol. Infect. 2001, 126, 317–321. [Google Scholar] [CrossRef]
- Sprygin, A.; Pestova, Y.; Wallace, D.B.; Tuppurainen, E.; Kononov, A.V. Transmission of Lumpy Skin Disease Virus: A Short Review. Virus Res. 2019, 269, 197637. [Google Scholar] [CrossRef] [PubMed]
- Kasem, S.; Saleh, M.; Qasim, I.; Hashim, O.; Alkarar, A.; Abu-Obeida, A.; Gaafer, A.; Hussien, R.; AL-Sahaf, A.; Al-Doweriej, A.; et al. Outbreak Investigation and Molecular Diagnosis of Lumpy Skin Disease among Livestock in Saudi Arabia 2016. Transbound. Emerg. Dis. 2018, 65, e494–e500. [Google Scholar] [CrossRef] [PubMed]
- Carn, V.M.; Kitching, R.P. An Investigation of Possible Routes of Transmission of Lumpy Skin Disease Virus (Neethling). Epidemiol. Infect. 1995, 114, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Magori-Cohen, R.; Louzoun, Y.; Herziger, Y.; Oron, E.; Arazi, A.; Tuppurainen, E.; Shpigel, N.Y.; Klement, E. Mathematical Modelling and Evaluation of the Different Routes of Transmission of Lumpy Skin Disease Virus. Vet. Res. 2012, 43, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A. A Review on Current Epidemiology and Molecular Studies of Lumpy Skin Disease Virus-an Emerging Worldwide Threat to Domestic Animals. J. Med. Pharm. Allied Sci. 2023, 12, 5635–5643. [Google Scholar] [CrossRef]
- Ali, H.; Ali, A.A.; Atta, M.S.; Cepica, A. Common, Emerging, Vector-Borne and Infrequent Abortogenic Virus Infections of Cattle. Transbound. Emerg. Dis. 2012, 59, 11–25. [Google Scholar] [CrossRef]
- Annandale, C.H.; Holm, D.E.; Ebersohn, K.; Venter, E.H. Seminal Transmission of Lumpy Skin Disease Virus in Heifers. Transbound. Emerg. Dis. 2014, 61, 443–448. [Google Scholar] [CrossRef]
- Rouby, S.; Aboulsoud, E. Evidence of Intrauterine Transmission of Lumpy Skin Disease Virus. Vet. J. 2016, 209, 193–195. [Google Scholar] [CrossRef]
- Salib, A.; Osman, F.H. Incidence of Lumpy Skin Disease among Egyptian Cattle in Giza Governorate, Egypt. Vet. World 2011, 4, 162–167. [Google Scholar]
- Lu, G.; Xie, J.; Luo, J.; Shao, R.; Jia, K.; Li, S. Lumpy Skin Disease Outbreaks in China, since 3 August 2019. Transbound. Emerg. Dis. 2021, 68, 216–219. [Google Scholar] [CrossRef]
- Lubinga, J.C.; Tuppurainen, E.S.M.; Mahlare, R.; Coetzer, J.A.W.; Stoltsz, W.H.; Venter, E.H. Evidence of Transstadial and Mechanical Transmission of Lumpy Skin Disease Virus by Amblyomma Hebraeum Ticks. Transbound. Emerg. Dis. 2015, 62, 174–182. [Google Scholar] [CrossRef] [PubMed]
- El-Kenawy, A.A.; El-Tholoth, M.S. Lumpy Skin Disease Virus Identification in Different Tissues of Naturally Infected Cattle and Chorioallantoic Membrane of Emberyonated Chicken Eggs Using Immunofluorescence, Immunoperoxidase Techniques and Polymerase Chain Reaction. Int. J. Virol. 2011, 7, 158–166. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Hussan, M.T.; Hashem, M.A.; Eliyas, M.; Anower, A.K.M.M. Lumpy Skin Disease Virus Infection: An Emerging Threat to Cattle Health in Bangladesh. Hosts Viruses 2020, 7, 97. [Google Scholar] [CrossRef]
- Fenner, F.; Bachmann, P.A.; Gibbs, E.P.J.; Murphy, F.A.; Studdert, M.J.; White, D.O. Veterinary Virology, 1st ed.; Academic Press: Cambridge, MA, USA, 1987; ISBN 9781483257815. [Google Scholar]
- Coetzer, J.A.W.; Tustin, R.C. Infectious Diseases of Livestock, 1st ed.; Oxford University Press: New York, NY, USA, 2004; Volume 2, ISBN 019578202X. [Google Scholar]
- Lumpy Skin Disease–CFSPH. Available online: https://www.cfsph.iastate.edu/diseaseinfo/disease/?disease=lumpy-skin-disease&lang=en (accessed on 25 May 2023).
- Lumpy Skin Disease: Pathogenesis of an African Capripox Virus Disease | Pashudhan Praharee. Available online: https://www.pashudhanpraharee.com/lumpy-skin-disease-pathogenesis-of-an-african-capripox-virus-disease/ (accessed on 25 May 2023).
- Gupta, T.; Patial, V.; Bali, D.; Angaria, S.; Sharma, M.; Chahota, R. A Review: Lumpy Skin Disease and Its Emergence in India. Vet. Res. Commun. 2020, 44, 111–118. [Google Scholar] [CrossRef]
- Abutarbush, S.M. Lumpy Skin Disease (Knopvelsiekte, Pseudo-Urticaria, Neethling Virus Disease, Exanthema Nodularis Bovis). In Emerging and Re-Emerging Infectious Diseases of Livestock; Bayry, J., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 309–326. [Google Scholar] [CrossRef]
- Jameel, G.H. Determination of Complications Decrease the Risk Factor in Cattle infected by lumpy skin disease virus in Diyala province, Iraq. Int. J. Micro Biol. Genet. Monocular Biol. Res. 2016, 2, 1–9. [Google Scholar]
- Abutarbush, S.M. Hematological and Serum Biochemical Findings in Clinical Cases of Cattle Naturally Infected with Lumpy Skin Disease. J. Infect. Dev. Ctries. 2015, 9, 283–288. [Google Scholar] [CrossRef]
- Neamat-allah, A.N.F. Studies on Cows Naturally Infected with Lumpy Skin Disease. Vet. World 2015, 8, 8–13. [Google Scholar] [CrossRef]
- Brooks, M.B.; Harr, K.E.; Seelig, D.M.; Wardrop, K.J.; Weiss, D.J. Schalm’s Veterinary Hematology, 6th ed.; Wiley-Blackwell: Ames, IA, USA, 2020; ISBN 9781119500537. [Google Scholar]
- Latimer, K.S. (Ed.) Duncan and Prasse’s Veterinary Laboratory Medicine: Clinical Pathology, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-1-119-94616-8. [Google Scholar]
- Kumar, V.; Abbas, A.; Aster, J. Robbins Basic Pathology, 10th ed.; Kumar, V., Abbas, A., Aster, J., Eds.; Elsevier Health Sciences: Amsterdam, The Netherland, 2017; ISBN 9780323394130. [Google Scholar]
- Coles, E.H. Veterinary Clinical Pathology, 4th ed.; Coles, E.H., Saunders, W.B., Eds.; Saunders Company: Philadelphia, PA, USA, 1986; ISBN 0721618286. [Google Scholar]
- Smith, B.P. Large Animal Internal Medicine; Smith, B.P., Ed.; Elsevier Mosby: St. Louis, MO, USA, 2015; ISBN 9780323088398. [Google Scholar]
- El-mandrawy, S.A.M.; Alam, R.T.M. Hematological, Biochemical and Oxidative Stress Studies of Lumpy Skin Disease Virus Infection in Cattle. J. Appl. Anim. Res. 2018, 46, 1073–1077. [Google Scholar] [CrossRef]
- Hassan, H. Immunobiochemical Profile in Cattle Infected with Lumpy Skin Disease. J. Basic Appl. Chem. 2011, 1, 21–25. [Google Scholar]
- Şevik, M.; Avci, O.; Doǧan, M.; Ince, Ö.B. Serum Biochemistry of Lumpy Skin Disease Virus-Infected Cattle. BioMed. Res. Int. 2016, 2016, 6257984. [Google Scholar] [CrossRef]
- Ahmed, W.M.; Zaher, K.S. Observations on Lumpy Skin Disease in Local Egyptian Cows with Emphasis on Its Impact on Ovarian Function. Afr. J. Microbiol. Res. 2008, 2, 252–257. [Google Scholar]
- Jaffersab, A.; Ravindra, B.G.; Halmandge, S.; Mallinath, K.C.; Doddagoudar, V.; Kasaralikar, V.R. A Study on Hematobiochemical Alterations in Cattle Affected with Lumpy Skin Disease in and around Bidar. Pharma Innov. J. 2022, SP-11, 958–960. [Google Scholar]
- Helal, M.; Marawan, M.; Bahgy, H. Clinico-Biochemical and Electrocardiographic Changes in Cattle Naturally Infected with Lumpy Skin Disease. Alex. J. Vet. Sci. 2019, 60, 41. [Google Scholar] [CrossRef]
- Stockham, S.L.; Scott, M.A.; Michael, A. Fundamentals of Veterinary Clinical Pathology; John Wiley & Sons: Hoboken, NJ, USA, 2008; p. 908. [Google Scholar]
- Marmor, A.T.; Klein, R.; Plich, M.; Groshar, D.; Schneeweiss, A. Elevated CK-MB Isoenzyme after Exercise Stress Test and Atrial Pacing in Patients with Ischemic Heart Disease. Chest 1988, 94, 1216–1220. [Google Scholar] [CrossRef]
- Tuppurainen, E.; Alexandrov, T.; Beltrán-Alcrudo, D. Lumpy Skin Disease Field Manual—A Manual for Veterinarians; FAO Animal Production and Health Manual No. 20; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2017; ISBN 9789251097762. [Google Scholar]
- Awad, W.S.; Ibrahim, A.K.; Salib, F.A. Using indirect ELISA to assess different antigens for the serodiagnosis of Fasciola gigantica infection in cattle, sheep and donkeys. Res. Vet. Sci. 2009, 86, 466–471. [Google Scholar] [CrossRef] [PubMed]
- El-Neweshy, M.S.; El-Shemey, T.M.; Youssef, S.A. Pathologic and Immunohistochemical Findings of Natural Lumpy Skin Disease in Egyptian Cattle. Pak. Vet. J. 2013, 33, 60–64. [Google Scholar]
- Body, M.; Singh, P.K.; Hussain, H.M.; Al-rawahi, A.; Al-maawali, M.; Al-lamki, K.; Al-habsy, S. Clinico-Histopathological Findings and PCR Based Diagnosis of Lumpy Skin Disease in the Sultanate of Oman. Pak. Vet. J. 2012, 32, 206–210. [Google Scholar]
- Sanz-Bernardo, B.; Haga, I.R.; Wijesiriwardana, N.; Basu, S.; Larner, W.; Diaz, A.V.; Langlands, Z.; Denison, E.; Stoner, J.; White, M.; et al. Quantifying and Modeling the Acquisition and Retention of Lumpy Skin Disease Virus by Hematophagus Insects Reveals Clinically but Not Subclinically Affected Cattle Are Promoters of Viral Transmission and Key Targets for Control of Disease Outbreaks. J. Virol. 2021, 95, 10–1128. [Google Scholar] [CrossRef]
- Şevik, M.; Doğan, M. Epidemiological and Molecular Studies on Lumpy Skin Disease Outbreaks in Turkey during 2014–2015. Transbound. Emerg. Dis. 2017, 64, 1268–1279. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Amina, A.D. Abattoir-Based Survey and Histopathological Findings of Lumpy Skin Disease in Cattle at Ismailia Abattoir. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 372–375. [Google Scholar]
- Prozesky, L.; Barnard, B.J. A Study of the Pathology of Lumpy Skin Disease in Cattle. Onderstepoort J. Vet. Res. 1982, 49, 167–175. [Google Scholar] [PubMed]
- Gammada, I.; Morshed, M.; Rabby, T.R.; Hossain, I. The Prevalence of Lumpy Skin Disease in the Cattle Population: A Brief Study. Int. J. Agric. Vet. Sci. 2022, 4, 55–67. [Google Scholar] [CrossRef]
- Davies, F.G. Observations on the Epidemiology of Lumpy Skin Disease in Kenya. J. Hyg. 1982, 88, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Modise, B.M.; Settypalli, T.B.K.; Kgotlele, T.; Xue, D.; Ntesang, K.; Kumile, K.; Naletoski, I.; Nyange, J.F.; Thanda, C.; Macheng, K.N.; et al. First Molecular Characterization of Poxviruses in Cattle, Sheep, and Goats in Botswana. Virol. J. 2021, 18, 167. [Google Scholar] [CrossRef] [PubMed]
- Capstick, P.B.; Coackley, W. Protection of Cattle Against Lumpy Skin Disease. Res. Vet. Sci. 1961, 2, 362–368. [Google Scholar] [CrossRef]
- Khalafalla, A.I.; Gaffar Elamin, M.A.; Abbas, Z. Lumpy Skin Disease: Observations on the Recent Outbreaks of the Disease in the Sudan. Rev. D’Elevage Médecine Vétérinaire Pays Trop. 1993, 46, 548–550. [Google Scholar]
- Ali, B.H.; Obeid, H.M. Investigation of the First Outbreaks of Lumpy Skin Disease in the Sudan. Br. Vet. J. 1977, 133, 184–189. [Google Scholar] [CrossRef]
- Wolff, J.; Tuppurainen, E.; Adedeji, A.; Meseko, C.; Asala, O.; Adole, J.; Atai, R.; Dogonyaro, B.; Globig, A.; Hoffmann, D.; et al. Characterization of a Nigerian Lumpy Skin Disease Virus Isolate after Experimental Infection of Cattle. Pathogens 2022, 11, 16. [Google Scholar] [CrossRef]
- Nawathe, D.R.; Asagba, M.O.; Abegunde, A.; Ajayi, S.A.; Durkwa, L. Some Observations on the Occurrence of Lumpy Skin Disease in Nigeria. Zentralblatt Für Veterinärmedizin Reihe B 1982, 29, 31–36. [Google Scholar] [CrossRef]
- Mebratu, G.Y.; Kassa, B.; Fikre, Y.; Berhanu, B. Observation on the Outbreak of Lumpy Skin Disease in Ethiopia. Rev. D’Elevage Médecine Vétérinaire Pays Trop. 1984, 37, 395–399. [Google Scholar]
- Rozstalnyy, A.; Tago, D.; Kamata, A.; Claudia, P. Introduction and Spread of Lumpy Skin Disease in South, East and Southeast Asia; Paper 183; Fao Animal Production and Health: Rome, Italy, 2020; ISBN 9789251335635. [Google Scholar]
- Rouby, S.R.; Safwat, N.M.; Hussein, K.H.; Abdel-Ra’ouf, A.M.; Madkour, B.S.; Abdel-Moneim, A.S.; Hosein, H.I. Lumpy Skin Disease Outbreaks in Egypt during 2017-2018 among Sheeppox Vaccinated Cattle: Epidemiological, Pathological, and Molecular Findings. PLoS ONE 2021, 16, e0258755. [Google Scholar] [CrossRef] [PubMed]
- Shimshony, A.; Economides, P. Disease Prevention and Preparedness for Animal Health Emergencies in the Middle East. OIE Rev. Sci. Tech. 2006, 25, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Calistri, P.; De Clercq, K.; Gubbins, S.; Klement, E.; Stegeman, A.; Cortiñas Abrahantes, J.; Marojevic, D.; Antoniou, S.E.; Broglia, A. Lumpy Skin Disease Epidemiological Report IV: Data Collection and Analysis. EFSA J. 2020, 18, e06010. [Google Scholar] [CrossRef] [PubMed]
- Mercier, A.; Arsevska, E.; Bournez, L.; Bronner, A.; Calavas, D.; Cauchard, J.; Falala, S.; Caufour, P.; Tisseuil, C.; Lefrançois, T.; et al. Spread Rate of Lumpy Skin Disease in the Balkans, 2015–2016. Transbound. Emerg. Dis. 2018, 65, 240–243. [Google Scholar] [CrossRef]
- Hasib, F.M.Y.; Islam, M.S.; Das, T.; Rana, E.A.; Uddin, M.H.; Bayzid, M.; Nath, C.; Hossain, M.A.; Masuduzzaman, M.; Das, S.; et al. Lumpy Skin Disease Outbreak in Cattle Population of Chattogram, Bangladesh. Vet. Med. Sci. 2021, 7, 1616–1624. [Google Scholar] [CrossRef]
- Mathivanan, D.E.; Raju, D.; Kavitha, M.D.R. Watching Brief. Learn. Disabil. Today 2022, 11, 11. [Google Scholar]
- Sai, C.H.; Goud, K.; Maheshwari, K.; Vigneshwari, K. Overview of Lumpy Skin Disease. Int. J. Pharm. Sci. Rev. Res. 2023, 79, 15–22. [Google Scholar] [CrossRef]
- Kumar, N.; Tripathi, B.N. A Serious Skin Virus Epidemic Sweeping through the Indian Subcontinent Is a Threat to the Livelihood of Farmers. Virulence 2022, 13, 1943–1944. [Google Scholar] [CrossRef]
- FAO. Emergence of Lumpy Skin Disease in the Eastern Mediterranean Basin Countries. Empres Watch. 2013, Volume 29. Available online: https://www.fao.org/documents/card/en/c/2a27bb10-8de5-510c-8303-c4c9d6414b3c (accessed on 27 July 2023).
- Mweene, A.S.; Pandey, G.S.; Sinyangwe, P.; Nambota, A.; Samui, K.; Kida, H. Viral diseases of livestock in Zambia. Jpn. J. Vet. Res. 1996, 44, 89–105. [Google Scholar] [PubMed]
- Mafirakureva, P.; Saidi, B.; Mbanga, J. Incidence and Molecular Characterisation of Lumpy Skin Disease Virus in Zimbabwe Using the P32 Gene. Trop. Anim. Health Prod. 2017, 49, 47–54. [Google Scholar] [CrossRef]
- Swiswa, S.; Masocha, M.; Pfukenyi, D.M.; Dhliwayo, S.; Chikerema, S.M. Long-Term Changes in the Spatial Distribution of Lumpy Skin Disease Hotspots in Zimbabwe. Trop. Anim. Health Prod. 2017, 49, 195–199. [Google Scholar] [CrossRef] [PubMed]
- HANDISTATUS II Multiannual Animal Disease Status. Available online: https://web.oie.int/hs2/sit_pays_mald_pl.asp?c_pays=30&c_mald=8 (accessed on 1 June 2023).
- Hunter, P.; Wallace, D. Lumpy Skin Disease in Southern Africa: A Review of the Disease and Aspects of Control. J. S. Afr. Vet. Assoc. 2001, 72, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Elsaim, N.A.G.E. Prevalence of Lumpy Skin Disease in Cattle, in Red Sea State, Sudan. Master’s Thesis, University of Khartoum, Khartoum, Sudan, 2007. [Google Scholar]
- El-Ansary, R.E.; El-Dabae, W.H.; Bream, A.S.; El Wakil, A. Isolation and Molecular Characterization of Lumpy Skin Disease Virus from Hard Ticks, Rhipicephalus (Boophilus) Annulatus in Egypt. BMC Vet. Res. 2022, 18, 302. [Google Scholar] [CrossRef]
- Yeruham, O.N.; Braveman, M.D.H. Spread of Lumpy Skinn Disease in Israeli. Vet. Rec. 1995, 137, 91–93. [Google Scholar] [CrossRef]
- Abutarbush, S.M.; Hananeh, W.M.; Ramadan, W.; Al Sheyab, O.M.; Alnajjar, A.R.; Al Zoubi, I.G.; Knowles, N.J.; Bachanek-Bankowska, K.; Tuppurainen, E.S.M. Adverse Reactions to Field Vaccination Against Lumpy Skin Disease in Jordan. Transbound. Emerg. Dis. 2016, 63, e213–e219. [Google Scholar] [CrossRef]
- Panel, E.; Ahaw, W. Scientific Opinion on Lumpy Skin Disease. EFSA J. 2015, 13, 3986. [Google Scholar] [CrossRef]
- Calistri, P.; DeClercq, K.; Gubbins, S.; Klement, E.; Stegeman, A.; Cortiñas Abrahantes, J.; Antoniou, S.E.; Broglia, A.; Gogin, A. Lumpy Skin Disease: III. Data Collection and Analysis. EFSA J. 2019, 17, e05638. [Google Scholar] [CrossRef] [PubMed]
- Bouchemla, F.; Agoltsov, V.A.; Larionov, S.V.; Popova, O.M.; Shvenk, E.V. Epizootiological Study on Spatiotemporal Clusters of Schmallenberg Virus and Lumpy Skin Diseases: The Case of Russia. Vet. World 2018, 11, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- EFSA, E.F.S.A. Lumpy Skin Disease: I. Data Collection and Analysis. EFSA J. 2017, 15, 1–54. [Google Scholar] [CrossRef]
- Ranjan Jena, B.; Kantale, R.A.; Dash, A.; Singh, P.; Basak, G.; Singh, S.; Jadhao, A. Emergence of Lumpy Skin Disease Virus (LSDV) Infection in Cattle and Buffaloes in India. Epidemiology 2022, 11, 1857–1861. [Google Scholar]
- Sudhakar, S.B.; Mishra, N.; Kalaiyarasu, S.; Jhade, S.K.; Singh, V.P. Genetic and Phylogenetic Analysis of Lumpy Skin Disease Viruses (LSDV) Isolated from the First and Subsequent Field Outbreaks in India during 2019 Reveals Close Proximity with Unique Signatures of Historical Kenyan NI-2490/Kenya/KSGP-like Field Strains. Transbound. Emerg. Dis. 2022, 69, e451–e462. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Doley, S.; Barlaskar, S.A.; Nath, A.J.; Yadav, S.N. Lumpy Skin Disease: An Emerging Bovine Viral Infection in India. Indian J. Anim. Health 2020, 59, 137–142. [Google Scholar] [CrossRef]
- Wei, Y.R.; Ma, W.G.; Wang, P.; Wang, W.; Su, X.H.; Yang, X.Y.; Mi, X.Y.; Wu, J.Y.; Huang, J. Retrospective Genomic Analysis of the First Lumpy Skin Disease Virus Outbreak in China (2019). Front. Vet. Sci. 2023, 9, 1073648. [Google Scholar] [CrossRef]
- Zan, X.; Huang, H.; Guo, Y.; Di, D.; Fu, C.; Wang, S.; Wu, Y.; Wang, J.; Wang, Y.; Ma, Y.; et al. Molecular Characterization of a Novel Subgenotype of Lumpy Skin Disease Virus Strain Isolated in Inner Mongolia of China. BMC Vet. Res. 2022, 18, 295. [Google Scholar] [CrossRef]
- An, Q.; Li, Y.; Sun, Z.; Gao, X.; Wang, H. Global Risk Assessment of the Occurrence of Bovine Lumpy Skin Disease: Based on an Ecological Niche Model. Transbound. Emerg. Dis. 2023, 2023, 2349173. [Google Scholar] [CrossRef]
- Koirala, P.; Meki, I.K.; Maharjan, M.; Settypalli, B.K.; Manandhar, S.; Yadav, S.K.; Cattoli, G.; Lamien, C.E. Molecular Characterization of the 2020 Outbreak of Lumpy Skin Disease in Nepal. Microorganisms 2022, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Kattel, P.; Kaphle, K. Review on Lumpy Skin Disease and Its Emerging Threat to Livestock in Nepal. Vet. Sci. Res. Rev. 2022, 8, 43–51. [Google Scholar] [CrossRef]
- Acharya, K.P.; Subedi, D. First Outbreak of Lumpy Skin Disease in Nepal. Transbound. Emerg. Dis. 2020, 67, 2280–2281. [Google Scholar] [CrossRef]
- Regmi, S. Lumpy Skin Disease (LSD) Outbreak in Nepal. Authorea Prepr. 2020, 1–4. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Truong, A.D.; Dang, A.K.; Ly, D.V.; Nguyen, C.T.; Chu, N.T.; Hoang, T.V.; Nguyen, H.T.; Nguyen, V.T.; Dang, H.V. Lumpy Skin Disease Outbreaks in Vietnam, 2020. Transbound. Emerg. Dis. 2021, 68, 977–980. [Google Scholar] [CrossRef]
- Mathijs, E.; Vandenbussche, F.; Nguyen, L.; Aerts, L.; Nguyen, T.; De Leeuw, I.; Quang, M.; Nguyen, H.D.; Philips, W.; Dam, T.V.; et al. Coding-Complete Sequences of Recombinant Lumpy Skin Disease Viruses Collected in 2020 from Four Outbreaks in Northern Vietnam. Microbiol. Resour. Announc. 2021, 10, 20–21. [Google Scholar] [CrossRef]
- Mehtani, Y. Study of Human Psychology for Introduction of Trult Intelligent. 2014, Volume 2021, pp. 1–2. Available online: http://data.conferenceworld.in/ICSTM-2015/101.pdf (accessed on 1 June 2023).
- Vinitchaikul, P.; Punyapornwithaya, V.; Seesupa, S.; Phuykhamsingha, S.; Arjkumpa, O.; Sansamur, C.; Jarassaeng, C. The First Study on the Impact of Lumpy Skin Disease Outbreaks on Monthly Milk Production on Dairy Farms in Khon Kaen, Thailand. Vet. World 2023, 16, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Arjkumpa, O.; Suwannaboon, M.; Boonrod, M.; Punyawan, I.; Liangchaisiri, S.; Laobannue, P.; Lapchareonwong, C.; Sansri, C.; Kuatako, N.; Panyasomboonying, P.; et al. The First Lumpy Skin Disease Outbreak in Thailand (2021): Epidemiological Features and Spatio-Temporal Analysis. Front. Vet. Sci. 2022, 8, 799065. [Google Scholar] [CrossRef] [PubMed]
- Sariya, L.; Paungpin, W.; Chaiwattanarungruengpaisan, S.; Thongdee, M.; Nakthong, C.; Jitwongwai, A.; Taksinoros, S.; Sutummaporn, K.; Boonmasawai, S.; Kornmatitsuk, B. Molecular Detection and Characterization of Lumpy Skin Disease Viruses from Outbreaks in Thailand in 2021. Transbound. Emerg. Dis. 2022, 69, e2145–e2152. [Google Scholar] [CrossRef]
- Manzoor, S.; Abubakar, M.; Rahman, A.U.; Syed, Z.; Ahmad, K.; Afzal, M. Molecular Characterization of Lumpy Skin Disease Virus from Recent Outbreaks in Pakistan. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Khatri, G.; Rai, A.; Aashish; Shahzaib; Hyder, S.; Priya; Hasan, M.M. Epidemic of Lumpy Skin Disease in Pakistan. Vet. Med. Sci. 2023, 9, 982–984. [Google Scholar] [CrossRef]
- Odonchimeg, M.; Erdenechimeg, D.; Tuvshinbayar, A.; Tsogtgerel, M.; Bazarragchaa, E.; Ulaankhuu, A.; Selenge, T.; Munkhgerel, D.; Munkhtsetseg, A.; Altanchimeg, A.; et al. Molecular Identification and Risk Factor Analysis of the First Lumpy Skin Disease Outbreak in Cattle in Mongolia. J. Vet. Med. Sci. 2022, 84, 1244–1252. [Google Scholar] [CrossRef]
- Lumpy Skin Disease (LSD) | Open Development Cambodia (ODC). Available online: https://opendevelopmentcambodia.net/tag/lumpy-skin-disease-lsd/#!/story=post-154955 (accessed on 17 July 2023).
- Lumpy Skin Disease Outbreak in Eastern Afghanistan Kills Hundreds of Livestock–Khaama Press. Available online: https://www.khaama.com/lumpy-skin-disease-outbreak-in-eastern-afghanistan-kills-hundreds-of-livestock-474833/ (accessed on 16 July 2023).
- Gongal, G.; Rahman, H.; Thakuri, K.C.; Vijayalakshmy, K. An Overview of Transboundary Animal Diseases of Viral Origin in South Asia: What Needs to Be Done? Vet. Sci. 2022, 9, 586. [Google Scholar] [CrossRef]
- Ko, Y.-S.; Oh, Y.; Lee, T.G.; Bae, D.-Y.; Tark, D.; Cho, H.-S. Serological and Molecular Prevalence of Lumpy Skin Disease Virus in Korean Water Deer, Native and Dairy Cattle in Korea. Korean J. Vet. Serv. 2022, 45, 133–137. [Google Scholar] [CrossRef]
- FAO Regional Office for Asia and the Pacific 2023. Indonesia Moves Another Step Closer to Ending Costly Livestock Diseases with Support from FAO and Australia. Available online: https://www.fao.org/asiapacific/news/detail-events/en/c/1638419/ (accessed on 27 July 2023).
- Home–ProMED–ProMED-Mail. Available online: https://promedmail.org/ (accessed on 28 June 2023).
- Selim, A.; Manaa, E.; Khater, H. Seroprevalence and Risk Factors for Lumpy Skin Disease in Cattle in Northern Egypt. Trop. Anim. Health Prod. 2021, 53, 350. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Bernardo, B.; Haga, I.R.; Wijesiriwardana, N.; Hawes, P.C.; Simpson, J.; Morrison, L.R.; MacIntyre, N.; Brocchi, E.; Atkinson, J.; Haegeman, A.; et al. Lumpy Skin Disease Is Characterized by Severe Multifocal Dermatitis With Necrotizing Fibrinoid Vasculitis Following Experimental Infection. Vet. Pathol. 2020, 57, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Gruenberg, W. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 11th ed.; Saunders Company: Philadelphia, PA, USA, 2016. [Google Scholar]
- Balinsky, C.A.; Delhon, G.; Smoliga, G.; Prarat, M.; French, R.A.; Geary, S.J.; Rock, D.L.; Rodriguez, L.L. Rapid Preclinical Detection of Sheeppox Virus by a Real-Time PCR Assay. J. Clin. Microbiol. 2008, 46, 438–442. [Google Scholar] [CrossRef]
- Lamien, C.E.; Lelenta, M.; Goger, W.; Silber, R.; Tuppurainen, E.; Matijevic, M.; Luckins, A.G.; Diallo, A. Real Time PCR Method for Simultaneous Detection, Quantitation and Differentiation of Capripoxviruses. J. Virol. Methods 2011, 171, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Leliso, S.A. Overview on Diagnostic Techniques and Economic Importance of Lumpy Skin Disease. J. Vet. Med. Res. 2021, 8, 1218. [Google Scholar]
- Amin, D.M.; Shehab, G.; Emran, R.; Hassanien, R.T.; Alagmy, G.N.; Hagag, N.M.; Abd-El-Moniem, M.I.I.; Habashi, A.R.; Ibraheem, E.M.; Shahein, M.A. Diagnosis of Naturally Occurring Lumpy Skin Disease Virus Infection in Cattle Using Virological, Molecular, and Immunohistopathological Assays. Vet. World 2021, 14, 2230–2237. [Google Scholar] [CrossRef]
- Choudhari, A.N.; Moregaonkar, S.D.; Gangane, G.R.; Markandeya, N.M.; Narladkar, B.W. Lumpy Skin Disease (LSD), an Emerging Disease in India: A Review. Agric. Rev. 2020, 41, 398–402. [Google Scholar] [CrossRef]
- Wallace, D.B.; Viljoen, G.J. Immune Responses to Recombinants of the South African Vaccine Strain of Lumpy Skin Disease Virus Generated by Using Thymidine Kinase Gene Insertion. Vaccine 2005, 23, 3061–3067. [Google Scholar] [CrossRef]
- Kitching, R.P. Vaccines for Lumpy Skin Disease, Sheep Pox and Goat Pox. Dev. Biol. 2003, 114, 161–167. [Google Scholar]
- Hamdi, J.; Munyanduki, H.; Tadlaoui, K.O.; Harrak, M.E.; Fihri, O.F. Capripoxvirus Infections in Ruminants: A Review. Microorganisms 2021, 9, 902. [Google Scholar] [CrossRef]
- Tuppurainen, E.S.M.; Pearson, C.R.; Bachanek-Bankowska, K.; Knowles, N.J.; Amareen, S.; Frost, L.; Henstock, M.R.; Lamien, C.E.; Diallo, A.; Mertens, P.P.C. Characterization of Sheep Pox Virus Vaccine for Cattle against Lumpy Skin Disease Virus. Antivir. Res. 2014, 109, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ayelet, G.; Haftu, R.; Jemberie, S.; Belay, A.; Gelaye, E.; Sibhat, B.; Skjerve, E.; Asmare, K. Lumpy Skin Disease in Cattle in Central Ethiopia: Outbreak Investigation and Isolation and Molecular Detection of the Virus. OIE Rev. Sci. Tech. 2014, 33, 877–887. [Google Scholar] [CrossRef] [PubMed]
- OIE. Frequently Asked Questions (FAQ) on Lumpy Skin Disease (LSD) Vaccination. 2022. Available online: https://www.woah.org/app/uploads/2022/06/faq-lsd-faired-v2-4forpublication.pdf (accessed on 27 July 2023).
- Varshovi, H. Immune Response Characteristics of Capri Pox Virus Vaccines Following Emergency Vaccination of Cattle against Lumpy Skin Disease Virus. Iran. J. Vet. Sci. Technol. 2018, 9, 33–40. [Google Scholar] [CrossRef]
- Brenner, J.; Bellaiche, M.; Gross, E.; Elad, D.; Oved, Z.; Haimovitz, M.; Wasserman, A.; Friedgut, O.; Stram, Y.; Bumbarov, V.; et al. Appearance of Skin Lesions in Cattle Populations Vaccinated against Lumpy Skin Disease: Statutory Challenge. Vaccine 2009, 27, 1500–1503. [Google Scholar] [CrossRef] [PubMed]
- Haegeman, A.; De Leeuw, I.; Saduakassova, M.; Campe, W.V.; Aerts, L.; Philips, W.; Sultanov, A.; Mostin, L.; De Clercq, K. The Importance of Quality Control of LSDV Live Attenuated Vaccines for Its Safe Application in the Field. Vaccines 2021, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- Boumart, Z.; Daouam, S.; Belkourati, I.; Rafi, L.; Tuppurainen, E.; Tadlaoui, K.O.; El Harrak, M. Comparative Innocuity and Efficacy of Live and Inactivated Sheeppox Vaccines. BMC Vet. Res. 2016, 12, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Sprygin, A.; Pestova, Y.; Bjadovskaya, O.; Prutnikov, P.; Zinyakov, N.; Kononova, S.; Ruchnova, O.; Lozovoy, D.; Chvala, I.; Kononov, A. Evidence of Recombination of Vaccine Strains of Lumpy Skin Disease Virus with Field Strains, Causing Disease. PLoS ONE 2020, 15, e0232584. [Google Scholar] [CrossRef]
- Gershon, P.D.; Black, D.N. A capripoxvirus pseudogene whose only intact homologs are in other poxvirus genomes. Virology 1989, 172, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Favis, N.; Famulski, J.; Evans, D.H. Evolution of and Evolutionary Relationships between Extant Vaccinia Virus Strains. J. Virol. 2015, 89, 1809–1824. [Google Scholar] [CrossRef]
- Bedson, H.S.; Dumbell, K.R. Hybrids Derived from the Viruses of Variola Major and Cowpox. J. Hyg. 1964, 62, 147–158. [Google Scholar] [CrossRef]
- Saltykov, Y.V.; Kolosova, A.A.; Filonova, N.N.; Chichkin, A.N.; Feodorova, V.A. Genetic Evidence of Multiple Introductions of Lumpy Skin Disease Virus into Saratov Region, Russia. Pathogens 2021, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, F.; Mathijs, E.; Philips, W.; Saduakassova, M.; De Leeuw, I.; Sultanov, A.; Haegeman, A.; De Clercq, K. Recombinant LSDV Strains in Asia: Vaccine Spillover or Natural Emergence? Viruses 2022, 14, 1429. [Google Scholar] [CrossRef] [PubMed]
- Haegeman, A.; De Leeuw, I.; Philips, W.; De Regge, N. Development and Validation of a New DIVA Real-Time PCR Allowing to Differentiate Wild-Type Lumpy Skin Disease Virus Strains, Including the Asian Recombinant Strains, from Neethling-Based Vaccine Strains. Viruses 2023, 15, 870. [Google Scholar] [CrossRef] [PubMed]
- Farra, D.; De Nardi, M.; Lets, V.; Holopura, S.; Klymenok, O.; Stephan, R.; Boreiko, O. Qualitative Assessment of the Probability of Introduction and Onward Transmission of Lumpy Skin Disease in Ukraine. Microb. Risk Anal. 2022, 20, 100200. [Google Scholar] [CrossRef]
- Hailu, B. Economic Importance and Control Techniques of Lumpy Skin Diseases. Anim. Vet. Sci. 2015, 3, 58. [Google Scholar] [CrossRef]
- Chouhan, C.S.; Parvin, M.S.; Ali, M.Y.; Sadekuzzaman, M.; Chowdhury, M.G.A.; Ehsan, M.A.; Islam, M.T. Epidemiology and Economic Impact of Lumpy Skin Disease of Cattle in Mymensingh and Gaibandha Districts of Bangladesh. Transbound. Emerg. Dis. 2022, 69, 3405–3418. [Google Scholar] [CrossRef]
- Foot and Mouth Disease: Virus Now in Bali a “$100b” Timebomb for Aussie Farmers. Available online: https://www.9news.com.au/national/foot-and-mouth-disease-in-bali-risks-100-billion-time-bomb-for-australia-cattle-livestock-exports-indsutry/353895ba-ede5-4924-a1c3-710ea4e43844 (accessed on 18 July 2023).
- Kononov, A.; Prutnikov, P.; Shumilova, I.; Kononova, S.; Nesterov, A.; Byadovskaya, O.; Pestova, Y.; Diev, V.; Sprygin, A. Determination of Lumpy Skin Disease Virus in Bovine Meat and Offal Products Following Experimental Infection. Transbound. Emerg. Dis. 2019, 66, 1332–1340. [Google Scholar] [CrossRef]
- Saegerman, C.; Bertagnoli, S.; Meyer, G.; Ganière, J.P.; Caufour, P.; De Clercq, K.; Jacquiet, P.; Hautefeuille, C.; Etore, F.; Casal, J. Risk of Introduction of Lumpy Skin Disease into France through Imports of Cattle. Transbound. Emerg. Dis. 2019, 66, 957–967. [Google Scholar] [CrossRef]
- Animal Health Australia. Response Strategy: Lumpy Skin Disease (Version 5.0). Australian Veterinary Emergency Plan (AUSVETPLAN), 5th ed.; ACT: Canberra, Australia, 2022. [Google Scholar]
- Li, Y.; An, Q.; Sun, Z.; Gao, X.; Wang, H. Risk Factors and Spatiotemporal Distribution of Lumpy Skin Disease Occurrence in the Asian Continent during 2012–2022: An Ecological Niche Model. Transbound. Emerg. Dis. 2023, 2023, 1–10. [Google Scholar] [CrossRef]
- Alemayehu, G.; Zewde, G.; Admassu, B. Risk Assessments of Lumpy Skin Diseases in Borena Bull Market Chain and Its Implication for Livelihoods and International Trade. Trop. Anim. Health Prod. 2013, 45, 1153–1159. [Google Scholar] [CrossRef]
- Animal Health Australia. National Biosecurity Manual for Beef Cattle Feedlots; Animal Health Australia: Lyneham, Australia, 2013; ISBN 9781921958182. Available online: https://www.farmbiosecurity.com.au/wp-content/uploads/2019/03/National-Biosecurity-Manual-for-Beef-Cattle-Feedlots1.pdf (accessed on 27 July 2023).
- LSD-Free Country List, Department of Agriculture, Fisheries and Forestry, Australian Government. 2022. Available online: https://www.agriculture.gov.au/sites/default/files/documents/lsd-free-country-list.pdf (accessed on 18 July 2023).
- Tuppurainen, E.; Lumpy Skin Disease. CABI Compendium. 2022. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.76780 (accessed on 27 July 2023).
- New Modelling Shows Lumpy Skin Disease Risk Reduced for Australia, as Cattle Industry Warns against Complacency–ABC News. Available online: https://www.abc.net.au/news/2023-05-08/lumpy-skin-foot-and-mouth-disease-risk-lower-livestock-australia/102308890 (accessed on 18 July 2023).
- Bianchini, J.; Simons, X.; Humblet, M.-F.; Saegerman, C. Lumpy Skin Disease: A Systematic Review of Mode of Transmission, Risk of Emergence and Risk Entry Pathway. Viruses 2023, 15, 1622. [Google Scholar] [CrossRef] [PubMed]
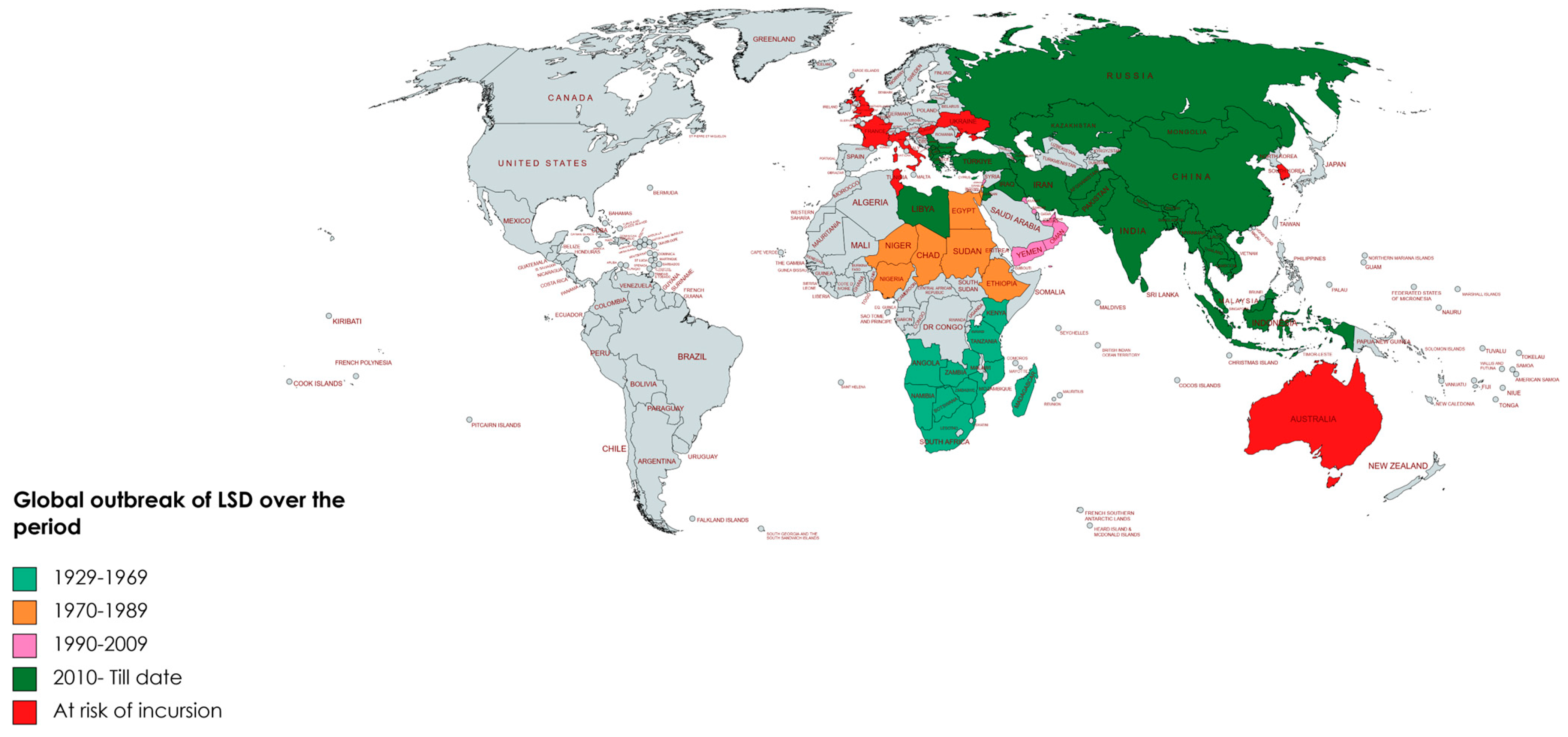
| Year | Country | Probable Origin | Strain, Vaccine and Vaccine Complication | Brief Epidemiological Data Including Economic Importance | Re-Emergence and Possibilities of Re-Emerging | Global Threat | Reference |
|---|---|---|---|---|---|---|---|
| 1929 | Zambia | Not clear | Limited data on strain Insufficient vaccination | Acute, Subacute or inapparent Countrywide distribution (Eastern, Northern and Central province) | 33 outbreaks occur in 1987 | Other African countries | [125,126] |
| 1943 | Botswana | Zambia | Neethling strain, controlled through vaccination with live attenuated vaccines (LSDV Neethling strain) and South African LSDV field isolate (Lumpyvax) | Named as ‘Ngamiland Cattle Disease’ Endemic type, transmission occurred by biting arthropods | Outbreaks occur in almost every year since 1943 | Zimbabwe and South Africa | [109] |
| 1944–1945 | Zimbabwe | Zambia and Botswana | Neethling virus, live attenuated virus vaccine Neethling strain: immunity conferred lasts up to 3 years | Cattle movement from communal areas into the previous commercial farms. After land reform program launched in 2000, outbreaks occurred on large-scale commercial cattle farms | Re-emergence occurs almost every year | Infected animals exporting countries | [127,128] |
| South Africa | Neethling-type strain Live attenuated South African LSDV field isolate | Known As ‘knopvelsiekte’, transportation of cattle is the possible route of entry and biting flies (Stomoxys calcitrans and Musca confiscate) are the transmitting vector. Approximately 8 million cattle were affected and massive economic loss due to reduced production | Severe outbreak occurred in 1953–1954 and epizootics continued to 1968 | Sudan and Ethiopia | [129,130] | ||
| 1946–1956 | Mozambique, Angola, Madagascar, Namibia, Tanzania and Uganda | Zambia and Botswana | Neethling-type strain Limited available data of vaccination | Outbreak spreads slowly and erratically Cattle transportation is the mode of transmission. No detailed information is available | Re-emergence in almost every year with minimal outbreak | Other cattle transporting, border-side countries | [131] |
| 1957 | Kenya | No record found in online search | LSD/240 strain of lumpy skin disease virus, identical with the Neethling strain Vaccine derived from Kedong Valley strain of sheep pox virus | Primarily epizootic in nature but subsequently sporadic, vector borne infection persists in the areas of high-altitude indigenous forest in a fairly high rainfall zone with very lower morbidity of 1–2% but initiate considerable economic losses by decreasing production performances | Re-emergence in almost every year with minimal outbreak | Several African territories by entry of exotic breed | [8,110] |
| 1971 | Sudan | Limited data available | Neethling-type strain Live attenuated local strain of LSD used as vaccine | Epizootic in nature and later become endemic of a milder form for local breed but fatal for exotic, transmitted mainly by biting arthropods (Tick, Amblyomma spp.) and aerosol transmission, affect mostly larger dairy farm and usually after rainy season | several outbreaks in Khartoum, Gezira and the River Nile States during the period 2004–2006 | Limited data available | [111,112,131] |
| 1973–1974 | Cand, Niger and Nigeria | Cameroon through Gongola State (Nigeria) | LSDV V/281-Nigeria field strain Live attenuated homologous and heterologous vaccines were used | Epizootic initially with gradual turn into endemic pattern, outbreaks occurred in rainy season and spread by combined effect of wind and movement of host and vector with very insignificant direct loss but persistent economic losses was recorded | In 1979–1980 more severe and widespread outbreaks were recorded | Limited data available | [113,114] |
| 1981–1983 | Ethiopia | Sudan | Neethling virus prototype strain Live attenuated Kenyan sheep and goat pox (KSGP 0–180) vaccines produced effective control approach | It is epizootic in almost all the regions and agro-ecological zones of Ethiopia, highly associated with climatic conditions, mainly heavy rainfall, favoring the increased vector population (Biting insects), sero-prevalence was higher in the midland agro-climate zones. The financial losses reflect the loss both animal and animal products | Major epidemic outbreaks were in 2000–2001 in and epidemics recorded up to 2010. | Limited data available | [35,49,115] |
| 1988–1989 | Egypt | Africa | Similar to the African, Asian and European strains A heterologous vaccine (Romanian sheep pox vaccine) provided sufficient levels of protection. | Is typical exotic disease, usually entered through importation of live animals. Within a few short years, it transformed into enzootic status, arthropod vectors are main source of infection and the activity of vector increased in the wet weather of rainy season | Re-emerged with enzootic nature in 2006, 2011 and 2014 and going on with endemic nature | European countries and specially Israel | [72,117,132] |
| 1889 | Israel | Egypt | No reported data found about strain. Vaccination was failed with Yugoslavian RM 65 sheep pox strain vaccine, but effectiveness was derived by repeated vaccination with RM 65 sheep pox strain vaccine | Epizootics of this disease were associated with high humidity with warm and moist condition and possibly spread by stable flies (Stomoxys calcitrans), charecteristics with mild clinical form and lower morbidity and mortality | Outbreaks occurred in Israel in 1989, 2006, 2007, and 2012 subsequently. | Limited data available | [41,133] |
| 1990–2010 | Middle eastern countries # | Egypt and Israel | Neethling-type strain Unclear data about the maintenance during inter-epidemic periods but proper vaccination, strict biosecurity and slaughter policies were noted for eradication | This disease was epizootic in nature in all the countries, considered as the transboundary transmitted mainly, having a significant effect on trade and food security and there was much variation in morbidity, mortality and disease spread rate | Several occurrences sporadically with the cross-boundary transmission | All the surrounding countries were subjected to be pandemic exploration | [50] |
| 2012–2014 | Middle eastern countries ## | Syria and Iraq | Neethling-type strain, Vaccination with Bakirkoy sheep pox strain, RM-65 sheep pox strain and the unlabeled LSD strain specially in Jordan were used for control | Primarily an epizootic disease having a risk of being endemic in each country, without or with a very low abundance of arthropod vectors but was likely to be associated with the illegal movement of clinically sick or asymptomatic infected animals and vectors | Several occurrences were recorded in different provinces of individual country | Greece and Bulgaria | [134,135] |
| 2015–2017 | Russia | Turkey, Azerbaijan, Iran, and Kazakhstan | Neethling-type strain, A heterologous (SGPV strain vaccine) has been used with a coverage of approximately 70% protection | Epizootics nature was responsible for significant damage with high morbidity and low mortality rates, usually spread occurred by illegal, infected animal movement, had a severe attack with approximately 7% infected animals died | Subsequent re-emergence in following years up to 2019 | Northern regions of Europe | [136,137] |
| Balkan Countries ### | Turkey | Neethling-type strain, A live attenuated homologous vaccine (Neethling strain and SIS Neethling type) was used and reported a better immunity | This epidemic had a strong seasonal pattern, with a summer peak and a winter drop, along with a abundance of arthropod vector resulting a faster spread of over 7600 LSD outbreaks with approximately 12,800 affected animals were reported in 2015 | No outbreaks were reported in 2018 | Central and South Asian countries | [39,121,138] | |
| 2019 | Bangladesh | Unresolved but may be from neighboring countries | LSDV are 99.99% homologous with two old African field strains-Neethling 2490 and KSGP 0–240 Prophylactic vaccination with attenuated LSD or goat pox viruses are being practicing | An emerging threat to cattle health with major socioeconomic impact by production losses, added treatment costs, chronic debilitation and death of the animals, massively influenced by the geographical distribution and seasonal pattern and mechanical transmission by arthropod vector | Re-emergence was found in 2020 with huge high morbidity and mortality was also recorded. | India, Myanmar | [23,76,121] |
| India | LSDV were very closely related to the Neethling NI-2490/1958, Kenya/1958 and KSGP-like strains. Live attenuated vaccines of capripoxvirus (Kenyan sheep and goat pox strain (KS-1), Yugoslavian RM-65 sheep pox strain, Romanian sheep pox strain and South African strain) are currently being using | Endemic occurrence was carried out by mechanical arthropod vector (mosquitoes, biting flies, Culicoides, midges and blood sucking hard ticks) and vector abundance is influenced by wet and warmer condition of summer and autumn months, causes approximately 2 hundred thousand cattle infection with death of approximately 97,000 cattle resulting severe economic losses by the means of individual death and reduced milk yield of 20% till 2022 | Outbreaks are ongoing in different states of India | Nepal and Bhutan | [139,140,141] | ||
| China | Kazakhstan and Russia | Three strains were found (LSDV/China/XJ01/2019 China/GD01/2020 and LSDV/Hongkong/2021) and related to the Neethling strain. Live attenuated goat pox vaccines are used | The epidemic nature outbreak occurs in different provinces and believed to be spread through the arthropod vector and uncontrol cattle and animal products movement, caused a devastation for the cattle industry. The morbidity and mortality of each outbreaks ranged 6.6–100% and 0–16.7%, respectively | LSD was spread to Southeast China in 2020 and caused outbreaks in multiple provinces | Taiwan | [142,143,144] | |
| 2020 | Nepal | India and China | LSDV NI-2490 strain is identical to the strain isolated from Kenya, Bangladesh and India Lack of available vaccines Prevention and control is maintained by proper biosecurity, vector control, movement restriction and treatment of diseased animals | This re-emerging disease can spread rapidly during summer and autumn months, in moist and warm environment (favorable for the growth and reproduction of houseflies, mosquitoes, etc.). The outbreaks are considered to be caused mainly by mechanical vector transmission. Along with 3–7% morbidity rate it can causes great economic losses by restricted animal products to the global trade and costly control and eradication measures | Regular outbreaks are recorded till to date | Data not found | [145,146,147,148] |
| Vietnam | China | LSDV of the Vietnamese LSD samples shows a similarity with Neethling virus strains and an identity with Chinese and Russian LSD strains. Both homologues (Neethling strain) and heterologous (Gorgan strain and RM65 strain) vaccines are the possible controlling option | This easily spreading disease spread mainly through insect bites such as mosquitoes, flies, ticks and can also be transmitted through transport of pathogen carrying animals and then share drinkers, feeding areas, milk, semen with others. Although morbidity was recorded approximately 10–20% but the economic effects were not properly mentioned | A total of 93 LSD outbreaks were reported in 93 communes of 36 districts. | Data not available | [149,150,151] | |
| 2021 | Thailand | Not mentioned | The Thailand strain is similar to the China/GD01/2020 and Hong Kong/2020 isolates. Live attenuated Neethling LSD vaccines have been disseminated for disease control | The emergence was supposed to get entry through illegal movements of infected and carrier animals from the source country and possible inter-farm transmission was occur with the insect vectors such as stable flies and mosquitoes. The overall morbidity and mortality rates were 40.5% and 1.2%, respectively | Several outbreaks were recorded in different provinces till date | Data not available | [152,153,154] |
| Pakistan | India | LSDV strains have shared the highest genomic homology with strains reported from India, China, and Bangladesh No successful vaccination protocol was found | LSD was noted as most dangerous, devastating, endemic disease, transmitted by transboundary transmission with allowing the infected population and vectors were observed in all the affected farms It causes serious economic implications by reduced milk production, infertility in cows and bulls, emaciation, abortion, skin damage and death | Still, it is the major threat for livestock in Pakistan because of its rapid spreading nature | Not clearly mentioned | [10,154,155,156] | |
| Mongolia | Russia and China | Mongolian isolates shared 100% identity to Chinese, Vietnamese, Russian, and Kazakhstan isolates. No vaccination was reported, only control and preventive measures were adopted | The clinical prevalence of LSD in cattle was approximately 6%, that leads a huge economic loss including restrictions on international trade of live animals and losses animal products such as milk, meat, and hide. | Data not available | China and India | [157] | |
| Cambodia | Strain data are not clearly reported but collaborative vaccination to domestic livestock was performed with Lumpyvax TM | Not clearly mentioned but continuous outbreak was recorded in different parts of the country | [158] | ||||
| 2022 | Afghanistan | Unknown or inconclusive | Not reported in detail Control measures were carried out at the event level with ante and postmortem inspections, vectors control, movement control, selective killing and disposal, slaughtering, surveillance within and outside the restricted zone | Limited data available | [159,160] | ||
| Korea | China and Nepal | A serological and molecular prevalence study of LSDV in Korean water deer, native and dairy cattle was performed in South Korea, but no positive reactor was detected. No data is available about LSDV in North Korea. | Limited data available | [161] | |||
| Indonesia | India | Limited data available. LSD has infected more than 22,000 animals in 13 provinces including Bali in Indonesia and the outbreaks continue. As Indonesia shares a border with northern Australia, it has become a great threat for the Australian cattle industry | [162] | ||||
| 2023 | Libya | Unknown or inconclusive | Not detected yet and no information available yet on vaccination | Zonal infection was noted, 10 infected cases were recorded among 26 susceptible cattle with 3 death case | The infection may have already spread beyond the observed/recorded foci, either by vectors or by movements of animals | Tunisia, and other North-West African (Maghreb) countries | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akther, M.; Akter, S.H.; Sarker, S.; Aleri, J.W.; Annandale, H.; Abraham, S.; Uddin, J.M. Global Burden of Lumpy Skin Disease, Outbreaks, and Future Challenges. Viruses 2023, 15, 1861. https://doi.org/10.3390/v15091861
Akther M, Akter SH, Sarker S, Aleri JW, Annandale H, Abraham S, Uddin JM. Global Burden of Lumpy Skin Disease, Outbreaks, and Future Challenges. Viruses. 2023; 15(9):1861. https://doi.org/10.3390/v15091861
Chicago/Turabian StyleAkther, Mahfuza, Syeda Hasina Akter, Subir Sarker, Joshua W. Aleri, Henry Annandale, Sam Abraham, and Jasim M. Uddin. 2023. "Global Burden of Lumpy Skin Disease, Outbreaks, and Future Challenges" Viruses 15, no. 9: 1861. https://doi.org/10.3390/v15091861
APA StyleAkther, M., Akter, S. H., Sarker, S., Aleri, J. W., Annandale, H., Abraham, S., & Uddin, J. M. (2023). Global Burden of Lumpy Skin Disease, Outbreaks, and Future Challenges. Viruses, 15(9), 1861. https://doi.org/10.3390/v15091861







