Cytomegalovirus Infection after Allogeneic Hematopoietic Cell Transplantation under 100-Day Letermovir Prophylaxis: A Real-World 1-Year Follow-Up Study
Abstract
:1. Introduction
2. Materials and Methods
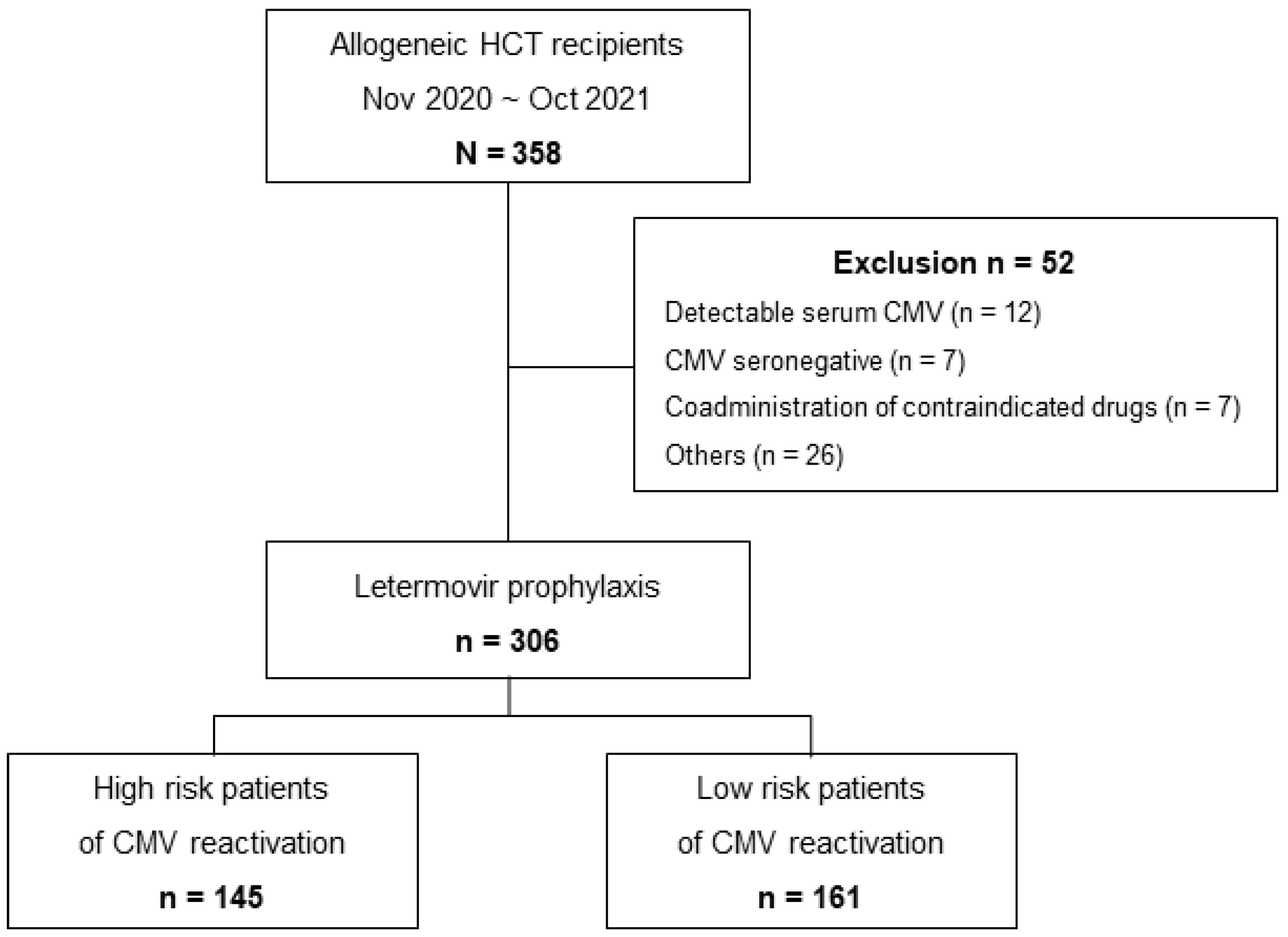
2.1. Patients and Study Design
2.2. Institutional Protocol
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Study Population and Baseline Characteristics
3.2. Discontinuation of Letermovir
3.3. CMV Reactivation and Mortality
3.4. Risk Factors of Clinically Significant CMV Infection
3.5. Letermovir Breakthrough CMV Reactivation
3.6. Refractory/Probable Refractory CMV Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, S.Y.; Lee, D.G.; Kim, H.J. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int. J. Mol. Sci. 2019, 20, 2666. [Google Scholar] [CrossRef] [PubMed]
- Chemaly, R.F.; Ullmann, A.J.; Stoelben, S.; Richard, M.P.; Bornhauser, M.; Groth, C.; Einsele, H.; Silverman, M.; Mullane, K.M.; Brown, J.; et al. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N. Engl. J. Med. 2014, 370, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; de la Camara, R.; Robin, C.; Crocchiolo, R.; Einsele, H.; Hill, J.A.; Hubacek, P.; Navarro, D.; Cordonnier, C.; Ward, K.N. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 2019, 19, e260–e272. [Google Scholar] [CrossRef] [PubMed]
- Goldner, T.; Hewlett, G.; Ettischer, N.; Ruebsamen-Schaeff, H.; Zimmermann, H.; Lischka, P. The novel anticytomegalovirus compound AIC246 (Letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase. J. Virol. 2011, 85, 10884–10893. [Google Scholar] [CrossRef]
- Heming, J.D.; Conway, J.F.; Homa, F.L. Herpesvirus Capsid Assembly and DNA Packaging. Cell Biol. Herpes Viruses 2017, 223, 119–142. [Google Scholar]
- Ligat, G.; Cazal, R.; Hantz, S.; Alain, S. The human cytomegalovirus terminase complex as an antiviral target: A close-up view. FEMS Microbiol. Rev. 2018, 42, 137–145. [Google Scholar] [CrossRef]
- Lischka, P.; Hewlett, G.; Wunberg, T.; Baumeister, J.; Paulsen, D.; Goldner, T.; Ruebsamen-Schaeff, H.; Zimmermann, H. In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246. Antimicrob. Agents Chemother. 2010, 54, 1290–1297. [Google Scholar] [CrossRef]
- Marschall, M.; Stamminger, T.; Urban, A.; Wildum, S.; Ruebsamen-Schaeff, H.; Zimmermann, H.; Lischka, P. In vitro evaluation of the activities of the novel anticytomegalovirus compound AIC246 (letermovir) against herpesviruses and other human pathogenic viruses. Antimicrob. Agents Chemother. 2012, 56, 1135–1137. [Google Scholar] [CrossRef]
- Marty, F.M.; Ljungman, P.; Chemaly, R.F.; Maertens, J.; Dadwal, S.S.; Duarte, R.F.; Haider, S.; Ullmann, A.J.; Katayama, Y.; Brown, J.; et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2017, 377, 2433–2444. [Google Scholar] [CrossRef]
- Vyas, A.; Raval, A.D.; Kamat, S.; LaPlante, K.; Tang, Y.; Chemaly, R.F. Real-World Outcomes Associated With Letermovir Use for Cytomegalovirus Primary Prophylaxis in Allogeneic Hematopoietic Cell Transplant Recipients: A Systematic Review and Meta-analysis of Observational Studies. Open Forum Infect. Dis. 2023, 10, ofac687. [Google Scholar] [CrossRef]
- Choi, S.R.; Kim, K.R.; Kim, D.S.; Kang, J.M.; Kim, S.J.; Kim, J.M.; Oh, S.Y.; Kang, C.I.; Chung, D.R.; Peck, K.R.; et al. Changes in Cytomegalovirus Seroprevalence in Korea for 21 Years: A Single Center Study. Pediatr. Infect. Vaccine 2018, 25, 123–131. [Google Scholar] [CrossRef]
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 2019, 29, e2034. [Google Scholar] [CrossRef] [PubMed]
- Huntley, D.; Talaya, A.; Giménez, E.; Martínez, A.; Hernández-Boluda, J.C.; Hernani, R.; Torres, I.; Alberola, J.; Albert, E.; Piñana, J.L.; et al. Features of Cytomegalovirus DNAemia Blips in Allogeneic Hematopoietic Stem Cell Transplant Recipients: Implications for Optimization of Preemptive Antiviral Therapy Strategies. Biol. Blood Marrow Transplant. 2020, 26, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Lodding, I.P.; Mocroft, A.; da Cunha Bang, C.; Gustafsson, F.; Iversen, M.; Kirkby, N.; Perch, M.; Rasmussen, A.; Sengeløv, H.; Sørensen, S.S.; et al. Impact of CMV PCR Blips in Recipients of Solid Organ and Hematopoietic Stem Cell Transplantation. Transplant. Direct 2018, 4, e355. [Google Scholar] [CrossRef]
- Royston, L.; Royston, E.; Masouridi-Levrat, S.; Chalandon, Y.; Van Delden, C.; Neofytos, D. Predictors of breakthrough clinically significant cytomegalovirus infection during letermovir prophylaxis in high-risk hematopoietic cell transplant recipients. Immun. Inflamm. Dis. 2021, 9, 771–776. [Google Scholar] [CrossRef]
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V.; Griffiths, P.D. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin. Infect. Dis. 2017, 64, 87–91. [Google Scholar]
- Chemaly, R.F.; Chou, S.; Einsele, H.; Griffiths, P.; Avery, R.; Razonable, R.R.; Mullane, K.M.; Kotton, C.; Lundgren, J.; Komatsu, T.E.; et al. Definitions of Resistant and Refractory Cytomegalovirus Infection and Disease in Transplant Recipients for Use in Clinical Trials. Clin. Infect. Dis. 2019, 68, 1420–1426. [Google Scholar] [CrossRef]
- Chae, S.; Kim, H.S.; Cho, S.Y.; Nho, D.; Lee, R.; Lee, D.G.; Kim, M.; Kim, Y. Genetic Variants Associated with Drug Resistance of Cytomegalovirus in Hematopoietic Cell Transplantation Recipients. Viruses 2023, 15, 1286. [Google Scholar] [CrossRef]
- Choi, S.-M.; Lee, D.-G.; Park, S.H.; Kim, S.-H.; Kim, Y.-J.; Min, C.-K.; Kim, H.-J.; Lee, S.; Choi, J.-H.; Yoo, J.-H.; et al. Characteristics of Cytomegalovirus Diseases among Hematopoietic Stem Cell Transplant Recipients: A 10-year Experience at an University Hospital in Korea. Infect. Chemother. 2009, 41, 9–19. [Google Scholar] [CrossRef]
- Kim, S.H.; Kee, S.Y.; Lee, D.G.; Choi, S.M.; Park, S.H.; Kwon, J.C.; Eom, K.S.; Kim, Y.J.; Kim, H.J.; Lee, S.; et al. Infectious complications following allogeneic stem cell transplantation: Reduced-intensity vs. myeloablative conditioning regimens. Transpl. Infect. Dis. 2013, 15, 49–59. [Google Scholar] [CrossRef]
- Choi, J.K.; Cho, S.Y.; Yoon, S.S.; Moon, J.H.; Kim, S.H.; Lee, J.H.; Kim, J.S.; Cheong, J.W.; Jang, J.H.; Seo, B.J.; et al. Epidemiology and Risk Factors for Invasive Fungal Diseases among Allogeneic Hematopoietic Stem Cell Transplant Recipients in Korea: Results of “RISK” Study. Biol. Blood Marrow Transplant. 2017, 23, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Extension of Letermovir (LET) from Day 100 to Day 200 Post-Transplant for the Prevention of Cytomegalovirus (CMV) Infection in Hematopoietic Stem Cell Transplant (HSCT) Participants (MK-8228-040). Available online: https://clinicaltrials.gov/ct2/show/NCT03930615 (accessed on 15 July 2023).
- Allice, T.; Busca, A.; Locatelli, F.; Falda, M.; Pittaluga, F.; Ghisetti, V. Valganciclovir as pre-emptive therapy for cytomegalovirus infection post-allogenic stem cell transplantation: Implications for the emergence of drug-resistant cytomegalovirus. J. Antimicrob. Chemother. 2009, 63, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Lurain, N.S.; Chou, S. Antiviral drug resistance of human cytomegalovirus. Clin. Microbiol. Rev. 2010, 23, 689–712. [Google Scholar] [CrossRef] [PubMed]
- Borst, E.M.; Kleine-Albers, J.; Gabaev, I.; Babic, M.; Wagner, K.; Binz, A.; Degenhardt, I.; Kalesse, M.; Jonjic, S.; Bauerfeind, R.; et al. The human cytomegalovirus UL51 protein is essential for viral genome cleavage-packaging and interacts with the terminase subunits pUL56 and pUL89. J. Virol. 2013, 87, 1720–1732. [Google Scholar] [CrossRef]
- Muller, C.; Tilloy, V.; Frobert, E.; Feghoul, L.; Garrigue, I.; Lepiller, Q.; Mirand, A.; Sidorov, E.; Hantz, S.; Alain, S. First clinical description of letermovir resistance mutation in cytomegalovirus UL51 gene and potential impact on the terminase complex structure. Antivir. Res. 2022, 204, 105361. [Google Scholar] [CrossRef]
- Cassaniti, I.; Colombo, A.A.; Bernasconi, P.; Malagola, M.; Russo, D.; Iori, A.P.; Girmenia, C.; Greco, R.; Peccatori, J.; Ciceri, F.; et al. Positive HCMV DNAemia in stem cell recipients undergoing letermovir prophylaxis is expression of abortive infection. Am. J. Transplant. 2021, 21, 1622–1628. [Google Scholar] [CrossRef]



| (N = 306), n (%) | |
|---|---|
| Age (years), median (range) | 50 (18–73) |
| Male gender | 156 (51.0%) |
| Underlying disease | |
| Acute myeloid leukemia | 133 (43.5%) |
| Acute lymphocytic leukemia | 72 (23.5%) |
| Myelodysplastic syndrome | 53 (17.3%) |
| Myeloproliferative neoplasm | 29 (9.5%) |
| Multiple myeloma | 5 (1.6%) |
| Other diseases 1 | 14 (4.6%) |
| Type of conditioning regimen | |
| Myeloablative conditioning | 165 (53.9%) |
| Non-myeloablative conditioning | 141 (46.1%) |
| Use of antithymocyte globulin | 243 (79.4%) |
| Type of donor | |
| Matched sibling donor | 84 (27.5%) |
| Matched unrelated donor | 117 (38.2%) |
| Family mismatched transplantation | 80 (26.1%) |
| Double cord blood transplantation | 25 (8.2%) |
| Donor/Recipient CMV serostatus | |
| D+/R+ | 242 (79.1%) |
| D−/R+ | 39 (12.7%) |
| Unknown | 25 (8.2%) |
| Risk stratification for CMV reactivation | |
| High risk | 145 (47.4%) |
| Low risk | 161 (52.6%) |
| Start day of letermovir after HCT (days), median (range) | 2 (0–27) |
| Acute GVHD of grade ≥ 2 at initiation of letermovir | 1 (0.3%) |
| (N = 306), n (%) | |||
|---|---|---|---|
| 14 Weeks | 24 Weeks | 1 Year | |
| Any level of CMV reactivation | 81 (26.5%) | 196 (64.1%) | 226 (73.9%) |
| CS-CMVi, received pre-emptive therapy | 35 (11.4%) | 97 (31.7%) | 113 (36.9%) |
| CMV end-organ disease | 8 (2.6%) | 20 (6.5%) | 25 (8.2%) |
| CMV DNAemia, not requiring pre-emptive therapy | 46 (15.0%) | 99 (32.4%) | 113 (36.9%) |
| All-cause mortality | 22 (7.2%) | 32 (10.5%) | 66 (21.6%) |
| CMV-related mortality | 1 (0.3%) | 3 (1.0%) | 5 (1.6%) |
| Follow-up loss | 2 (0.3%) | 4 (1.3%) | 9 (2.9%) |
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| Myeloablative conditioning | 1.054 | 0.648–1.712 | 0.833 |
| Lymphoid lineage malignancies | 1.355 | 0.794–2.314 | 0.266 |
| Matched sibling donor HCT | 0.937 | 0.542–1.618 | 0.815 |
| CMV seronegative donor | 0.845 | 0.402–1.778 | 0.657 |
| GVHD (≥grade 2) | 3.64 | 2.036–6.510 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nho, D.; Lee, R.; Cho, S.-Y.; Lee, D.-G.; Kim, E.-J.; Park, S.; Lee, S.-E.; Cho, B.-S.; Kim, Y.-J.; Lee, S.; et al. Cytomegalovirus Infection after Allogeneic Hematopoietic Cell Transplantation under 100-Day Letermovir Prophylaxis: A Real-World 1-Year Follow-Up Study. Viruses 2023, 15, 1884. https://doi.org/10.3390/v15091884
Nho D, Lee R, Cho S-Y, Lee D-G, Kim E-J, Park S, Lee S-E, Cho B-S, Kim Y-J, Lee S, et al. Cytomegalovirus Infection after Allogeneic Hematopoietic Cell Transplantation under 100-Day Letermovir Prophylaxis: A Real-World 1-Year Follow-Up Study. Viruses. 2023; 15(9):1884. https://doi.org/10.3390/v15091884
Chicago/Turabian StyleNho, Dukhee, Raeseok Lee, Sung-Yeon Cho, Dong-Gun Lee, Eun-Jin Kim, Silvia Park, Sung-Eun Lee, Byung-Sik Cho, Yoo-Jin Kim, Seok Lee, and et al. 2023. "Cytomegalovirus Infection after Allogeneic Hematopoietic Cell Transplantation under 100-Day Letermovir Prophylaxis: A Real-World 1-Year Follow-Up Study" Viruses 15, no. 9: 1884. https://doi.org/10.3390/v15091884
APA StyleNho, D., Lee, R., Cho, S.-Y., Lee, D.-G., Kim, E.-J., Park, S., Lee, S.-E., Cho, B.-S., Kim, Y.-J., Lee, S., & Kim, H.-J. (2023). Cytomegalovirus Infection after Allogeneic Hematopoietic Cell Transplantation under 100-Day Letermovir Prophylaxis: A Real-World 1-Year Follow-Up Study. Viruses, 15(9), 1884. https://doi.org/10.3390/v15091884





