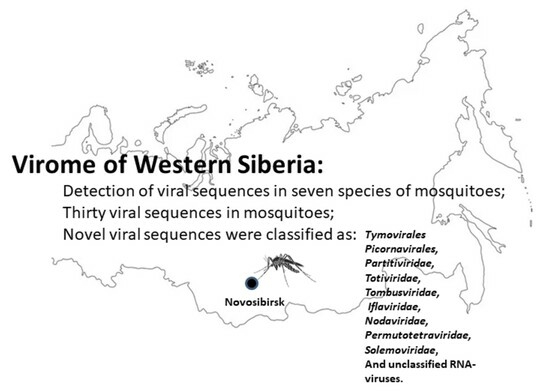
The Viromes of Mosquitoes from the Natural Landscapes of Western Siberia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mosquito Samples
2.2. Sample Preparation
2.3. NGS Sequencing and Phylogenetic Analysis
3. Results
3.1. Mosquito Species
3.2. NGS Sequencing
3.2.1. Tymovirales (Positive ssRNA)
3.2.2. Partitiviridae (dsRNA)
3.2.3. Totiviridae (Monopartite dsRNA)
3.2.4. Tombusviridae (Positive ssRNA)
3.2.5. Picornavirales (Positive ssRNA)
3.2.6. Iflaviridae (Positive ssRNA)
3.2.7. Nodaviridae (Bi-Partite Positive-Sense, ssRNA, Sometimes Associated with Subviral RNA Molecule Capable of Replicating Itself)
3.2.8. Permutotetraviridae (dsRNA)
3.2.9. Other Viruses and Viral Sequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizzoli, A.; Jimenez-Clavero, M.A.; Barzon, L.; Cordioli, P.; Figuerola, J.; Koraka, P.; Martina, B.; Moreno, A.; Nowotny, N.; Pardigon, N.; et al. The challenge of West Nile virus in Europe: Knowledge gaps and research priorities. Euro Surveill. 2015, 20, 21135. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S.; Morens, D.M. Zika Virus in the Americas—Yet Another Arbovirus Threat. N. Engl. J. Med. 2016, 374, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.; Dermody, T.S. Chikungunya virus: Epidemiology, replication, disease mechanisms, and prospective intervention strategies. J. Clin. Investig. 2017, 127, 737–749. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Scott, T.W.; Zou, C.; Pigott, D.M.; Duda, K.A.; Bhatt, S.; Katzelnick, L.; Howes, R.E.; Battle, K.E.; et al. Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 2014, 22, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.D.; Tian, J.H.; Chen, L.J.; Chen, X.; Li, C.X.; Qin, X.C.; Li, J.; Cao, J.P.; Eden, J.S.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef]
- Sadeghi, M.; Altan, E.; Deng, X.; Barker, C.M.; Fang, Y.; Coey, L.L.; Delwart, E. Virome of >12 thousand Culex mosquitoes from throughout California. Virology 2018, 523, 74–88. [Google Scholar] [CrossRef]
- Li, C.X.; Shi, M.; Tian, J.H.; Lin, X.D.; Kang, Y.J.; Chen, L.J.; Qin, X.C.; Xu, J.; Holmes, E.C.; Zhang, Y.Z. Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. eLife 2015, 4, e05378. [Google Scholar] [CrossRef] [PubMed]
- Frey, K.G.; Biser, T.; Hamilton, T.; Santos, C.J.; Pimentel, G.; Mokashi, V.P.; Bishop-Lilly, K.A. Bioinformatic Characterization of Mosquito Viromes within the Eastern United States and Puerto Rico: Discovery of Novel Viruses. Evol. Bioinform. 2016, 12, 1–12. [Google Scholar] [CrossRef]
- Junglen, S.; Drosten, C. Virus discovery and recent insights into virus diversity in arthropods. Curr. Opin. Microbiol. 2013, 16, 507–513. [Google Scholar] [CrossRef]
- Webster, C.L.; Longdon, B.; Lewis, S.H.; Obbard, D.J. Twenty-Five New Viruses Associated with the Drosophilidae (Diptera). Evol. Bioinform. 2016, 12, 13–25. [Google Scholar] [CrossRef]
- Bolling, B.G.; Olea-Popelka, F.J.; Eisen, L.; Moore, C.G.; Blair, C.D. Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology 2012, 427, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Hobson-Peters, J.; Yam, A.W.; Lu, J.W.; Setoh, Y.X.; May, F.J.; Kurucz, N.; Walsh, S.; Prow, N.A.; Davis, S.S.; Weir, R.; et al. A new insect-specific flavivirus from northern Australia suppresses replication of West Nile virus and Murray Valley encephalitis virus in co-infected mosquito cells. PLoS ONE 2013, 8, e56534. [Google Scholar] [CrossRef] [PubMed]
- Kenney, J.L.; Solberg, O.D.; Langevin, S.A.; Brault, A.C. Characterization of a novel insect-specific flavivirus from Brazil: Potential for inhibition of infection of arthropod cells with medically important flaviviruses. J. Gen. Virol. 2014, 95, 2796–2808. [Google Scholar] [CrossRef] [PubMed]
- Goenaga, S.; Kenney, J.L.; Duggal, N.K.; Delorey, M.; Ebel, G.D.; Zhang, B.; Levis, S.C.; Enria, D.A.; Brault, A.C. Potential for Co-Infection of a Mosquito-Specific Flavivirus, Nhumirim Virus, to Block West Nile Virus Transmission in Mosquitoes. Viruses 2015, 7, 5801–5812. [Google Scholar] [CrossRef]
- Nasar, F.; Erasmus, J.H.; Haddow, A.D.; Tesh, R.B.; Weaver, S.C. Eilat virus induces both homologous and heterologous interference. Virology 2015, 484, 51–58. [Google Scholar] [CrossRef]
- Hall-Mendelin, S.; McLean, B.J.; Bielefeldt-Ohmann, H.; Hobson-Peters, J.; Hall, R.A.; van den Hurk, A.F. The insect-specific Palm Creek virus modulates West Nile virus infection in and transmission by Australian mosquitoes. Parasit. Vectors 2016, 9, 414. [Google Scholar] [CrossRef]
- Marklewitz, M.; Zirkel, F.; Kurth, A.; Drosten, C.; Junglen, S. Evolutionary and phenotypic analysis of live virus isolates suggests arthropod origin of a pathogenic RNA virus family. Proc. Natl. Acad. Sci. USA 2015, 112, 7536–7541. [Google Scholar] [CrossRef]
- Crochu, S.; Cook, S.; Attoui, H.; Charrel, R.N.; De Chesse, R.; Belhouchet, M.; Lemasson, J.J.; de Micco, P.; de Lamballerie, X. Sequences of flavivirus-related RNA viruses persist in DNA form integrated in the genome of Aedes spp. mosquitoes. J. Gen. Virol. 2004, 85, 1971–1980. [Google Scholar] [CrossRef]
- Cholleti, H.; Hayer, J.; Abilio, A.P.; Mulandane, F.C.; Verner-Carlsson, J.; Falk, K.I.; Fafetine, J.M.; Berg, M.; Blomstrom, A.L. Discovery of Novel Viruses in Mosquitoes from the Zambezi Valley of Mozambique. PLoS ONE 2016, 11, e0162751. [Google Scholar] [CrossRef]
- Shi, M.; Neville, P.; Nicholson, J.; Eden, J.S.; Imrie, A.; Holmes, E.C. High-Resolution Metatranscriptomics Reveals the Ecological Dynamics of Mosquito-Associated RNA Viruses in Western Australia. J. Virol. 2017, 91, e00680-17. [Google Scholar] [CrossRef]
- Ternovoĭ, V.A.; Shchelkanov, M.I.; Shestopalov, A.M.; Aristova, V.A.; Protopopova, E.V.; Gromashevskiĭ, V.L.; Druziaka, A.V.; Slavskiĭ, A.A.; Zolotykh, S.I.; Loktev, V.B.; et al. Detection of West Nile virus in birds in the territories of Baraba and Kulunda lowlands (West Siberian migration way) during summer-autumn of 2002. Vopr. Virusol. 2004, 49, 52–56. (In Russian) [Google Scholar] [PubMed]
- Korobitsyn, I.G.; Moskvitina, N.S.; Tyutenkov, O.Y.; Gashkov, S.I.; Kononova, Y.; Moskvitin, S.S.; Romanenko, V.N.; Mikryukova, T.P.; Protopopova, E.V.; Kartashov, M.Y.; et al. Detection of tick-borne pathogens in wild birds and their ticks in Western Siberia and high level of their mismatch. Folia Parasitol. 2021, 68, 024. [Google Scholar] [CrossRef] [PubMed]
- Ternovoi, V.A.; Shvalov, A.N.; Kartashov, M.Y.; Ponomareva, E.P.; Tupota, N.L.; Khoroshavin, Y.A.; Bayandin, R.B.; Gladysheva, A.V.; Mikryukova, T.P.; Tregubchak, T.V. Cases of West Nile fever in Novosibirsk region in 2004, and the genotyping of its viral pathogen. Vestn. Ross. Akad. Med. Nauk. 2007, 1, 21–26. (In Russian) [Google Scholar]
- Rodgers, M.A.; Wilkinson, E.; Vallari, A.; McArthur, C.; Sthreshley, L.; Brennan, C.A.; Cloherty, G.; de Oliveira, T. Sensitive Next-Generation Sequencing Method Reveals Deep Genetic Diversity of HIV-1 in the Democratic Republic of the Congo. J. Virol. 2017, 91, e01841-16. [Google Scholar] [CrossRef]
- Ding, S.W.; Howe, J.; Keese, P.; Mackenzie, A.; Meek, D.; Osorio-Keese, M.; Skotnicki, M.; Srifah, P.; Torronen, M.; Gibbs, A. The tymobox, a sequence shared by most tymoviruses: Its use in molecular studies of tymoviruses. Nucleic Acids Res. 1990, 18, 1181–1187. [Google Scholar] [CrossRef]
- Kreuze, J.; Koenig, R.; De Souza, J.; Vetten, H.J.; Muller, G.; Flores, B.; Ziebell, H.; Cuellar, W. The complete genome sequences of a Peruvian and a Colombian isolate of Andean potato latent virus and partial sequences of further isolates suggest the existence of two distinct potato-infecting tymovirus species. Virus Res. 2013, 173, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Carino, E.; Bera, S.; Gao, F.; May, J.P.; Simon, A.E. Structural Analysis and Whole Genome Mapping of a New Type of Plant Virus Subviral RNA: Umbravirus-Like Associated RNAs. Viruses 2021, 13, 646. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, S.; Käfer, S.; Zirkel, F.; Donath, A.; Petersen, M.; Liu, S.; Zhou, X.; Drosten, C.; Misof, B.; Junglen, S. Viromics of extant insect orders unveil the evolution of the flavi-like superfamily. Virus Evol. 2021, 7, veab030. [Google Scholar] [CrossRef]
- Bera, S.; Ilyas, M.; Mikkelsen, A.A.; Simon, A.E. Conserved Structure Associated with Different 3’CITEs Is Important for Translation of Umbraviruses. Viruses 2023, 15, 638. [Google Scholar] [CrossRef]
- Öhlund, P.; Hayer, J.; Lundén, H.; Hesson, J.C.; Blomström, A.L. Viromics Reveal a Number of Novel RNA Viruses in Swedish Mosquitoes. Viruses 2019, 11, 1027. [Google Scholar] [CrossRef]
- Sanborn, M.A.; Klein, T.A.; Kim, H.-C.; Fung, C.K.; Figueroa, K.L.; Yang, Y.; Asafo-Adjei, E.A.; Jarman, R.G.; Hang, J. Metagenomic Analysis Reveals Three Novel and Prevalent Mosquito Viruses from a Single Pool of Aedes vexans nipponii Collected in the Republic of Korea. Viruses 2019, 11, 222. [Google Scholar] [CrossRef]
- Walker, P.J.; Firth, C.; Widen, S.G.; Blasdell, K.R.; Guzman, H.; Wood, T.G.; Paradkar, P.N.; Holmes, E.C.; Tesh, R.B.; Vasilakis, N. Evolution of genome size and complexity in the Rhabdoviridae. PLoS Pathog. 2015, 11, e1004664. [Google Scholar] [CrossRef]
- Presti, R.M.; Zhao, G.; Beatty, W.L.; Mihindukulasuriya, K.A.; da Rosa, A.P.T.; Popov, V.L.; Wang, D. Quaranfil, Johnston Atoll, and Lake Chad viruses are novel members of the family Orthomyxoviridae. J. Virol. 2009, 83, 11599–11606. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Y.; Atoni, E.; Zhang, B.; Yuan, Z. Mosquito-associated viruses in China. Virol. Sin. 2018, 33, 5–20. [Google Scholar] [CrossRef]
- Attoui, H.; Jaafar, F.M.; Belhouchet, M.; Tao, S.; Chen, B.; Liang, G.; de Lamballerie, X. Liao ning virus, a new Chinese seadornavirus that replicates in transformed and embryonic mammalian cells. J. Gen. Virol. 2006, 87, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, N.; Forrester, N.L.; Palacios, G.; Nasar, F.; Savji, N.; Rossi, S.L.; Guzman, H.; Wood, T.G.; Popov, V.; Gorchakov, R.; et al. Negevirus: A proposed new taxon of insect-specific viruses with wide geographic distribution. J. Virol. 2013, 87, 2475–2488. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Han, J.; Zhang, Y.; Li, C.; Guo, X.; Wen, S.; Tian, M.; Li, Y.; Wang, M.; Liu, H.; et al. Metagenomic Analysis of Flaviviridae in Mosquito Viromes Isolated from Yunnan Province in China Reveals Genes from Dengue and Zika Viruses. Front. Cell. Infect. Microbiol. 2018, 8, 359. [Google Scholar] [CrossRef]
- Klungthong, C.; Gibbons, R.V.; Thaisomboonsuk, B.; Nisalak, A.; Kalayanarooj, S.; Thirawuth, V.; Jarman, R.G. Dengue virus detection using whole blood for reverse transcriptase PCR and virus isolation. J. Clin. Microbiol. 2007, 45, 2480–2485. [Google Scholar] [CrossRef]
- LaBeaud, A.D.; Bashir, F.; King, C.H. Measuring the burden of arboviral diseases: The spectrum of morbidity and mortality from four prevalent infections. Popul. Health Metr. 2011, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.A.; Bielefeldt-Ohmann, H.; McLean, B.J.; O’Brien, C.A.; Colmant, A.M.; Piyasena, T.B.; Harrison, J.J.; Newton, N.D.; Barnard, R.T.; Prow, N.A.; et al. Commensal Viruses of Mosquitoes: Host Restriction, Transmission, and Interaction with Arboviral Pathogens. Evol. Bioinform. 2017, 12, 35–44. [Google Scholar] [CrossRef]
- Vasilakis, N.; Tesh, R.B. Insect-specific viruses and their potential impact on arbovirus transmission. Curr. Opin. Virol. 2015, 15, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging arboviruses: Why today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.M.; Waterman, S.H. A decade of arboviral activity—Lessons learned from the trenches. PLoS Negl. Trop. Dis. 2017, 11, e0005421. [Google Scholar] [CrossRef]
- Joganzen, B.G.; Petkevich, A.N. Acclimatization of fish in Western Siberia. Proc. Baraba Branch VNIORKH 1951, 5, 3–204. [Google Scholar]
- IuV, K.; Mirzaeva, A.G.; IuV, S.; Protopopova, E.V.; Dupal, T.A.; Ternovoĭ, V.A.; IuA, I.; Shestopalov, A.M.; Loktev, V.B. Species composition of mosquitoes (Diptera, Culicidae) and possibility of the West Nile virus natural foci formation in the South of Western Siberia. Parazitologiia 2007, 41, 459–470. (In Russian) [Google Scholar]




| Name of Virus | Viral Prototype (Identity, %) | Accession Number, GenBank | Genome (Fragment) Size (bp) | Coverage by NGS, Times | |
|---|---|---|---|---|---|
| Tymovirales (positive ssRNA) | |||||
| 1. | Inya insect-associated virus 1 | Insect-associated tymovirus 1/Andean potato mild mosaic virus (near 60%) | MW251314 | 6568 (polyprotein) | 155 |
| 2. | Inya insect-associated virus 2 | Insect-associated tymovirus 1/Andean potato mild mosaic virus (near 60%) | MW251315 | 6566 (polyprotein) | 105 |
| Partitiviridae (dsRNA) | |||||
| 3. | Partitivirus-like 1 | Partitivirus-like 1 (89%) | MW251327 | 1749 (RdRp) | 40 |
| 4. | Krahall insect-associated virus 1/01 | Atrato partiti-like virus 2 (63%) | MW389552 | 1490 (capsid) | 179 |
| Krahall insect-associated virus 1/02 | Atrato partiti-like virus 2 (61%) | MW389553 | 1512 (capsid) | 71 | |
| Krahall insect-associated virus 1/03 | Atrato partiti-like virus 2 (61%) | MW389554 | 1488 (capsid) | 35 | |
| 5. | Krahall insect-associated virus 2 | Atrato partiti-like virus 2 (60%) | MW389555 | 1531 (capsid) | 79 |
| 6. | Talaya insect virus 2/01 | Partitivirus-like 1 (68%) | MW251328 | 1426 (RdRp) | 51 |
| Talaya insect virus 2/02 | Partitivirus-like 1 (71%) | MW251329 | 1489 (RdRp) | 30 | |
| Talaya insect virus 2/03 | Partitivirus-like 1 (68%) | MW251330 | 1426 (RdRp) | 20 | |
| 7. | TarBrook virus | Sonnbo virus (79%) | MW251325 | 1742 (hypot. protein) | 75 |
| 8. | Zeyabrook partiti-like_virus 1/01 | Beihai partiti-like virus 2 (38%) | MW389559 | 1597 (capsid) | 99 |
| Zeyabrook partiti-like_virus 1/02 | Beihai partiti-like virus 2 (38%) | MW389560 | 1597 (capsid) | 31 | |
| 9. | Zeyabrook partiti-like_virus 2 | Beihai partiti-like virus 2 (42%) | MW389561 | 1458 (capsid) | 32 |
| Totiviridae (monopartite, dsRNA) | |||||
| 10. | Zyryana toti-like virus 2 | Fitzroy Crossing toti-like virus 1 (51%) | MW251336 | >5863 (RdRp, capsid) | 24 |
| Tombusviridae (positive, ssRNA) | |||||
| 11. | Hammarskog tombus-like virus | Hammarskog tombus-like virus (90) | MW251332 | 4317 (polyprotein) | 10 |
| 12. | Oyosh tombus-like virus | Hubei tombus-like virus 13 (62%) | MW251324 | >5640 (polyprotein) | 19 |
| Picornavirales (positive, ssRNA) | |||||
| 13. | Hammarskog picorna-like virus | Hammarskog picorna-like virus (98%) | MT753151 | 11506 (polyprotein) | 6952 |
| 14. | Miltyush picorna-like virus 1/01 | Halhan virus 3 (31%) | MW251320 | 9329 (polyprotein) | 31 |
| Miltyush picorna-like virus 1/02 | Halhan virus 3 (31%) | MW251321 | 9329 (polyprotein) | 19 | |
| 15. | Miltyush picorna-like virus 2 | Halhan virus 3 (36%) | MW251322 | 10,077 (polyprotein) | 49 |
| 16. | Isses picorna-like virus 1 | Washington bat picornavirus (64%) | MW251316 | 8992 (polyprotein) | 537 |
| Isses picorna-like virus 2 | Washington bat picornavirus (64%) | MW251317 | 8992 (polyprotein) | 134 | |
| 17. | Ora Rivulet insect-associated polycipivirus | Polycipiviridae sp (34%) | MW251323 | 10,879 (polyprotein) | 62 |
| 18. | Icha Creek insect virus | Solenopsis invicta virus 3 (32%) | MW251313 | 10,272 (polyprotein) | 24 |
| Iflaviridae (positive, ssRNA) | |||||
| 19. | Lymantria dispar iflavirus 1 | Lymantria dispar iflavirus 1 (99%) | MT753155 | 9996 (polyprotein) | 347 |
| Nodaviridae (Bi-partite positive-sense, ssRNA) | |||||
| 20. | Wenzhou noda-like virus 6 | Wenzhou noda-like virus 6 (87%) | MW251319 | 3098 (RdRp) | 159 |
| 21. | Mayapan virus 1/1 | Mayapan virus (90%) | MT753152 | 3024 (RdRp) | 292 |
| Mayapan virus 1/2 | Mayapan virus (88%) | MT753153 | 1378 (capsid) | 181 | |
| 22. | Mayzas noda-like virus RNA 2 | Mayapan virus segment RNA2 (43%) | MW389556 | 1239 (capsid) | 68 |
| 23. | Uzakla insect-associated virus | Mosinovirus (36%) | MW389557 | 2493 (capsid) | 46 |
| Permutotetraviridae (dsRNA) | |||||
| 24. | Sanxia permutotetra-like virus 1 | Sanxia permutotetra-like virus 1 (93%) | MT753154 | 4670 (polyprotein) | 378 |
| 25. | Uzakla mosquito-associated permutotetra-like virus | Vespa velutina permutotetra-like virus 2 (53%) | MW389558 | 1749 (capsid) | 207 |
| Other viruses and viral sequences | |||||
| 26. | Chaq virus-like 1/1 | Chaq virus-like 1 (82%) | MW251333 | 1495 (hypot. protein) | 451 |
| Chaq virus-like 1/2 | Chaq virus-like 1 (82%) | MW251334 | 1492 (hypot. protein) | 300 | |
| 27. | Tartas insect associate virus | Atrato Sobemo-like virus 6 (61%) | MW251326 | 3259 (polyprotein) | 464 |
| 28. | ZeyaBrook chaq-like_virus 2 | Chaq virus-like 3 (47%) | MW251335 | 1409 (hypot. protein) | 220 |
| 29. | Kamenka insect-associated virus | Hubei levi-like virus 3 (38%) | MW251318 | >3296 (polyprotein) | 17 |
| 30. | Uzakla insect virus | Hubei myriapoda virus 1 (33%) | MW251331 | 9614 (polyprotein) | 46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ternovoi, V.A.; Shvalov, A.N.; Kartashov, M.Y.; Ponomareva, E.P.; Tupota, N.L.; Khoroshavin, Y.A.; Bayandin, R.B.; Gladysheva, A.V.; Mikryukova, T.P.; Tregubchak, T.V.; et al. The Viromes of Mosquitoes from the Natural Landscapes of Western Siberia. Viruses 2023, 15, 1896. https://doi.org/10.3390/v15091896
Ternovoi VA, Shvalov AN, Kartashov MY, Ponomareva EP, Tupota NL, Khoroshavin YA, Bayandin RB, Gladysheva AV, Mikryukova TP, Tregubchak TV, et al. The Viromes of Mosquitoes from the Natural Landscapes of Western Siberia. Viruses. 2023; 15(9):1896. https://doi.org/10.3390/v15091896
Chicago/Turabian StyleTernovoi, Vladimir A., Alexander N. Shvalov, Mikhail Yu. Kartashov, Eugenia P. Ponomareva, Natalia L. Tupota, Yuri A. Khoroshavin, Roman B. Bayandin, Anastasia V. Gladysheva, Tamara P. Mikryukova, Tatyana V. Tregubchak, and et al. 2023. "The Viromes of Mosquitoes from the Natural Landscapes of Western Siberia" Viruses 15, no. 9: 1896. https://doi.org/10.3390/v15091896
APA StyleTernovoi, V. A., Shvalov, A. N., Kartashov, M. Y., Ponomareva, E. P., Tupota, N. L., Khoroshavin, Y. A., Bayandin, R. B., Gladysheva, A. V., Mikryukova, T. P., Tregubchak, T. V., & Loktev, V. B. (2023). The Viromes of Mosquitoes from the Natural Landscapes of Western Siberia. Viruses, 15(9), 1896. https://doi.org/10.3390/v15091896