IL-15 and N-803 for HIV Cure Approaches
Abstract
1. Introduction
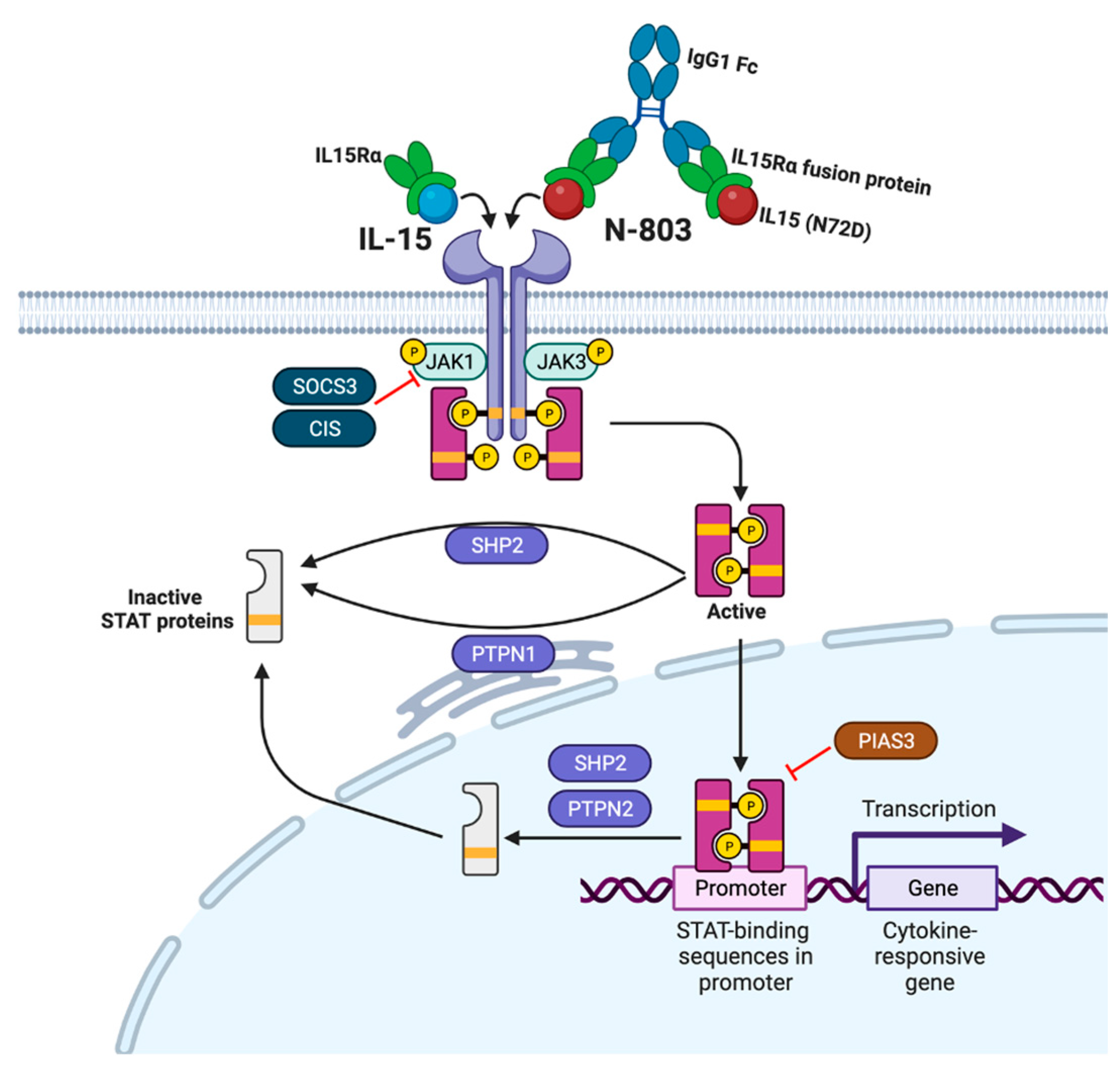
2. IL-15
3. N-803
4. Anti-HIV Activity of IL-15/N-803 In Vitro and Ex Vivo
5. Anti-HIV Activity of IL-15/N-803 in Animal Models
5.1. IL-15 Studies
5.2. N-803 Studies
6. N-803 Activity in Clinical Trials
7. Future Directions of IL-15/N-803 in HIV Cure Studies
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pennings, P.S. HIV Drug Resistance: Problems and Perspectives. Infect. Dis. Rep. 2013, 5 (Suppl. 1), e5. [Google Scholar] [CrossRef] [PubMed]
- Perelson, A.S.; Essunger, P.; Cao, Y.; Vesanen, M.; Hurley, A.; Saksela, K.; Markowitz, M.; Ho, D.D. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 1997, 387, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Finzi, D.; Blankson, J.; Siliciano, J.D.; Margolick, J.B.; Chadwick, K.; Pierson, T.; Smith, K.; Lisziewicz, J.; Lori, F.; Flexner, C.; et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 1999, 5, 512–517. [Google Scholar] [CrossRef]
- Chun, T.W.; Stuyver, L.; Mizell, S.B.; Ehler, L.A.; Mican, J.A.; Baseler, M.; Lloyd, A.L.; Nowak, M.A.; Fauci, A.S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 1997, 94, 13193–13197. [Google Scholar] [CrossRef] [PubMed]
- Finzi, D.; Hermankova, M.; Pierson, T.; Carruth, L.M.; Buck, C.; Chaisson, R.E.; Quinn, T.C.; Chadwick, K.; Margolick, J.; Brookmeyer, R.; et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997, 278, 1295–1300. [Google Scholar] [CrossRef]
- Wong, J.K.; Hezareh, M.; Günthard, H.F.; Havlir, D.V.; Ignacio, C.C.; Spina, C.A.; Richman, D.D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 1997, 278, 1291–1295. [Google Scholar] [CrossRef]
- Chun, T.W.; Carruth, L.; Finzi, D.; Shen, X.; DiGiuseppe, J.A.; Taylor, H.; Hermankova, M.; Chadwick, K.; Margolick, J.; Quinn, T.C.; et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 1997, 387, 183–188. [Google Scholar] [CrossRef]
- Davey, R.T., Jr.; Bhat, N.; Yoder, C.; Chun, T.W.; Metcalf, J.A.; Dewar, R.; Natarajan, V.; Lempicki, R.A.; Adelsberger, J.W.; Miller, K.D.; et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl. Acad. Sci. USA 1999, 96, 15109–15114. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Kajdas, J.; Finzi, D.; Quinn, T.C.; Chadwick, K.; Margolick, J.B.; Kovacs, C.; Gange, S.J.; Siliciano, R.F. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 2003, 9, 727–728. [Google Scholar] [CrossRef]
- Crooks, A.M.; Bateson, R.; Cope, A.B.; Dahl, N.P.; Griggs, M.K.; Kuruc, J.D.; Gay, C.L.; Eron, J.J.; Margolis, D.M.; Bosch, R.J.; et al. Precise Quantitation of the Latent HIV-1 Reservoir: Implications for Eradication Strategies. J. Infect. Dis. 2015, 212, 1361–1365. [Google Scholar] [CrossRef]
- Siliciano, R.F.; Greene, W.C. HIV latency. Cold Spring Harb. Perspect. Med. 2011, 1, a007096. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G. HIV: Shock and kill. Nature 2012, 487, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Chun, T.W.; Engel, D.; Mizell, S.B.; Hallahan, C.W.; Fischette, M.; Park, S.; Davey, R.T., Jr.; Dybul, M.; Kovacs, J.A.; Metcalf, J.A.; et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat. Med. 1999, 5, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Prins, J.M.; Jurriaans, S.; van Praag, R.M.; Blaak, H.; van Rij, R.; Schellekens, P.T.; ten Berge, I.J.; Yong, S.L.; Fox, C.H.; Roos, M.T.; et al. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS 1999, 13, 2405–2410. [Google Scholar] [CrossRef]
- van Praag, R.M.; Prins, J.M.; Roos, M.T.; Schellekens, P.T.; Ten Berge, I.J.; Yong, S.L.; Schuitemaker, H.; Eerenberg, A.J.; Jurriaans, S.; de Wolf, F.; et al. OKT3 and IL-2 treatment for purging of the latent HIV-1 reservoir in vivo results in selective long-lasting CD4+ T cell depletion. J. Clin. Immunol. 2001, 21, 218–226. [Google Scholar] [CrossRef]
- Dybul, M.; Hidalgo, B.; Chun, T.W.; Belson, M.; Migueles, S.A.; Justement, J.S.; Herpin, B.; Perry, C.; Hallahan, C.W.; Davey, R.T.; et al. Pilot study of the effects of intermittent interleukin-2 on human immunodeficiency virus (HIV)-specific immune responses in patients treated during recently acquired HIV infection. J. Infect. Dis. 2002, 185, 61–68. [Google Scholar] [CrossRef]
- Scripture-Adams, D.D.; Brooks, D.G.; Korin, Y.D.; Zack, J.A. Interleukin-7 induces expression of latent human immunodeficiency virus type 1 with minimal effects on T-cell phenotype. J. Virol. 2002, 76, 13077–13082. [Google Scholar] [CrossRef]
- Vandergeeten, C.; Fromentin, R.; DaFonseca, S.; Lawani, M.B.; Sereti, I.; Lederman, M.M.; Ramgopal, M.; Routy, J.P.; Sekaly, R.P.; Chomont, N. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood 2013, 121, 4321–4329. [Google Scholar] [CrossRef]
- Bosque, A.; Famiglietti, M.; Weyrich, A.S.; Goulston, C.; Planelles, V. Homeostatic proliferation fails to efficiently reactivate HIV-1 latently infected central memory CD4+ T cells. PLoS Pathog. 2011, 7, e1002288. [Google Scholar] [CrossRef]
- Levy, Y.; Sereti, I.; Tambussi, G.; Routy, J.P.; Lelievre, J.D.; Delfraissy, J.F.; Molina, J.M.; Fischl, M.; Goujard, C.; Rodriguez, B.; et al. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: Results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin. Infect. Dis. 2012, 55, 291–300. [Google Scholar] [CrossRef]
- Thiebaut, R.; Jarne, A.; Routy, J.P.; Sereti, I.; Fischl, M.; Ive, P.; Speck, R.F.; D’Offizi, G.; Casari, S.; Commenges, D.; et al. Repeated Cycles of Recombinant Human Interleukin 7 in HIV-Infected Patients With Low CD4 T-Cell Reconstitution on Antiretroviral Therapy: Results of 2 Phase II Multicenter Studies. Clin. Infect. Dis. 2016, 62, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Katlama, C.; Lambert-Niclot, S.; Assoumou, L.; Papagno, L.; Lecardonnel, F.; Zoorob, R.; Tambussi, G.; Clotet, B.; Youle, M.; Achenbach, C.J.; et al. EraMune-01 study, t. Treatment intensification followed by interleukin-7 reactivates HIV without reducing total HIV DNA: A randomized trial. AIDS 2016, 30, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Grabstein, K.H.; Eisenman, J.; Shanebeck, K.; Rauch, C.; Srinivasan, S.; Fung, V.; Beers, C.; Richardson, J.; Schoenborn, M.A.; Ahdieh, M.; et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 1994, 264, 965–968. [Google Scholar] [CrossRef]
- Li, X.C.; Demirci, G.; Ferrari-Lacraz, S.; Groves, C.; Coyle, A.; Malek, T.R.; Strom, T.B. IL-15 and IL-2: A matter of life and death for T cells in vivo. Nat. Med. 2001, 7, 114–118. [Google Scholar] [CrossRef]
- Rochman, Y.; Spolski, R.; Leonard, W.J. New insights into the regulation of T cells by gamma(c) family cytokines. Nat. Rev. Immunol. 2009, 9, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Mattei, F.; Schiavoni, G.; Belardelli, F.; Tough, D.F. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J. Immunol. 2001, 167, 1179–1187. [Google Scholar] [CrossRef]
- Catalfamo, M.; Wilhelm, C.; Tcheung, L.; Proschan, M.; Friesen, T.; Park, J.H.; Adelsberger, J.; Baseler, M.; Maldarelli, F.; Davey, R.; et al. CD4 and CD8 T cell immune activation during chronic HIV infection: Roles of homeostasis, HIV, type I IFN, and IL-7. J. Immunol. 2011, 186, 2106–2116. [Google Scholar] [CrossRef]
- Dubois, S.; Mariner, J.; Waldmann, T.A.; Tagaya, Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity 2002, 17, 537–547. [Google Scholar] [CrossRef]
- Stonier, S.W.; Schluns, K.S. Trans-presentation: A novel mechanism regulating IL-15 delivery and responses. Immunol. Lett. 2010, 127, 85–92. [Google Scholar] [CrossRef]
- Lodolce, J.P.; Burkett, P.R.; Koka, R.M.; Boone, D.L.; Ma, A. Regulation of lymphoid homeostasis by interleukin-15. Cytokine Growth Factor. Rev. 2002, 13, 429–439. [Google Scholar] [CrossRef]
- Delconte, R.B.; Kolesnik, T.B.; Dagley, L.F.; Rautela, J.; Shi, W.; Putz, E.M.; Stannard, K.; Zhang, J.G.; Teh, C.; Firth, M.; et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat. Immunol. 2016, 17, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yun, S.; Piao, Z.H.; Jeong, M.; Kim, D.O.; Jung, H.; Lee, J.; Kim, M.J.; Kim, M.S.; Chung, J.W.; et al. Suppressor of cytokine signaling 2 regulates IL-15-primed human NK cell function via control of phosphorylated Pyk2. J. Immunol. 2010, 185, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A. The CIS family: Negative regulators of JAK-STAT signaling. Cytokine Growth Factor Rev. 1998, 9, 197–204. [Google Scholar] [CrossRef]
- Kim, W.S.; Kim, M.J.; Kim, D.O.; Byun, J.E.; Huy, H.; Song, H.Y.; Park, Y.J.; Kim, T.D.; Yoon, S.R.; Choi, E.J.; et al. Suppressor of Cytokine Signaling 2 Negatively Regulates NK Cell Differentiation by Inhibiting JAK2 Activity. Sci. Rep. 2017, 7, 46153. [Google Scholar] [CrossRef]
- Babon, J.J.; Kershaw, N.J.; Murphy, J.M.; Varghese, L.N.; Laktyushin, A.; Young, S.N.; Lucet, I.S.; Norton, R.S.; Nicola, N.A. Suppression of cytokine signaling by SOCS3: Characterization of the mode of inhibition and the basis of its specificity. Immunity 2012, 36, 239–250. [Google Scholar] [CrossRef]
- Schmidt, D.; Muller, S. PIAS/SUMO: New partners in transcriptional regulation. Cell Mol. Life Sci. 2003, 60, 2561–2574. [Google Scholar] [CrossRef]
- Shuai, K. Regulation of cytokine signaling pathways by PIAS proteins. Cell Res. 2006, 16, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Rycyzyn, M.A.; Clevenger, C.V. The intranuclear prolactin/cyclophilin B complex as a transcriptional inducer. Proc. Natl. Acad. Sci. USA 2002, 99, 6790–6795. [Google Scholar] [CrossRef]
- Tonks, N.K. Protein tyrosine phosphatases: From genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006, 7, 833–846. [Google Scholar] [CrossRef]
- Gurzov, E.N.; Stanley, W.J.; Brodnicki, T.C.; Thomas, H.E. Protein tyrosine phosphatases: Molecular switches in metabolism and diabetes. Trends Endocrinol. Metab. 2015, 26, 30–39. [Google Scholar] [CrossRef]
- Alonso, A.; Sasin, J.; Bottini, N.; Friedberg, I.; Friedberg, I.; Osterman, A.; Godzik, A.; Hunter, T.; Dixon, J.; Mustelin, T. Protein tyrosine phosphatases in the human genome. Cell 2004, 117, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.N.; Jansen, P.G.; Echwald, S.M.; Mortensen, O.H.; Fukada, T.; Del Vecchio, R.; Tonks, N.K.; Moller, N.P. A genomic perspective on protein tyrosine phosphatases: Gene structure, pseudogenes, and genetic disease linkage. FASEB J. 2004, 18, 8–30. [Google Scholar] [CrossRef] [PubMed]
- Streuli, M.; Krueger, N.X.; Thai, T.; Tang, M.; Saito, H. Distinct functional roles of the two intracellular phosphatase like domains of the receptor-linked protein tyrosine phosphatases LCA and LAR. EMBO J. 1990, 9, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Felberg, J.; Johnson, P. Characterization of recombinant CD45 cytoplasmic domain proteins. Evidence for intramolecular and intermolecular interactions. J. Biol. Chem. 1998, 273, 17839–17845. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.E.; Cooke, F.T.; Parker, P.J. Sac phosphatase domain proteins. Biochem. J. 2000, 350 Pt 2, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Jin, Y.J.; Burakoff, S.J. Cytosolic tyrosine dephosphorylation of STAT5. Potential role of SHP-2 in STAT5 regulation. J. Biol. Chem. 2000, 275, 599–604. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, R.; Yang, S.; Schuman, J.; Zhang, E.E.; Yi, T.; Feng, G.S.; Wang, D. Identification of Shp-2 as a Stat5A phosphatase. J. Biol. Chem. 2003, 278, 16520–16527. [Google Scholar] [CrossRef]
- Chen, J.; Yu, W.M.; Bunting, K.D.; Qu, C.K. A negative role of SHP-2 tyrosine phosphatase in growth factor-dependent hematopoietic cell survival. Oncogene 2004, 23, 3659–3669. [Google Scholar] [CrossRef][Green Version]
- Myers, M.P.; Andersen, J.N.; Cheng, A.; Tremblay, M.L.; Horvath, C.M.; Parisien, J.P.; Salmeen, A.; Barford, D.; Tonks, N.K. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 2001, 276, 47771–47774. [Google Scholar] [CrossRef]
- Zabolotny, J.M.; Bence-Hanulec, K.K.; Stricker-Krongrad, A.; Haj, F.; Wang, Y.; Minokoshi, Y.; Kim, Y.B.; Elmquist, J.K.; Tartaglia, L.A.; Kahn, B.B.; et al. PTP1B regulates leptin signal transduction in vivo. Dev. Cell 2002, 2, 489–495. [Google Scholar] [CrossRef]
- Cheng, A.; Uetani, N.; Simoncic, P.D.; Chaubey, V.P.; Lee-Loy, A.; McGlade, C.J.; Kennedy, B.P.; Tremblay, M.L. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev. Cell 2002, 2, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Dubé, N.; Kim, J.W.; Cheng, A.; Ibarra-Sanchez Mde, J.; Tremblay, M.L.; Boisclair, Y.R. Protein tyrosine phosphatase 1B attenuates growth hormone-mediated JAK2-STAT signaling. Mol. Cell Biol. 2003, 23, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Simoncic, P.D.; Lee-Loy, A.; Barber, D.L.; Tremblay, M.L.; McGlade, C.J. The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr. Biol. 2002, 12, 446–453. [Google Scholar] [CrossRef]
- Xu, D.; Qu, C.K. Protein tyrosine phosphatases in the JAK/STAT pathway. Front. Biosci. 2008, 13, 4925–4932. [Google Scholar] [CrossRef] [PubMed]
- Gurzov, E.N.; Tran, M.; Fernandez-Rojo, M.A.; Merry, T.L.; Zhang, X.; Xu, Y.; Fukushima, A.; Waters, M.J.; Watt, M.J.; Andrikopoulos, S.; et al. Hepatic oxidative stress promotes insulin-STAT-5 signaling and obesity by inactivating protein tyrosine phosphatase N2. Cell Metab. 2014, 20, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Younes, S.-A.; Freeman, M.L.; Mudd, J.C.; Shive, C.L.; Reynaldi, A.; Panigrahi, S.; Estes, J.D.; Deleage, C.; Lucero, C.; Anderson, J.; et al. IL-15 promotes activation and expansion of CD8+ T cells in HIV-1 infection. J. Clin. Investig. 2016, 126, 2745–2756. [Google Scholar] [CrossRef]
- Ahmad, R.; Sindhu, S.T.; Toma, E.; Morisset, R.; Ahmad, A. Studies on the production of IL-15 in HIV-infected/AIDS patients. J. Clin. Immunol. 2003, 23, 81–90. [Google Scholar] [CrossRef]
- d’Ettorre, G.; Forcina, G.; Lichtner, M.; Mengoni, F.; D’Agostino, C.; Massetti, A.P.; Mastroianni, C.M.; Vullo, V. Interleukin-15 in HIV infection: Immunological and virological interactions in antiretroviral-naive and -treated patients. AIDS 2002, 16, 181–188. [Google Scholar] [CrossRef]
- Kacani, L.; Stoiber, H.; Dierich, M.P. Role of IL-15 in HIV-1-associated hypergammaglobulinaemia. Clin. Exp. Immunol. 1997, 108, 14–18. [Google Scholar] [CrossRef]
- Stacey, A.R.; Norris, P.J.; Qin, L.; Haygreen, E.A.; Taylor, E.; Heitman, J.; Lebedeva, M.; DeCamp, A.; Li, D.; Grove, D.; et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol. 2009, 83, 3719–3733. [Google Scholar] [CrossRef]
- Swaminathan, S.; Qiu, J.; Rupert, A.W.; Hu, Z.; Higgins, J.; Dewar, R.L.; Stevens, R.; Rehm, C.A.; Metcalf, J.A.; Sherman, B.T.; et al. Interleukin-15 (IL-15) Strongly Correlates with Increasing HIV-1 Viremia and Markers of Inflammation. PLoS ONE 2016, 11, e0167091. [Google Scholar] [CrossRef]
- Roberts, L.; Passmore, J.A.; Williamson, C.; Little, F.; Bebell, L.M.; Mlisana, K.; Burgers, W.A.; van Loggerenberg, F.; Walzl, G.; Djoba Siawaya, J.F.; et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS 2010, 24, 819–831. [Google Scholar] [CrossRef]
- Tarkowski, M.; Ferraris, L.; Martone, S.; Strambio de Castillia, F.; Misciagna, D.; Mazzucchelli, R.I.; Lattuada, E.; Paraninfo, G.; Galli, M.; Riva, A.; et al. Expression of interleukin-15 and interleukin-15Ralpha in monocytes of HIV type 1-infected patients with different courses of disease progression. AIDS Res. Hum. Retroviruses 2012, 28, 693–701. [Google Scholar] [CrossRef]
- Maksoud, S.; El Hokayem, J. The cytokine/chemokine response in Leishmania/HIV infection and co-infection. Heliyon 2023, 9, e15055. [Google Scholar] [CrossRef]
- Ranson, T.; Vosshenrich, C.A.; Corcuff, E.; Richard, O.; Muller, W.; Di Santo, J.P. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood 2003, 101, 4887–4893. [Google Scholar] [CrossRef]
- Alves, N.L.; Hooibrink, B.; Arosa, F.A.; van Lier, R.A. IL-15 induces antigen-independent expansion and differentiation of human naive CD8+ T cells in vitro. Blood 2003, 102, 2541–2546. [Google Scholar] [CrossRef]
- Berard, M.; Brandt, K.; Bulfone-Paus, S.; Tough, D.F. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J. Immunol. 2003, 170, 5018–5026. [Google Scholar] [CrossRef] [PubMed]
- Carson, W.E.; Giri, J.G.; Lindemann, M.J.; Linett, M.L.; Ahdieh, M.; Paxton, R.; Anderson, D.; Eisenmann, J.; Grabstein, K.; Caligiuri, M.A. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 1994, 180, 1395–1403. [Google Scholar] [CrossRef]
- Ye, W.; Young, J.D.; Liu, C.C. Interleukin-15 induces the expression of mRNAs of cytolytic mediators and augments cytotoxic activities in primary murine lymphocytes. Cell Immunol. 1996, 174, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; van Hoef, V.; Zhang, X.; Wennerberg, E.; Lorent, J.; Witt, K.; Masvidal, L.; Liang, S.; Murray, S.; Larsson, O.; et al. IL-15 activates mTOR and primes stress-activated gene expression leading to prolonged antitumor capacity of NK cells. Blood 2016, 128, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Marcus, W.D.; Xu, W.; Lee, H.I.; Han, K.; Egan, J.O.; Yovandich, J.L.; Rhode, P.R.; Wong, H.C. Novel human interleukin-15 agonists. J. Immunol. 2009, 183, 3598–3607. [Google Scholar] [CrossRef] [PubMed]
- Han, K.P.; Zhu, X.; Liu, B.; Jeng, E.; Kong, L.; Yovandich, J.L.; Vyas, V.V.; Marcus, W.D.; Chavaillaz, P.A.; Romero, C.A.; et al. IL-15:IL-15 receptor alpha superagonist complex: High-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine 2011, 56, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jones, M.; Liu, B.; Zhu, X.; Johnson, C.B.; Edwards, A.C.; Kong, L.; Jeng, E.K.; Han, K.; Marcus, W.D.; et al. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: Interleukin-15 receptor alphaSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer Res. 2013, 73, 3075–3086. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Giacoia, E.; Miyake, M.; Goodison, S.; Sriharan, A.; Zhang, G.; You, L.; Egan, J.O.; Rhode, P.R.; Parker, A.S.; Chai, K.X.; et al. Intravesical ALT-803 and BCG treatment reduces tumor burden in a carcinogen induced bladder cancer rat model; a role for cytokine production and NK cell expansion. PLoS ONE 2014, 9, e96705. [Google Scholar] [CrossRef]
- Mathios, D.; Park, C.K.; Marcus, W.D.; Alter, S.; Rhode, P.R.; Jeng, E.K.; Wong, H.C.; Pardoll, D.M.; Lim, M. Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model. Int. J. Cancer 2016, 138, 187–194. [Google Scholar] [CrossRef]
- Rhode, P.R.; Egan, J.O.; Xu, W.; Hong, H.; Webb, G.M.; Chen, X.; Liu, B.; Zhu, X.; Wen, J.; You, L.; et al. Comparison of the Superagonist Complex, ALT-803, to IL15 as Cancer Immunotherapeutics in Animal Models. Cancer Immunol. Res. 2016, 4, 49–60. [Google Scholar] [CrossRef]
- Chehimi, J.; Marshall, J.D.; Salvucci, O.; Frank, I.; Chehimi, S.; Kawecki, S.; Bacheller, D.; Rifat, S.; Chouaib, S. IL-15 enhances immune functions during HIV infection. J. Immunol. 1997, 158, 5978–5987. [Google Scholar] [CrossRef]
- Jones, R.B.; Mueller, S.; O’Connor, R.; Rimpel, K.; Sloan, D.D.; Karel, D.; Wong, H.C.; Jeng, E.K.; Thomas, A.S.; Whitney, J.B.; et al. A Subset of Latency-Reversing Agents Expose HIV-Infected Resting CD4+ T-Cells to Recognition by Cytotoxic T-Lymphocytes. PLoS Pathog. 2016, 12, e1005545. [Google Scholar] [CrossRef]
- Covino, D.A.; Desimio, M.G.; Doria, M. Impact of IL-15 and latency reversing agent combinations in the reactivation and NK cell-mediated suppression of the HIV reservoir. Sci. Rep. 2022, 12, 18567. [Google Scholar] [CrossRef]
- Kanai, T.; Thomas, E.K.; Yasutomi, Y.; Letvin, N.L. IL-15 stimulates the expansion of AIDS virus-specific CTL. J. Immunol. 1996, 157, 3681–3687. [Google Scholar] [CrossRef]
- Mueller, Y.M.; Bojczuk, P.M.; Halstead, E.S.; Kim, A.H.; Witek, J.; Altman, J.D.; Katsikis, P.D. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood 2003, 101, 1024–1029. [Google Scholar] [CrossRef]
- McCann, C.D.; van Dorp, C.H.; Danesh, A.; Ward, A.R.; Dilling, T.R.; Mota, T.M.; Zale, E.; Stevenson, E.M.; Patel, S.; Brumme, C.J.; et al. A participant-derived xenograft model of HIV enables long-term evaluation of autologous immunotherapies. J. Exp. Med. 2021, 218, e20201908. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; O’Connor, R.; Mueller, S.; Foley, M.; Szeto, G.L.; Karel, D.; Lichterfeld, M.; Kovacs, C.; Ostrowski, M.A.; Trocha, A.; et al. Histone deacetylase inhibitors impair the elimination of HIV-infected cells by cytotoxic T-lymphocytes. PLoS Pathog. 2014, 10, e1004287. [Google Scholar] [CrossRef]
- Seay, K.; Church, C.; Zheng, J.H.; Deneroff, K.; Ochsenbauer, C.; Kappes, J.C.; Liu, B.; Jeng, E.K.; Wong, H.C.; Goldstein, H. In Vivo Activation of Human NK Cells by Treatment with an Interleukin-15 Superagonist Potently Inhibits Acute In Vivo HIV-1 Infection in Humanized Mice. J. Virol. 2015, 89, 6264–6274. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Abad-Fernandez, M.; Tuyishime, M.; Pollara, J.J.; Ferrari, G.; Soriano-Sarabia, N.; Margolis, D.M. Interleukin-15-Stimulated Natural Killer Cells Clear HIV-1-Infected Cells following Latency Reversal Ex Vivo. J. Virol. 2018, 92, 12. [Google Scholar] [CrossRef]
- Fisher, L.; Zinter, M.; Stanfield-Oakley, S.; Carpp, L.N.; Edwards, R.W.; Denny, T.; Moodie, Z.; Laher, F.; Bekker, L.G.; McElrath, M.J.; et al. Vaccine-Induced Antibodies Mediate Higher Antibody-Dependent Cellular Cytotoxicity after Interleukin-15 Pretreatment of Natural Killer Effector Cells. Front. Immunol. 2019, 10, 2741. [Google Scholar] [CrossRef] [PubMed]
- Loubeau, M.; Ahmad, A.; Toma, E.; Menezes, J. Enhancement of natural killer and antibody-dependent cytolytic activities of the peripheral blood mononuclear cells of HIV-infected patients by recombinant IL-15. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol 1997, 16, 137–145. [Google Scholar] [CrossRef]
- Macedo, A.B.; Levinger, C.; Nguyen, B.N.; Richard, J.; Gupta, M.; Cruz, C.R.Y.; Finzi, A.; Chiappinelli, K.B.; Crandall, K.A.; Bosque, A. The HIV Latency Reversal Agent HODHBt Enhances NK Cell Effector and Memory-like Functions by Increasing Interleukin-15-Mediated STAT Activation. J. Virol. 2022, 96, e0037222. [Google Scholar] [CrossRef]
- d’Ettorre, G.; Andreotti, M.; Carnevalini, M.; Andreoni, C.; Zaffiri, L.; Vullo, V.; Vella, S.; Mastroianni, C.M. Interleukin-15 enhances the secretion of IFN-gamma and CC chemokines by natural killer cells from HIV viremic and aviremic patients. Immunol. Lett. 2006, 103, 192–195. [Google Scholar] [CrossRef]
- Lin, S.J.; Roberts, R.L.; Ank, B.J.; Nguyen, Q.H.; Thomas, E.K.; Stiehm, E.R. Human immunodeficiency virus (HIV) type-1 GP120-specific cell-mediated cytotoxicity (CMC) and natural killer (NK) activity in HIV-infected (HIV+) subjects: Enhancement with interleukin-2(IL-2), IL-12, and IL-15. Clin. Immunol. Immunopathol. 1997, 82, 163–173. [Google Scholar] [CrossRef]
- Mueller, Y.M.; Petrovas, C.; Bojczuk, P.M.; Dimitriou, I.D.; Beer, B.; Silvera, P.; Villinger, F.; Cairns, J.S.; Gracely, E.J.; Lewis, M.G.; et al. Interleukin-15 increases effector memory CD8+ T cells and NK Cells in simian immunodeficiency virus-infected macaques. J. Virol. 2005, 79, 4877–4885. [Google Scholar] [CrossRef]
- Mueller, Y.M.; Do, D.H.; Altork, S.R.; Artlett, C.M.; Gracely, E.J.; Katsetos, C.D.; Legido, A.; Villinger, F.; Altman, J.D.; Brown, C.R.; et al. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J. Immunol. 2008, 180, 350–360. [Google Scholar] [CrossRef]
- Lugli, E.; Mueller, Y.M.; Lewis, M.G.; Villinger, F.; Katsikis, P.D.; Roederer, M. IL-15 delays suppression and fails to promote immune reconstitution in virally suppressed chronically SIV-infected macaques. Blood 2011, 118, 2520–2529. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.C.; Moysi, E.; Valentin, A.; Bergamaschi, C.; Devasundaram, S.; Fortis, S.P.; Bear, J.; Chertova, E.; Bess, J., Jr.; Sowder, R.; et al. Treatment with native heterodimeric IL-15 increases cytotoxic lymphocytes and reduces SHIV RNA in lymph nodes. PLoS Pathog. 2018, 14, e1006902. [Google Scholar]
- Ellis-Connell, A.L.; Balgeman, A.J.; Zarbock, K.R.; Barry, G.; Weiler, A.; Egan, J.O.; Jeng, E.K.; Friedrich, T.; Miller, J.S.; Haase, A.T.; et al. ALT-803 Transiently Reduces Simian Immunodeficiency Virus Replication in the Absence of Antiretroviral Treatment. J. Virol. 2018, 92, 3. [Google Scholar] [CrossRef] [PubMed]
- Webb, G.M.; Li, S.; Mwakalundwa, G.; Folkvord, J.M.; Greene, J.M.; Reed, J.S.; Stanton, J.J.; Legasse, A.W.; Hobbs, T.; Martin, L.D.; et al. The human IL-15 superagonist ALT-803 directs SIV-specific CD8+ T cells into B-cell follicles. Blood Adv. 2018, 2, 76–84. [Google Scholar] [CrossRef]
- Webb, G.M.; Molden, J.; Busman-Sahay, K.; Abdulhaqq, S.; Wu, H.L.; Weber, W.C.; Bateman, K.B.; Reed, J.S.; Northrup, M.; Maier, N.; et al. The human IL-15 superagonist N-803 promotes migration of virus-specific CD8+ T and NK cells to B cell follicles but does not reverse latency in ART-suppressed, SHIV-infected macaques. PLoS Pathog. 2020, 16, e1008339. [Google Scholar] [CrossRef] [PubMed]
- McBrien, J.B.; Mavigner, M.; Franchitti, L.; Smith, S.A.; White, E.; Tharp, G.K.; Walum, H.; Busman-Sahay, K.; Aguilera-Sandoval, C.R.; Thayer, W.O.; et al. Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8+ cells. Nature 2020, 578, 154–159. [Google Scholar] [CrossRef]
- McBrien, J.B.; Wong, A.K.H.; White, E.; Carnathan, D.G.; Lee, J.H.; Safrit, J.T.; Vanderford, T.H.; Paiardini, M.; Chahroudi, A.; Silvestri, G. Combination of CD8beta Depletion and Interleukin-15 Superagonist N-803 Induces Virus Reactivation in Simian-Human Immunodeficiency Virus-Infected, Long-Term ART-Treated Rhesus Macaques. J. Virol. 2020, 94, 19. [Google Scholar] [CrossRef]
- Romee, R.; Cooley, S.; Berrien-Elliott, M.M.; Westervelt, P.; Verneris, M.R.; Wagner, J.E.; Weisdorf, D.J.; Blazar, B.R.; Ustun, C.; DeFor, T.E.; et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 2018, 131, 2515–2527. [Google Scholar] [CrossRef]
- Das, B.; Dobrowolski, C.; Luttge, B.; Valadkhan, S.; Chomont, N.; Johnston, R.; Bacchetti, P.; Hoh, R.; Gandhi, M.; Deeks, S.G.; et al. Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc. Natl. Acad. Sci. USA 2018, 115, E7795–E7804. [Google Scholar] [PubMed]
- Miller, J.S.; Davis, Z.B.; Helgeson, E.; Reilly, C.; Thorkelson, A.; Anderson, J.; Lima, N.S.; Jorstad, S.; Hart, G.T.; Lee, J.H.; et al. Safety and virologic impact of the IL-15 superagonist N-803 in people living with HIV: A phase 1 trial. Nat. Med. 2022, 28, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Scholz, E.M.B.; Kashuba, A.D.M. The Lymph Node Reservoir: Physiology, HIV Infection, and Antiretroviral Therapy. Clin. Pharmacol. Ther. 2021, 109, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Bosque, A.; Nilson, K.A.; Macedo, A.B.; Spivak, A.M.; Archin, N.M.; Van Wagoner, R.M.; Martins, L.J.; Novis, C.L.; Szaniawski, M.A.; Ireland, C.M.; et al. Benzotriazoles Reactivate Latent HIV-1 through Inactivation of STAT5 SUMOylation. Cell Rep. 2017, 18, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, E.S.; Macedo, A.B.; Resop, R.S.; Howard, J.N.; Nell, R.; Sarabia, I.; Newman, D.; Ren, Y.; Jones, R.B.; Planelles, V.; et al. Structure-Activity Relationship Analysis of Benzotriazine Analogues as HIV-1 Latency-Reversing Agents. Antimicrob. Agents Chemother. 2020, 64, 8. [Google Scholar] [CrossRef] [PubMed]
- Copertino, D.C., Jr.; Holmberg, C.S.; Weiler, J.; Ward, A.R.; Howard, J.N.; Levinger, C.; Pang, A.P.; Corley, M.J.; Dundar, F.; Zumbo, P.; et al. The latency reversing agent HODHBt synergizes with IL-15 to enhance cytotoxic function of HIV-specific CD8+ T-cells. JCI Insight 2023, in press. [Google Scholar] [CrossRef]
- Howard, J.N.; Zaikos, T.D.; Levinger, C.; Rivera, E.; McMahon, E.K.; Sanz, M.; Copertino, D.C.; Wang, W.; Soriano-Sarabia, N.; Jones, R.B.; et al. Characterization of novel dual PTPN1 and PTPN2 inhibitors. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Liang, S.; Tran, E.; Du, X.; Dong, J.; Sudholz, H.; Chen, H.; Qu, Z.; Huntington, N.D.; Babon, J.J.; Kershaw, N.J.; et al. A small molecule inhibitor of PTP1B and PTPN2 enhances T cell anti-tumor immunity. Nat. Commun. 2023, 14, 4524. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howard, J.N.; Bosque, A. IL-15 and N-803 for HIV Cure Approaches. Viruses 2023, 15, 1912. https://doi.org/10.3390/v15091912
Howard JN, Bosque A. IL-15 and N-803 for HIV Cure Approaches. Viruses. 2023; 15(9):1912. https://doi.org/10.3390/v15091912
Chicago/Turabian StyleHoward, J. Natalie, and Alberto Bosque. 2023. "IL-15 and N-803 for HIV Cure Approaches" Viruses 15, no. 9: 1912. https://doi.org/10.3390/v15091912
APA StyleHoward, J. N., & Bosque, A. (2023). IL-15 and N-803 for HIV Cure Approaches. Viruses, 15(9), 1912. https://doi.org/10.3390/v15091912




