Implication of the Autophagy-Related Protein Beclin1 in the Regulation of EcoHIV Replication and Inflammatory Responses
Abstract
:1. Introduction
2. Materials and Methods
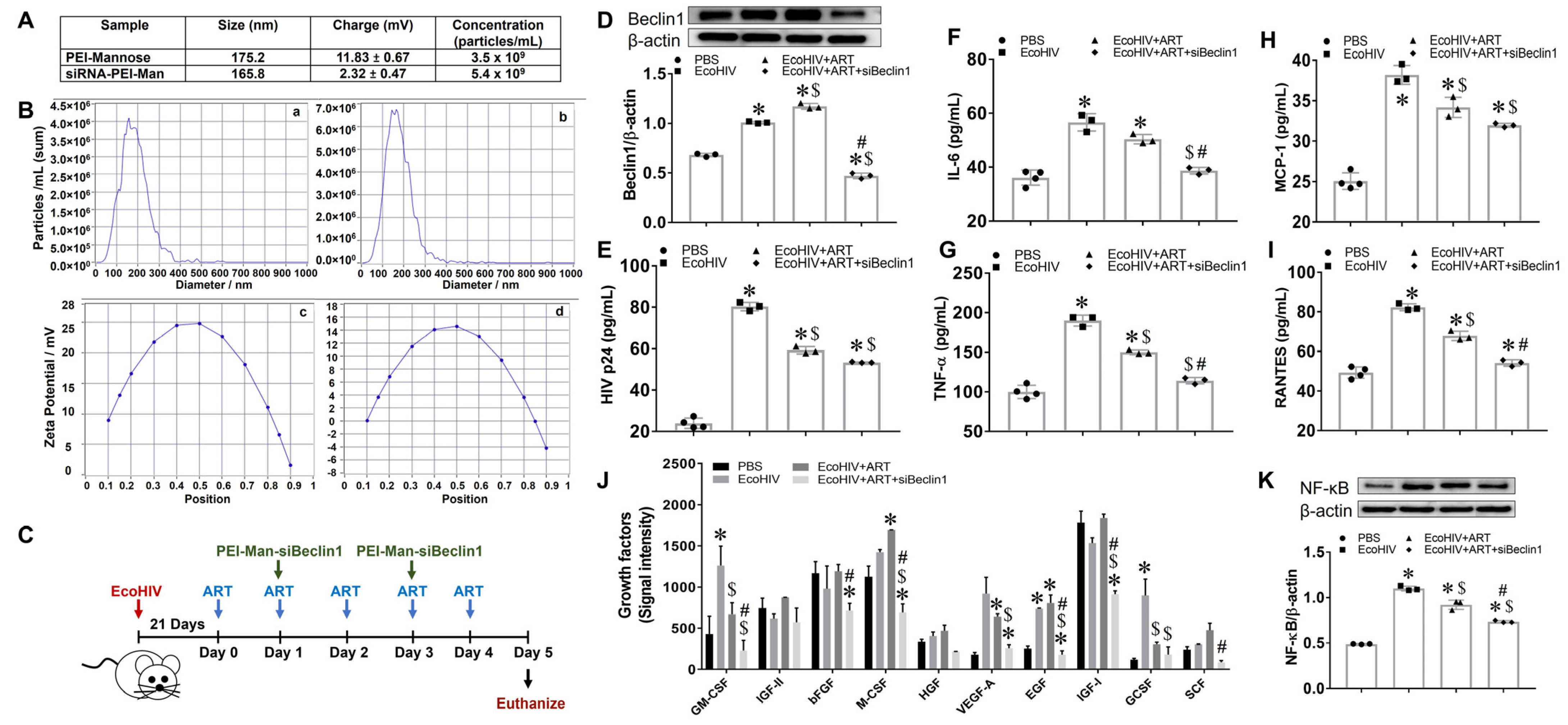
2.1. Characterization of siBeclin1-PEI Nanoplex
2.2. Animals
2.3. Viral Infection
2.4. Intranasal Administration of siBeclin1-PEI-Man Nanoplex into EcoHIV-Infected C57BL/6 Mice
2.5. ELISA
2.6. Growth Factor Antibody Array
2.7. Hematoxylin and Eosin (H&E) Staining
2.8. Nissl Staining
2.9. Behavioral Tests
2.10. Statistical Analysis
3. Results
3.1. Transient Inhibition of Beclin1 Reduces Viral Production and Attenuates Secreted Viral-Induced Inflammatory Molecules in EcoHIV-Infected Mice Co-Administered with Antiretroviral Drugs
3.2. Minimal Anti-Inflammatory Action in Peripheral Organs after Transient Inhibition of Beclin1
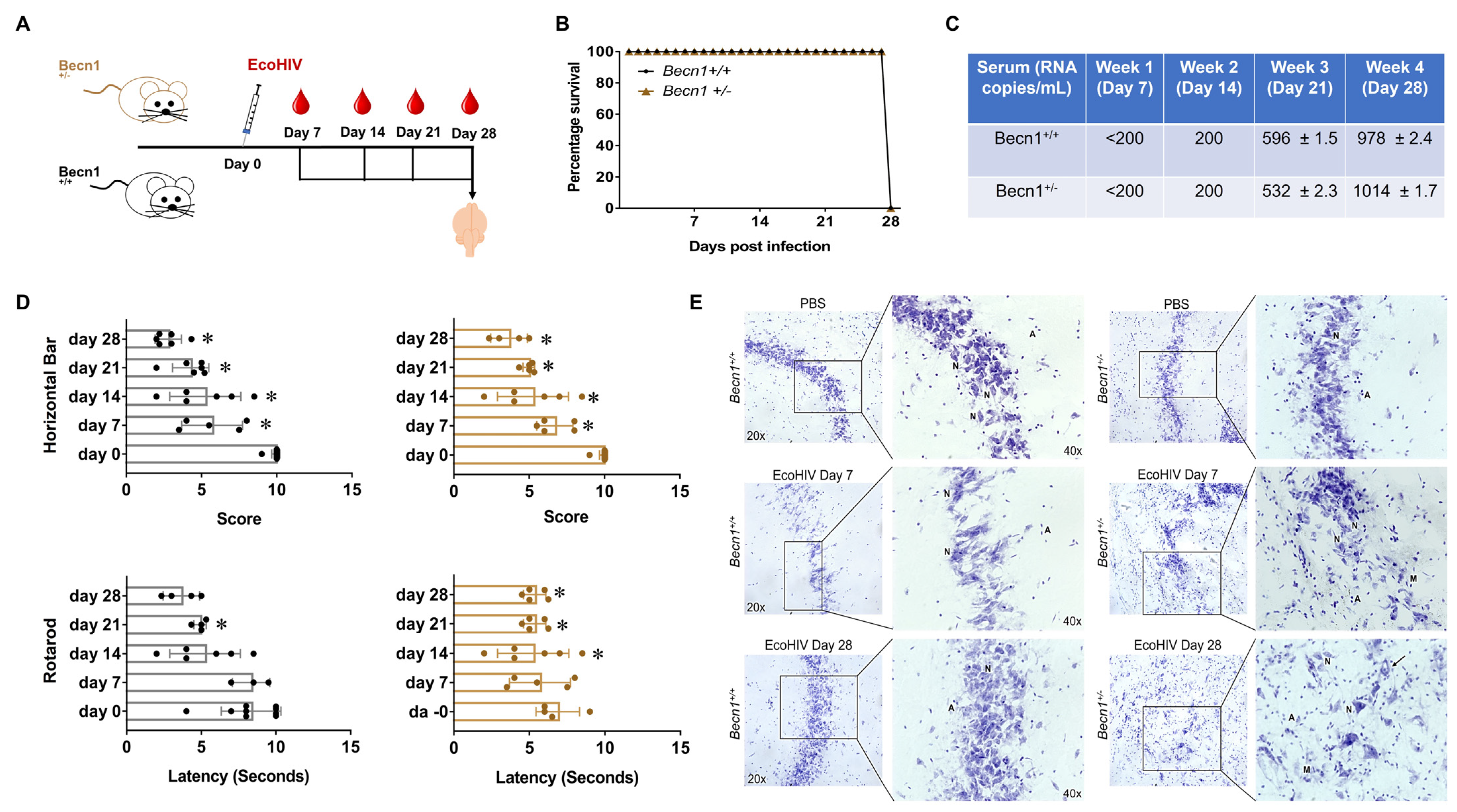
3.3. EcoHIV-Infected Becn1+/− Transgenic Mice Confirmed the Role of Beclin1 in the Regulation of HIV-Induced Cytokine Secretion but Not in HIV-Induced Locomotor Impairments
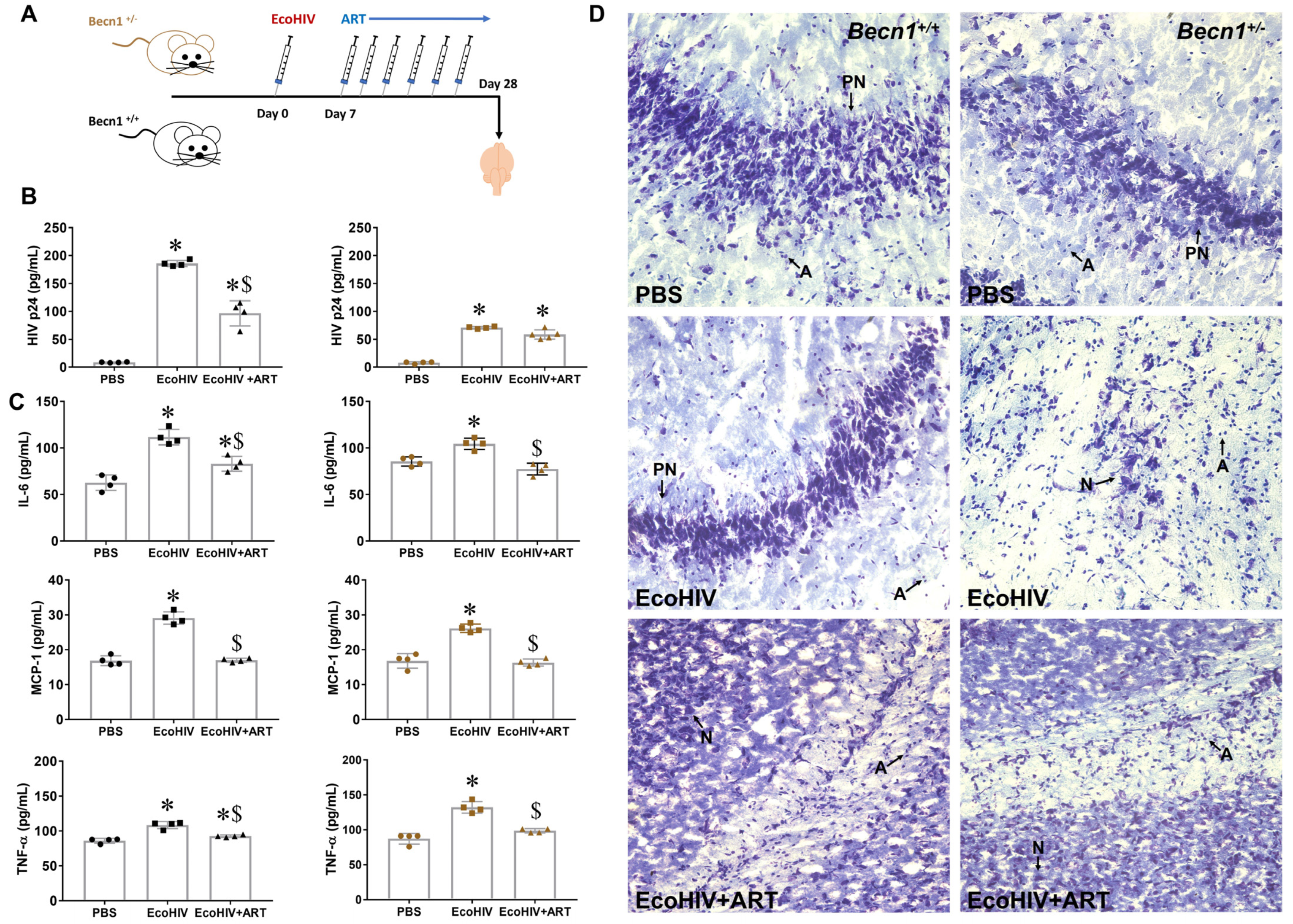
3.4. Effect of Combined ART on Viral Load in Brain Recovered from Becn1+/− versus C57BL/6J (Becn1+/+) Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R., Jr.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef]
- Simioni, S.; Cavassini, M.; Annoni, J.M.; Rimbault Abraham, A.; Bourquin, I.; Schiffer, V.; Calmy, A.; Chave, J.P.; Giacobini, E.; Hirschel, B.; et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010, 24, 1243–1250. [Google Scholar] [CrossRef]
- Cysique, L.A.; Maruff, P.; Brew, B.J. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: A combined study of two cohorts. J. Neurovirol. 2004, 10, 350–357. [Google Scholar] [CrossRef]
- Harezlak, J.; Buchthal, S.; Taylor, M.; Schifitto, G.; Zhong, J.; Daar, E.; Alger, J.; Singer, E.; Campbell, T.; Yiannoutsos, C.; et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 2011, 25, 625–633. [Google Scholar] [CrossRef]
- Molsberry, S.A.; Cheng, Y.; Kingsley, L.; Jacobson, L.; Levine, A.J.; Martin, E.; Miller, E.N.; Munro, C.A.; Ragin, A.; Sacktor, N.; et al. Neuropsychological phenotypes among men with and without HIV disease in the multicenter AIDS cohort study. AIDS 2018, 32, 1679–1688. [Google Scholar] [CrossRef]
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef]
- Hong, S.; Banks, W.A. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav. Immun. 2015, 45, 1–12. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Baehrecke, E.H.; Brumell, J.H.; Chu, C.T.; Codogno, P.; Cuervo, A.M.; Debnath, J.; Deretic, V.; Elazar, Z.; Eskelinen, E.-L.; et al. A comprehensive glossary of autophagy-related molecules and processes (2nd edition). Autophagy 2011, 7, 1273–1294. [Google Scholar] [CrossRef]
- Lapierre, J.; Rodriguez, M.; Ojha, C.R.; El-Hage, N. Critical Role of Beclin1 in HIV Tat and Morphine-Induced Inflammation and Calcium Release in Glial Cells from Autophagy Deficient Mouse. J. Neuroimmune Pharmacol. 2018, 13, 355–370. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Eskelinen, E.-L.; Saftig, P. Autophagy: A lysosomal degradation pathway with a central role in health and disease. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2009, 1793, 664–673. [Google Scholar] [CrossRef]
- Rodriguez, M.; Lapierre, J.; Ojha, C.R.; Estrada-Bueno, H.; Dever, S.M.; Gewirtz, D.A.; Kashanchi, F.; El-Hage, N. Importance of Autophagy in Mediating Human Immunodeficiency Virus (HIV) and Morphine-Induced Metabolic Dysfunction and Inflammation in Human Astrocytes. Viruses 2017, 9, 201. [Google Scholar] [CrossRef]
- Rodriguez, M.; Kaushik, A.; Lapierre, J.; Dever, S.M.; El-Hage, N.; Nair, M. Electro-Magnetic Nano-Particle Bound Beclin1 siRNA Crosses the Blood-Brain Barrier to Attenuate the Inflammatory Effects of HIV-1 Infection in Vitro. J. Neuroimmune Pharmacol. 2017, 12, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Lapierre, J.; Ojha, C.R.; Kaushik, A.; Batrakova, E.; Kashanchi, F.; Dever, S.M.; Nair, M.; El-Hage, N. Intranasal drug delivery of small interfering RNA targeting Beclin1 encapsulated with polyethylenimine (PEI) in mouse brain to achieve HIV attenuation. Sci. Rep. 2017, 7, 1862. [Google Scholar] [CrossRef]
- El-Hage, N.; Rodriguez, M.; Dever, S.M.; Masvekar, R.R.; Gewirtz, D.A.; Shacka, J.J. HIV-1 and morphine regulation of autophagy in microglia: Limited interactions in the context of HIV-1 infection and opioid abuse. J. Virol. 2015, 89, 1024–1035. [Google Scholar] [CrossRef]
- Rodriguez, M.; Soler, Y.; Muthu Karuppan, M.K.; Zhao, Y.; Batrakova, E.V.; El-Hage, N. Targeting Beclin1 as an Adjunctive Therapy against HIV Using Mannosylated Polyethylenimine Nanoparticles. Pharmaceutics 2021, 13, 223. [Google Scholar] [CrossRef]
- Potash, M.J.; Chao, W.; Bentsman, G.; Paris, N.; Saini, M.; Nitkiewicz, J.; Belem, P.; Sharer, L.; Brooks, A.I.; Volsky, D.J. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc. Natl. Acad. Sci. USA 2005, 102, 3760–3765. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.-J.; Borjabad, A.; Hadas, E.; Kelschenbach, J.; Kim, B.-H.; Chao, W.; Arancio, O.; Suh, J.; Polsky, B.; McMillan, J.; et al. EcoHIV infection of mice establishes latent viral reservoirs in T cells and active viral reservoirs in macrophages that are sufficient for induction of neurocognitive impairment. PLoS Pathog. 2018, 14, e1007061. [Google Scholar] [CrossRef]
- Soler, Y.; Rodriguez, M.; Austin, D.; Gineste, C.; Gelber, C.; El-Hage, N. SERPIN-Derived Small Peptide (SP16) as a Potential Therapeutic Agent against HIV-Induced Inflammatory Molecules and Viral Replication in Cells of the Central Nervous System. Cells 2023, 12, 632. [Google Scholar] [CrossRef]
- Nath, A. Eradication of human immunodeficiency virus from brain reservoirs. J. Neurovirol. 2015, 21, 227–234. [Google Scholar] [CrossRef]
- Morgello, S. HIV neuropathology. Handb. Clin. Neurol. 2018, 152, 3–19. [Google Scholar] [CrossRef]
- Gendelman, H.E.; Lipton, S.A.; Tardieu, M.; Bukrinsky, M.I.; Nottet, H.S. The neuropathogenesis of HIV-1 infection. J. Leukoc. Biol. 1994, 56, 389–398. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Sharer, L.R.; Chao, W.; Gu, C.J.; Borjabad, A.; Hadas, E.; Kelschenbach, J.; Ichiyama, K.; Do, M.; Potash, M.J.; et al. Enhanced human immunodeficiency virus Type 1 expression and neuropathogenesis in knockout mice lacking Type I interferon responses. J. Neuropathol. Exp. Neurol. 2014, 73, 59–71. [Google Scholar] [CrossRef]
- Jones, L.D.; Jackson, J.W.; Maggirwar, S.B. Modeling HIV-1 Induced Neuroinflammation in Mice: Role of Platelets in Mediating Blood-Brain Barrier Dysfunction. PLoS ONE 2016, 11, e0151702. [Google Scholar] [CrossRef]
- Kelschenbach, J.L.; Saini, M.; Hadas, E.; Gu, C.J.; Chao, W.; Bentsman, G.; Hong, J.P.; Hanke, T.; Sharer, L.R.; Potash, M.J.; et al. Mice chronically infected with chimeric HIV resist peripheral and brain superinfection: A model of protective immunity to HIV. J. Neuroimmune Pharmacol. 2012, 7, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Jin, S.; Yang, C.; Levine, A.J.; Heintz, N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. USA 2003, 100, 15077. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Zou, Z.; Sun, Q.; Luby-Phelps, K.; Cheng, P.; Hogan, R.N.; Gilpin, C.; Levine, B. Autophagy Gene-Dependent Clearance of Apoptotic Cells during Embryonic Development. Cell 2007, 128, 931–946. [Google Scholar] [CrossRef]
- Justice, J.N.; Carter, C.S.; Beck, H.J.; Gioscia-Ryan, R.A.; McQueen, M.; Enoka, R.M.; Seals, D.R. Battery of behavioral tests in mice that models age-associated changes in human motor function. Age 2014, 36, 583–592. [Google Scholar] [CrossRef]
- Kaushik, A.; Jayant, R.D.; Nikkhah-Moshaie, R.; Bhardwaj, V.; Roy, U.; Huang, Z.; Ruiz, A.; Yndart, A.; Atluri, V.; El-Hage, N.; et al. Magnetically guided central nervous system delivery and toxicity evaluation of magneto-electric nanocarriers. Sci. Rep. 2016, 6, 25309. [Google Scholar] [CrossRef]
- Lapierre, J.; Karuppan, M.K.M.; Perry, M.; Rodriguez, M.; El-Hage, N. Different Roles of Beclin1 in the Interaction Between Glia and Neurons after Exposure to Morphine and the HIV- Trans-Activator of Transcription (Tat) Protein. J. Neuroimmune Pharmacol. 2021, 17, 470–486. [Google Scholar] [CrossRef]
- Gills, J.J.; Lopiccolo, J.; Tsurutani, J.; Shoemaker, R.H.; Best, C.J.; Abu-Asab, M.S.; Borojerdi, J.; Warfel, N.A.; Gardner, E.R.; Danish, M.; et al. Nelfinavir, A lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin. Cancer Res. 2007, 13, 5183–5194. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, N.; Gomez-Sucerquia, L.J.; Gortat, A.; Blas-Garcia, A.; Esplugues, J.V. Compromising mitochondrial function with the antiretroviral drug efavirenz induces cell survival-promoting autophagy. Hepatology 2011, 54, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M.J. Measuring motor coordination in mice. J. Vis. Exp. 2013, e2609. [Google Scholar] [CrossRef]
- Takeshita, H.; Yamamoto, K.; Nozato, S.; Inagaki, T.; Tsuchimochi, H.; Shirai, M.; Yamamoto, R.; Imaizumi, Y.; Hongyo, K.; Yokoyama, S.; et al. Modified forelimb grip strength test detects aging-associated physiological decline in skeletal muscle function in male mice. Sci. Rep. 2017, 7, 42323. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, A.; Andersson, D.C.; Waning, D.L. Assessment of muscle mass and strength in mice. Bonekey Rep. 2015, 4, 732. [Google Scholar] [CrossRef] [PubMed]
- Killian, M.S. Dual role of autophagy in HIV-1 replication and pathogenesis. AIDS Res. Ther. 2012, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Leymarie, O.; Lepont, L.; Berlioz-Torrent, C. Canonical and Non-Canonical Autophagy in HIV-1 Replication Cycle. Viruses 2017, 9, 270. [Google Scholar] [CrossRef]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- Boven, L.A.; Middel, J.; Portegies, P.; Verhoef, J.; Jansen, G.H.; Nottet, H.S.L.M. Overexpression of nerve growth factor and basic fibroblast growth factor in AIDS dementia complex. J. Neuroimmunol. 1999, 97, 154–162. [Google Scholar] [CrossRef]
- Buch, S. Growth factor signaling: Implications for disease & therapeutics. J. Neuroimmune Pharmacol. 2014, 9, 65–68. [Google Scholar] [CrossRef]
- Numakawa, T.; Kajihara, R. Neurotrophins and Other Growth Factors in the Pathogenesis of Alzheimer’s Disease. Life 2023, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Schabitz, W.R.; Kruger, C.; Pitzer, C.; Weber, D.; Laage, R.; Gassler, N.; Aronowski, J.; Mier, W.; Kirsch, F.; Dittgen, T.; et al. A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF). J. Cereb. Blood Flow Metab. 2008, 28, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Murakami, T.; Nakamura, T.; Morimoto, C.; Arai, K. Human GM-CSF induces HIV-1 LTR by multiple signalling pathways. Biochimie 2002, 84, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Kedzierska, K.; Maerz, A.; Warby, T.; Jaworowski, A.; Chan, H.; Mak, J.; Sonza, S.; Lopez, A.; Crowe, S. Granulocyte-macrophage colony-stimulating factor inhibits HIV-1 replication in monocyte-derived macrophages. AIDS 2000, 14, 1739–1748. [Google Scholar] [CrossRef]
- Clark-Lewis, I.; Lopez, A.F.; To, L.B.; Vadas, M.A.; Schrader, J.W.; Hood, L.E.; Kent, S.B. Structure-function studies of human granulocyte-macrophage colony-stimulating factor. Identification of residues required for activity. J. Immunol. 1988, 141, 881–889. [Google Scholar] [CrossRef]
- Kaplan, L.D.; Kahn, J.O.; Crowe, S.; Northfelt, D.; Neville, P.; Grossberg, H.; Abrams, D.I.; Tracey, J.; Mills, J.; Volberding, P.A. Clinical and virologic effects of recombinant human granulocyte-macrophage colony-stimulating factor in patients receiving chemotherapy for human immunodeficiency virus-associated non-Hodgkin’s lymphoma: Results of a randomized trial. J. Clin. Oncol. 1991, 9, 929–940. [Google Scholar] [CrossRef]
- Bhalla, S.; Mehan, S.; Khan, A.; Rehman, M.U. Protective role of IGF-1 and GLP-1 signaling activation in neurological dysfunctions. Neurosci. Biobehav. Rev. 2022, 142, 104896. [Google Scholar] [CrossRef]
- Germinario, R.J.; DeSantis, T.; Wainberg, M.A. Insulin-like growth factor 1 and insulin inhibit HIV type 1 replication in cultured cells. AIDS Res. Hum. Retroviruses 1995, 11, 555–561. [Google Scholar] [CrossRef]
- Perkins, N.D.; Agranoff, A.B.; Duckett, C.S.; Nabel, G.J. Transcription factor AP-2 regulates human immunodeficiency virus type 1 gene expression. J. Virol. 1994, 68, 6820–6823. [Google Scholar] [CrossRef]
- Rao, K.S.; Ghorpade, A.; Labhasetwar, V. Targeting anti-HIV drugs to the CNS. Expert Opin. Drug Deliv. 2009, 6, 771–784. [Google Scholar] [CrossRef]
- Marban, C.; Forouzanfar, F.; Ait-Ammar, A.; Fahmi, F.; El Mekdad, H.; Daouad, F.; Rohr, O.; Schwartz, C. Targeting the Brain Reservoirs: Toward an HIV Cure. Front. Immunol. 2016, 7, 397. [Google Scholar] [CrossRef] [PubMed]
- Omeragic, A.; Kayode, O.; Hoque, M.T.; Bendayan, R. Potential pharmacological approaches for the treatment of HIV-1 associated neurocognitive disorders. Fluids Barriers CNS 2020, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, P.; Hadas, E.; Kim, B.H.; Dabo, A.J.; Volsky, D.J.; Foronjy, R. HIV infection model of chronic obstructive pulmonary disease in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L500–L509. [Google Scholar] [CrossRef]
- Saylor, D.; Dickens, A.M.; Sacktor, N.; Haughey, N.; Slusher, B.; Pletnikov, M.; Mankowski, J.L.; Brown, A.; Volsky, D.J.; McArthur, J.C. HIV-associated neurocognitive disorder-pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016, 12, 234–248. [Google Scholar] [CrossRef]
- Kelschenbach, J.; He, H.; Kim, B.-H.; Borjabad, A.; Gu, C.-J.; Chao, W.; Do, M.; Sharer, L.R.; Zhang, H.; Arancio, O.; et al. Efficient Expression of HIV in Immunocompetent Mouse Brain Reveals a Novel Nonneurotoxic Viral Function in Hippocampal Synaptodendritic Injury and Memory Impairment. mBio 2019, 10, e00591-19. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, M.; Owens, F.; Perry, M.; Stone, N.; Soler, Y.; Almohtadi, R.; Zhao, Y.; Batrakova, E.V.; El-Hage, N. Implication of the Autophagy-Related Protein Beclin1 in the Regulation of EcoHIV Replication and Inflammatory Responses. Viruses 2023, 15, 1923. https://doi.org/10.3390/v15091923
Rodriguez M, Owens F, Perry M, Stone N, Soler Y, Almohtadi R, Zhao Y, Batrakova EV, El-Hage N. Implication of the Autophagy-Related Protein Beclin1 in the Regulation of EcoHIV Replication and Inflammatory Responses. Viruses. 2023; 15(9):1923. https://doi.org/10.3390/v15091923
Chicago/Turabian StyleRodriguez, Myosotys, Florida Owens, Marissa Perry, Nicole Stone, Yemmy Soler, Rianna Almohtadi, Yuling Zhao, Elena V. Batrakova, and Nazira El-Hage. 2023. "Implication of the Autophagy-Related Protein Beclin1 in the Regulation of EcoHIV Replication and Inflammatory Responses" Viruses 15, no. 9: 1923. https://doi.org/10.3390/v15091923





