Performance Analysis of Serodiagnostic Tests to Characterize the Incline and Decline of the Individual Humoral Immune Response in COVID-19 Patients: Impact on Diagnostic Management
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Assessment of Humoral Responses in Acute COVID-19 Using Different Assays
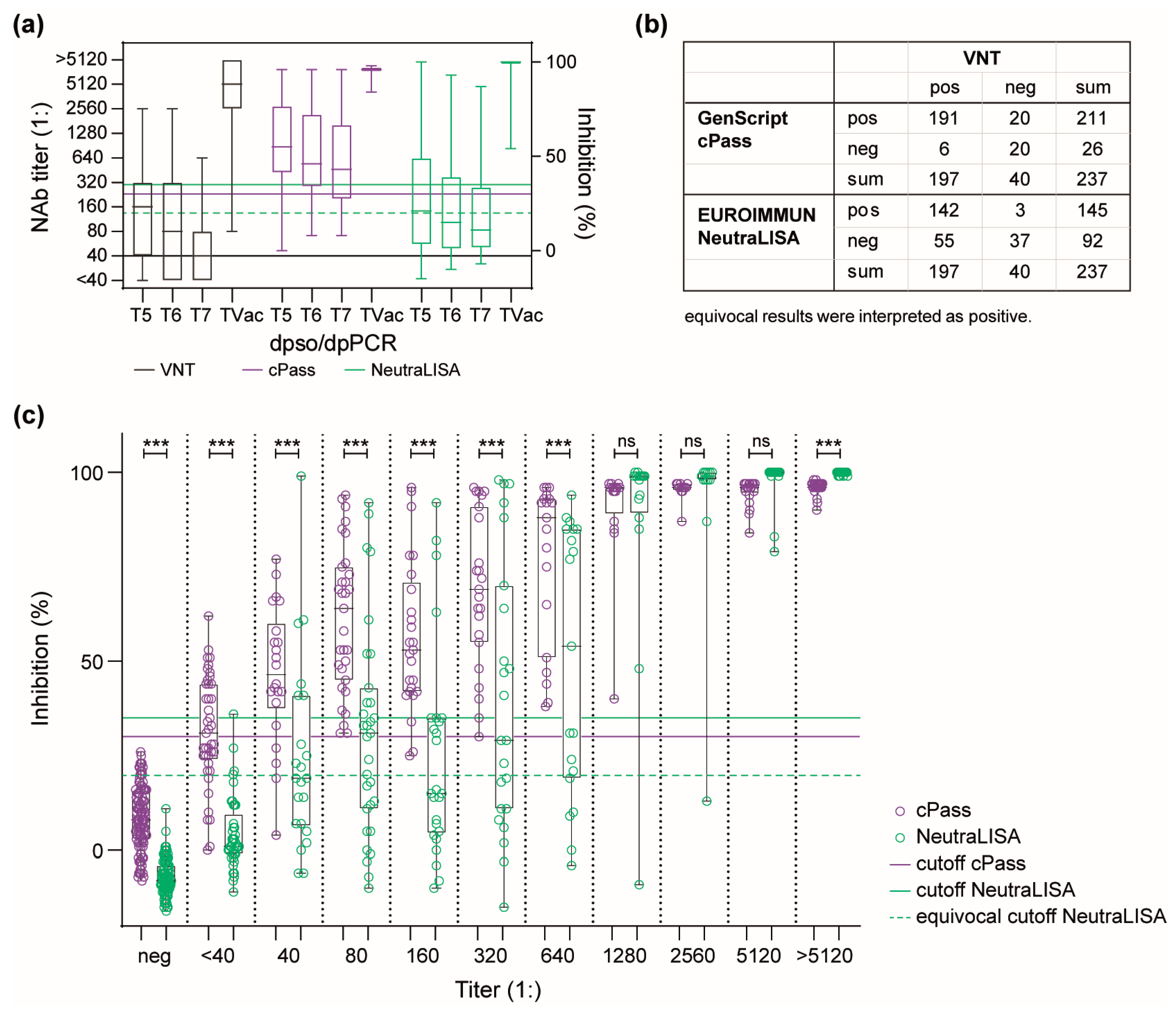
3.2. Dynamics of the Humoral Immune Response in a Convalescent COVID-19 Patient Cohort
3.3. Performance of Antibody Detection in Patient Samples after Infection with Seasonal hCoVs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Y.; Jiang, X.; Yang, L.; Chen, L.; Zeng, X.; Liu, G.; Tang, Y.; Qian, C.; Wang, X.; Cheng, F.; et al. SARS-CoV-2-specific immune response in COVID-19 convalescent individuals. Signal Transduct. Target. Ther. 2021, 6, 256. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. China Novel Coronavirus, I.; Research, T. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, S.M.; Yu, X.H.; Tang, S.L.; Tang, C.K. Coronavirus disease 2019 (COVID-19): Current status and future perspectives. Int. J. Antimicrob. Agents 2020, 55, 105951. [Google Scholar] [CrossRef] [PubMed]
- Boechat, J.L.; Chora, I.; Morais, A.; Delgado, L. The immune response to SARS-CoV-2 and COVID-19 immunopathology-Current perspectives. Pulmonology 2021, 27, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292 e6. [Google Scholar] [CrossRef]
- Lutomski, C.A.; El-Baba, T.J.; Bolla, J.R.; Robinson, C.V. Multiple Roles of SARS-CoV-2 N Protein Facilitated by Proteoform-Specific Interactions with RNA, Host Proteins, and Convalescent Antibodies. JACS Au 2021, 1, 1147–1157. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, C.; Zhu, Y.; Sun, F. Minireview of progress in the structural study of SARS-CoV-2 proteins. Curr. Res. Microb. Sci. 2020, 1, 53–61. [Google Scholar] [CrossRef]
- LeBlanc, J.J.; Gubbay, J.B.; Li, Y.; Needle, R.; Arneson, S.R.; Marcino, D.; Charest, H.; Desnoyers, G.; Dust, K.; Fattouh, R.; et al. Real-time PCR-based SARS-CoV-2 detection in Canadian laboratories. J. Clin. Virol. 2020, 128, 104433. [Google Scholar] [CrossRef]
- Vandenberg, O.; Martiny, D.; Rochas, O.; van Belkum, A.; Kozlakidis, Z. Considerations for diagnostic COVID-19 tests. Nat. Rev. Microbiol. 2021, 19, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Safiabadi Tali, S.H.; LeBlanc, J.J.; Sadiq, Z.; Oyewunmi, O.D.; Camargo, C.; Nikpour, B.; Armanfard, N.; Sagan, S.M.; Jahanshahi-Anbuhi, S. Tools and Techniques for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)/COVID-19 Detection. Clin. Microbiol. Rev. 2021, 34, e00228-20. [Google Scholar] [CrossRef] [PubMed]
- Emmerich, P.; von Possel, R.; Hemmer, C.J.; Fritzsche, C.; Geerdes-Fenge, H.; Menge, B.; Messing, C.; Borchardt-Loholter, V.; Deschermeier, C.; Steinhagen, K. Longitudinal detection of SARS-CoV-2-specific antibody responses with different serological methods. J. Med. Virol. 2021, 93, 5816–5824. [Google Scholar] [CrossRef] [PubMed]
- Wolfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Muller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Dolscheid-Pommerich, R.; Bartok, E.; Renn, M.; Kummerer, B.M.; Schulte, B.; Schmithausen, R.M.; Stoffel-Wagner, B.; Streeck, H.; Saschenbrecker, S.; Steinhagen, K.; et al. Correlation between a quantitative anti-SARS-CoV-2 IgG ELISA and neutralization activity. J. Med. Virol. 2022, 94, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, N.; Grossegesse, M.; Neumann, M.; Schaade, L.; Nitsche, A. Evaluation of a commercial ELISA as alternative to plaque reduction neutralization test to detect neutralizing antibodies against SARS-CoV-2. Sci. Rep. 2022, 12, 3549. [Google Scholar] [CrossRef]
- Meyer, B.; Drosten, C.; Muller, M.A. Serological assays for emerging coronaviruses: Challenges and pitfalls. Virus Res. 2014, 194, 175–183. [Google Scholar] [CrossRef]
- Li, D.; Li, J. Immunologic Testing for SARS-CoV-2 Infection from the Antigen Perspective. J. Clin. Microbiol. 2021, 59, e02160-20. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.; Tiu, C.; Hu, Z.; Chen, V.C.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Muller, K.; Girl, P.; von Buttlar, H.; Dobler, G.; Wolfel, R. Comparison of two commercial surrogate ELISAs to detect a neutralising antibody response to SARS-CoV-2. J. Virol. Methods 2021, 292, 114122. [Google Scholar] [CrossRef]
- Munsterkotter, L.; Hollstein, M.M.; Hahn, A.; Kroger, A.; Schnelle, M.; Erpenbeck, L.; Gross, U.; Frickmann, H.; Zautner, A.E. Comparison of the Anti-SARS-CoV-2 Surrogate Neutralization Assays by TECOmedical and DiaPROPH-Med with Samples from Vaccinated and Infected Individuals. Viruses 2022, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Reimerink, J.; Torriani, G.; Brouwer, F.; Godeke, G.J.; Yerly, S.; Hoogerwerf, M.; Vuilleumier, N.; Kaiser, L.; Eckerle, I.; et al. Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT). Emerg. Microbes Infect. 2020, 9, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Adams, O.; Andree, M.; Hermsen, D.; Lubke, N.; Timm, J.; Schaal, H.; Muller, L. Comparison of commercial SARS-CoV-2 surrogate neutralization assays with a full virus endpoint dilution neutralization test in two different cohorts. J. Virol. Methods 2022, 307, 114569. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brunink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Emmerich, P.; Murawski, C.; Ehmen, C.; von Possel, R.; Pekarek, N.; Oestereich, L.; Duraffour, S.; Pahlmann, M.; Struck, N.; Eibach, D.; et al. Limited specificity of commercially available SARS-CoV-2 IgG ELISAs in serum samples of African origin. Trop. Med. Int. Health 2021, 26, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Brehm, T.T.; Pfefferle, S.; von Possel, R.; Kobbe, R.; Norz, D.; Schmiedel, S.; Grundhoff, A.; Olearo, F.; Emmerich, P.; Robitaille, A.; et al. SARS-CoV-2 Reinfection in a Healthcare Worker Despite the Presence of Detectable Neutralizing Antibodies. Viruses 2021, 13, 661. [Google Scholar] [CrossRef] [PubMed]
- Pak, A.; Adegboye, O.A.; Adekunle, A.I.; Rahman, K.M.; McBryde, E.S.; Eisen, D.P. Economic Consequences of the COVID-19 Outbreak: The Need for Epidemic Preparedness. Front. Public. Health 2020, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- Pavan, M.; Bassani, D.; Sturlese, M.; Moro, S. From the Wuhan-Hu-1 strain to the XD and XE variants: Is targeting the SARS-CoV-2 spike protein still a pharmaceutically relevant option against COVID-19? J. Enzyme Inhib. Med. Chem. 2022, 37, 1704–1714. [Google Scholar] [CrossRef]
- Amrun, S.N.; Lee, C.Y.; Lee, B.; Fong, S.W.; Young, B.E.; Chee, R.S.; Yeo, N.K.; Torres-Ruesta, A.; Carissimo, G.; Poh, C.M.; et al. Linear B-cell epitopes in the spike and nucleocapsid proteins as markers of SARS-CoV-2 exposure and disease severity. EBioMedicine 2020, 58, 102911. [Google Scholar] [CrossRef]
- Van Elslande, J.; Decru, B.; Jonckheere, S.; Van Wijngaerden, E.; Houben, E.; Vandecandelaere, P.; Indevuyst, C.; Depypere, M.; Desmet, S.; Andre, E.; et al. Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs. Clin. Microbiol. Infect. 2020, 26, 1557.e1–1557.e7. [Google Scholar] [CrossRef]
- Havervall, S.; Jernbom Falk, A.; Klingstrom, J.; Ng, H.; Greilert-Norin, N.; Gabrielsson, L.; Salomonsson, A.C.; Isaksson, E.; Rudberg, A.S.; Hellstrom, C.; et al. SARS-CoV-2 induces a durable and antigen specific humoral immunity after asymptomatic to mild COVID-19 infection. PLoS ONE 2022, 17, e0262169. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Matthews, P.C.; Stoesser, N.; Maddox, T.; Lorenzi, L.; Studley, R.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. Anti-spike antibody response to natural SARS-CoV-2 infection in the general population. Nat. Commun. 2021, 12, 6250. [Google Scholar] [CrossRef]
- Pedersen, J.; Koumakpayi, I.H.; Babuadze, G.; Baz, M.; Ndiaye, O.; Faye, O.; Diagne, C.T.; Dia, N.; Naghibosadat, M.; McGeer, A.; et al. Cross-reactive immunity against SARS-CoV-2 N protein in Central and West Africa precedes the COVID-19 pandemic. Sci. Rep. 2022, 12, 12962. [Google Scholar] [CrossRef] [PubMed]
- Thura, M.; Sng, J.X.E.; Ang, K.H.; Li, J.; Gupta, A.; Hong, J.M.; Hong, C.W.; Zeng, Q. Targeting intra-viral conserved nucleocapsid (N) proteins as novel vaccines against SARS-CoVs. Biosci. Rep. 2021, 41, BSR20211491. [Google Scholar] [CrossRef] [PubMed]
- Ahlen, G.; Frelin, L.; Nikouyan, N.; Weber, F.; Hoglund, U.; Larsson, O.; Westman, M.; Tuvesson, O.; Gidlund, E.K.; Cadossi, M.; et al. The SARS-CoV-2 N Protein Is a Good Component in a Vaccine. J. Virol. 2020, 94, e01279-20. [Google Scholar] [CrossRef] [PubMed]
- Haveri, A.; Ekstrom, N.; Solastie, A.; Virta, C.; Osterlund, P.; Isosaari, E.; Nohynek, H.; Palmu, A.A.; Melin, M. Persistence of neutralizing antibodies a year after SARS-CoV-2 infection in humans. Eur. J. Immunol. 2021, 51, 3202–3213. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Takahashi, M.; Ai, T.; Sinozuka, K.; Baba, Y.; Igawa, G.; Nojiri, S.; Yamamoto, T.; Yuri, M.; Takei, S.; Saito, K.; et al. Activation of SARS-CoV-2 neutralizing antibody is slower than elevation of spike-specific IgG, IgM, and nucleocapsid-specific IgG antibodies. Sci. Rep. 2022, 12, 14909. [Google Scholar] [CrossRef]
- Carrillo, J.; Izquierdo-Useros, N.; Avila-Nieto, C.; Pradenas, E.; Clotet, B.; Blanco, J. Humoral immune responses and neutralizing antibodies against SARS-CoV-2; implications in pathogenesis and protective immunity. Biochem. Biophys. Res. Commun. 2021, 538, 187–191. [Google Scholar] [CrossRef]
- Sun, J.; Tang, X.; Bai, R.; Liang, C.; Zeng, L.; Lin, H.; Yuan, R.; Zhou, P.; Huang, X.; Xiong, Q.; et al. The kinetics of viral load and antibodies to SARS-CoV-2. Clin. Microbiol. Infect. 2020, 26, 1690.e1–1690.e4. [Google Scholar] [CrossRef]
- Taylor, S.C.; Hurst, B.; Charlton, C.L.; Bailey, A.; Kanji, J.N.; McCarthy, M.K.; Morrison, T.E.; Huey, L.; Annen, K.; DomBourian, M.G.; et al. A New SARS-CoV-2 Dual-Purpose Serology Test: Highly Accurate Infection Tracing and Neutralizing Antibody Response Detection. J. Clin. Microbiol. 2021, 59, e02438-20. [Google Scholar] [CrossRef] [PubMed]
- Girl, P.; Zwirglmaier, K.; von Buttlar, H.; Wolfel, R.; Muller, K. Evaluation of Two Rapid Lateral Flow Tests and Two Surrogate ELISAs for the Detection of SARS-CoV-2 Specific Neutralizing Antibodies. Front. Med. 2022, 9, 820151. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.; Rose, R.; Rompke, J.; Grobe, O.; Lorentz, T.; Fickenscher, H.; Krumbholz, A. Development of SARS-CoV-2 Specific IgG and Virus-Neutralizing Antibodies after Infection with Variants of Concern or Vaccination. Vaccines 2021, 9, 700. [Google Scholar] [CrossRef] [PubMed]
- von Rhein, C.; Scholz, T.; Henss, L.; Kronstein-Wiedemann, R.; Schwarz, T.; Rodionov, R.N.; Corman, V.M.; Tonn, T.; Schnierle, B.S. Comparison of potency assays to assess SARS-CoV-2 neutralizing antibody capacity in COVID-19 convalescent plasma. J. Virol. Methods 2021, 288, 114031. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, L.; Park, Y.J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 2020, 183, 1024–1042.e21. [Google Scholar] [CrossRef]
- Kruttgen, A.; Lauen, M.; Klingel, H.; Imohl, M.; Kleines, M. Two novel SARS-CoV-2 surrogate virus neutralization assays are suitable for assessing successful immunization with mRNA-1273. J. Virol. Methods 2022, 299, 114297. [Google Scholar] [CrossRef]
- Valcourt, E.J.; Manguiat, K.; Robinson, A.; Chen, J.C.; Dimitrova, K.; Philipson, C.; Lamoureux, L.; McLachlan, E.; Schiffman, Z.; Drebot, M.A.; et al. Evaluation of a commercially-available surrogate virus neutralization test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Diagn. Microbiol. Infect. Dis. 2021, 99, 115294. [Google Scholar] [CrossRef]
- Embregts, C.W.E.; Verstrepen, B.; Langermans, J.A.M.; Boszormenyi, K.P.; Sikkema, R.S.; de Vries, R.D.; Hoffmann, D.; Wernike, K.; Smit, L.A.M.; Zhao, S.; et al. Evaluation of a multi-species SARS-CoV-2 surrogate virus neutralization test. One Health 2021, 13, 100313. [Google Scholar] [CrossRef]
- Ortiz de Montellano, P.R.; David, S.K.; Ator, M.A.; Tew, D. Mechanism-based inactivation of horseradish peroxidase by sodium azide. Formation of meso-azidoprotoporphyrin IX. Biochemistry 1988, 27, 5470–5476. [Google Scholar] [CrossRef]
- Springer, D.N.; Perkmann, T.; Jani, C.M.; Mucher, P.; Pruger, K.; Marculescu, R.; Reuberger, E.; Camp, J.V.; Graninger, M.; Borsodi, C.; et al. Reduced Sensitivity of Commercial Spike-Specific Antibody Assays after Primary Infection with the SARS-CoV-2 Omicron Variant. Microbiol. Spectr. 2022, 10, e0212922. [Google Scholar] [CrossRef]

| Panel A (Acute) | Panel B (Pre-Pandemic) | Panel C (Convalescent) | Panel D (Seasonal hCoV, Non-SARS-CoV-2) | |
|---|---|---|---|---|
| Total number of patients | 115 | 95 | 36 | 13 |
| Age (mean ± SD, range) [years] | 61.6 ± 18.6, 21–89 | unknown | 43.1 ± 13.4, 19–64 | 7.8 ± 4.2, 4–15 |
| 75.7 ± 0.5, 75–89 | ||||
| Sex (n females, n males, n unknown) | 58, 57, 0 | unknown | 24, 12, 0 | 3, 7, 0 |
| 1, 2, 0 | ||||
| N samples in the panel | 268 | 95 | 237 | 14 |
| Number of sequential samples per patient | 1–6 | - | 1–14 | 1–2 |
| dpso/dpPCR (mean ± SD, range, unknown) | 14.3 ± 20.6, 0–154, 31 | - | 199.1 ± 134.6, 10–474, 0 | unknown |
| 68.7 ± 46.2, -, - | ||||
| Total number of vaccinated patients in the cohort | - | - | 21 | - |
| N samples post-vaccination | - | - | 63 | - |
| Number of sequential samples post-vaccination | - | - | 1–9 | - |
| dpVac (mean ± SD, range) | - | - | 52.1 ± 40.7, 6–181 | - |
| Anti-SARS-CoV-2-IgG | Anti-SARS-CoV-2 NAb | ||||||
|---|---|---|---|---|---|---|---|
| In-House IIFT | EUROLINE Profile IgG | In-House VNT | GenScript cPass | EUROIMMUN SARS-CoV-2 NeutraLISA | |||
| Period (dpso/dpPCR) | N total | N pos (%) | N pos (%) | N pos (%) | N pos (%) | N pos (%) | N equ (%) |
| T1 (0–5) | 62 | 13 (21.0) | 5 (8.1) | 7 (11.3) | 8 (12.9) | 2 (3.2) | 2 (3.2) |
| T2 (6–10) | 80 | 49 (61.3) | 39 (48.8) | 49 (61.3) | 42 (52.2) | 23 (28.8) | 5 (6.3) |
| T3 (11–20) | 75 | 60 (80.0) | 54 (72.0) | 58 (77.3) | 57 (76.0) | 40 (53.3) | 9 (12.0) |
| T4 (>20) | 20 | 16 (80.0) | 17 (85.0) | 17 (85.0) | 17 (85.0) | 15 (75.0) | 2 (10.0) |
| Unknown | 31 | 15 (48.4) | 11 (35.5) | 14 (45.2) | 13 (41.9) | 11 (35.5) | 2 (6.5) |
| All samples incl. unknown | 268 | 153 (57.1) | 126 (47.0) | 145 (54.1) | 137 (51.1) | 91 (34.0) | 20 (7.5) |
| Positive agreement % a | reference | 78.4 | reference | 88.3 | 71.7 | ||
| 95% CI | 71.1–84.7 | 81.9–93.0 | 63.7–78.9 | ||||
| Negative agreement % | reference | 94.8 | reference | 92.7 | 95.9 | ||
| 95% CI | 86.1–98.1 | 83.6–96.6 | 88.2–98.7 | ||||
| Pre-pandemic sera | 95 | 0 | 0 | 0 | 0 | 0 | |
| Specificity % b | 100 | 100 | 100 | 100 | 100 | ||
| 95% CI | 96.2–100 | 96.2–100 | 96.2–100 | 96.2–100 | 96.2–100 | ||
| Anti-SARS-CoV-2-IgG | Anti-SARS-CoV-2 NAb | |||||||
|---|---|---|---|---|---|---|---|---|
| In-House IIFT | EUROLINE Profile IgG | In-House VNT | GenScript cPass | EUROIMMUN SARS-CoV-2 NeutraLISA | ||||
| dpso/dpPCR | N total | N pos (%) | N pos (%) | N equ (%) | N pos (%) | N pos (%) | N pos (%) | N equ (%) |
| (T5 (0–150) | 99 | 99 (100) | 89 (89.9) | 1 (1.0) | 81 (81.8) | 87 (87.9) | 35 (35.4) | 16 (16.2) |
| T6 (151–300) | 45 | 45 (100) | 39 (86.7) | 0 (0) | 33 (73.3) | 38 (84.4) | 11 (24.4) | 7 (15.6) |
| T7 (301–500) | 25 | 25 (100) | 21 (84.0) | 0 (0) | 15 (60.0) | 18 (72.0) | 6 (24) | 2 (8.0) |
| TVac (Vaccination) | 68 | 68 (100) | 68 (100) | 68 (100) | 68 (100) | 68 (100) | 0 (0) | |
| All samples | 237 | 237 (100) | 216 (91.1) | 1 (0.4) | 197 (83.1) | 211 (89.0) | 120 (50.6) | 25 (10.5) |
| Positive agreement % a | reference | 91.6 | reference | 97.0 | 72.1 | |||
| 95% CI | 87.3–94.8 | 93.5–98.9 | 65.3–78.2 | |||||
| Negative agreement % | reference | reference | 50.0 | 92.5 | ||||
| 95% CI | 33.8–66.2 | 79.6–98.4 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Possel, R.; Menge, B.; Deschermeier, C.; Fritzsche, C.; Hemmer, C.; Geerdes-Fenge, H.; Loebermann, M.; Schulz, A.; Lattwein, E.; Steinhagen, K.; et al. Performance Analysis of Serodiagnostic Tests to Characterize the Incline and Decline of the Individual Humoral Immune Response in COVID-19 Patients: Impact on Diagnostic Management. Viruses 2024, 16, 91. https://doi.org/10.3390/v16010091
von Possel R, Menge B, Deschermeier C, Fritzsche C, Hemmer C, Geerdes-Fenge H, Loebermann M, Schulz A, Lattwein E, Steinhagen K, et al. Performance Analysis of Serodiagnostic Tests to Characterize the Incline and Decline of the Individual Humoral Immune Response in COVID-19 Patients: Impact on Diagnostic Management. Viruses. 2024; 16(1):91. https://doi.org/10.3390/v16010091
Chicago/Turabian Stylevon Possel, Ronald, Babett Menge, Christina Deschermeier, Carlos Fritzsche, Christoph Hemmer, Hilte Geerdes-Fenge, Micha Loebermann, Anette Schulz, Erik Lattwein, Katja Steinhagen, and et al. 2024. "Performance Analysis of Serodiagnostic Tests to Characterize the Incline and Decline of the Individual Humoral Immune Response in COVID-19 Patients: Impact on Diagnostic Management" Viruses 16, no. 1: 91. https://doi.org/10.3390/v16010091
APA Stylevon Possel, R., Menge, B., Deschermeier, C., Fritzsche, C., Hemmer, C., Geerdes-Fenge, H., Loebermann, M., Schulz, A., Lattwein, E., Steinhagen, K., Tönnies, R., Ahrendt, R., & Emmerich, P. (2024). Performance Analysis of Serodiagnostic Tests to Characterize the Incline and Decline of the Individual Humoral Immune Response in COVID-19 Patients: Impact on Diagnostic Management. Viruses, 16(1), 91. https://doi.org/10.3390/v16010091






