Distribution of Wheat-Infecting Viruses and Genetic Variability of Wheat Streak Mosaic Virus and Barley Stripe Mosaic Virus in Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and RNA Extraction
2.2. Primer Design
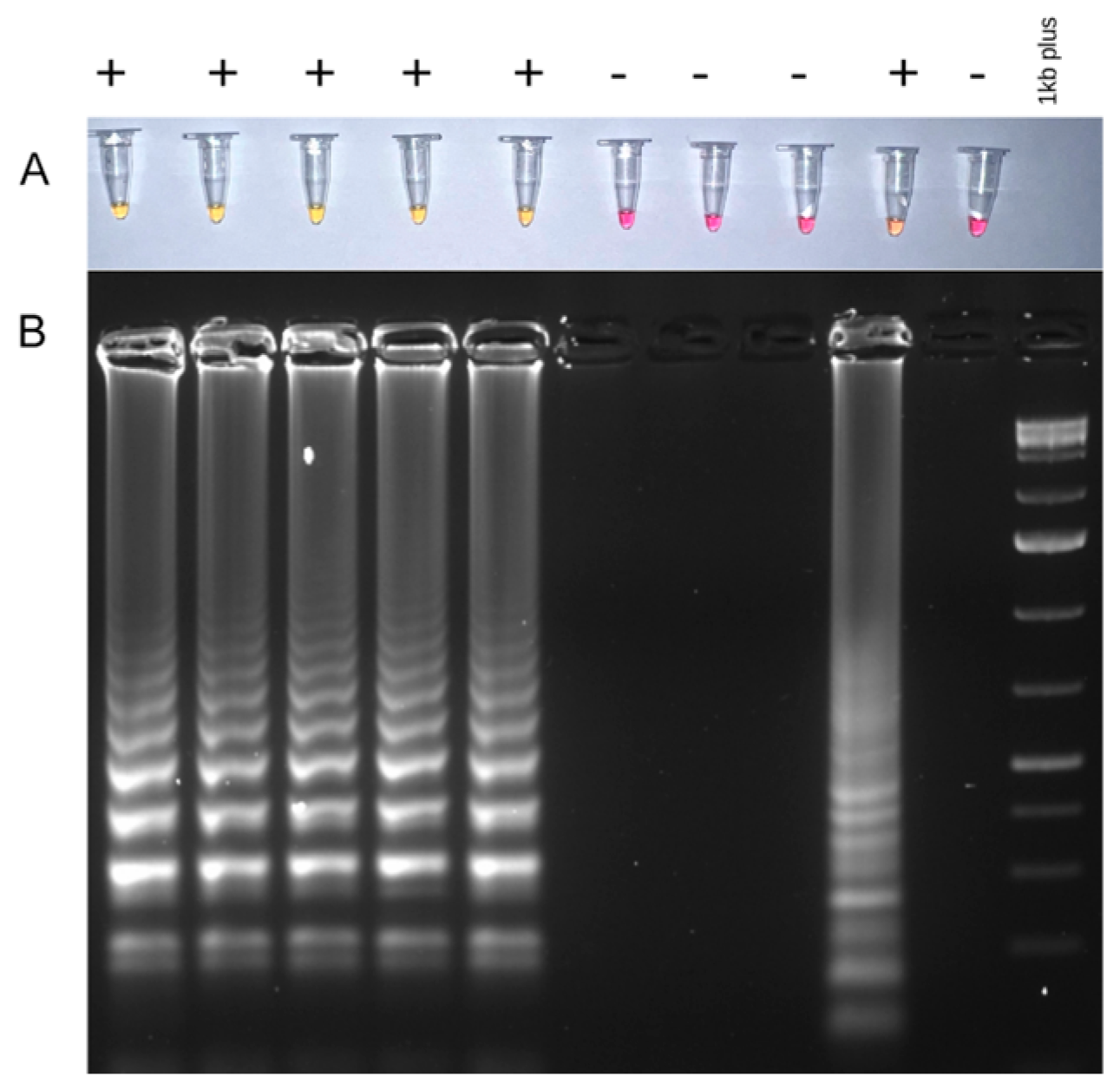
2.3. Detection of Viruses Using PCR and LAMP
2.4. Targeted Sequencing
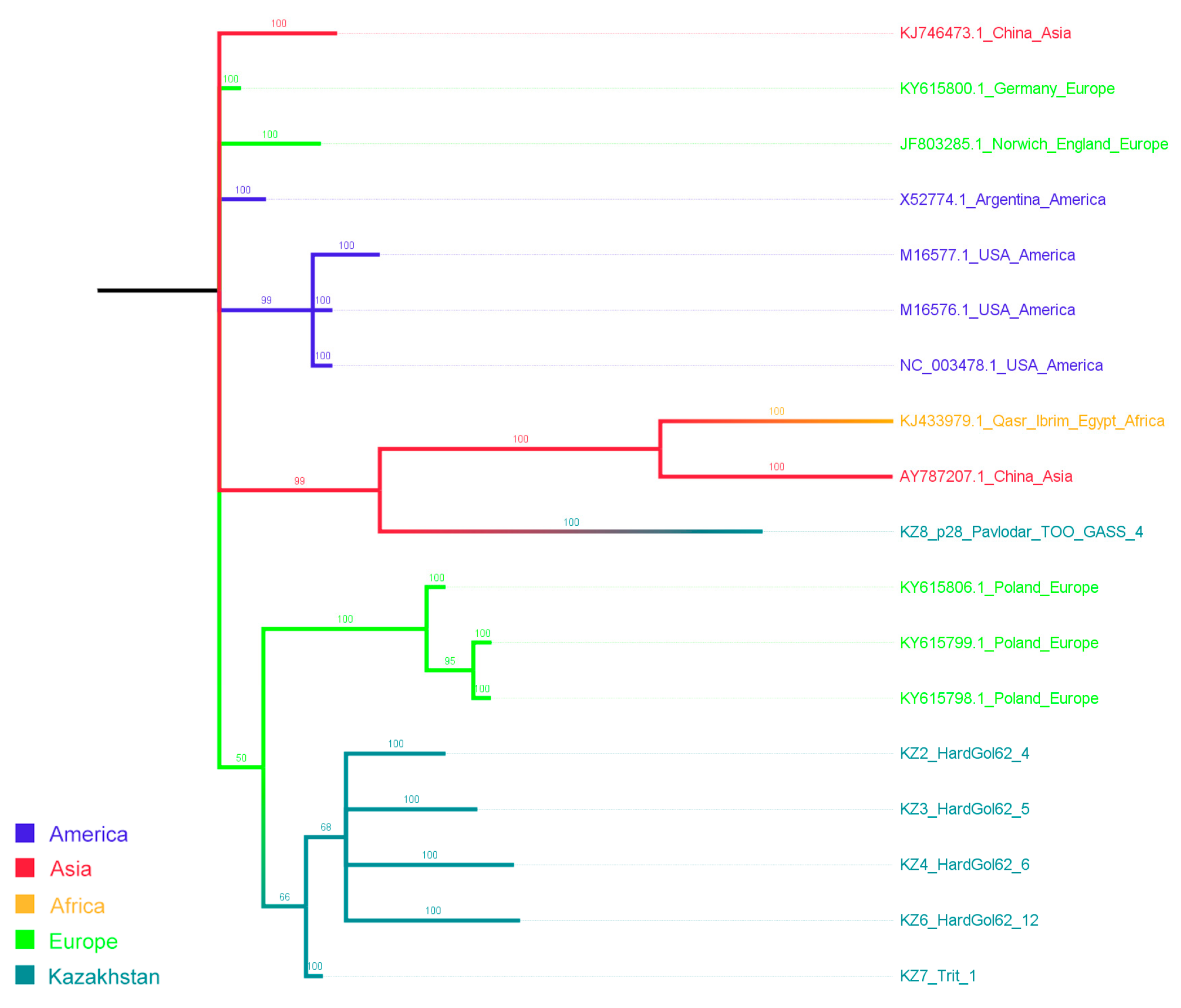
2.5. Phylogenetic Analysis
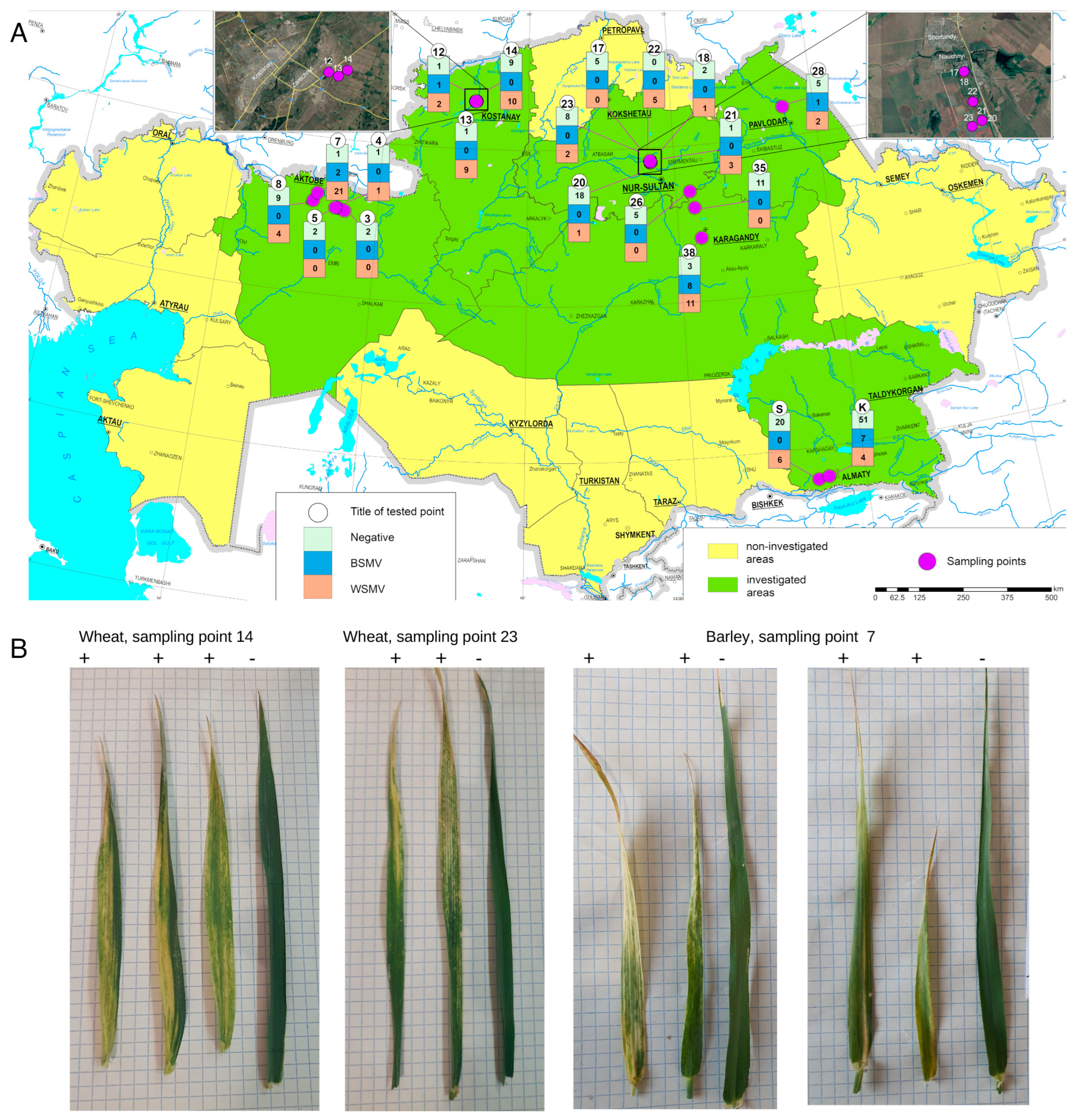
3. Results
3.1. RT-PCR and LAMP Detection of Wheat Viruses
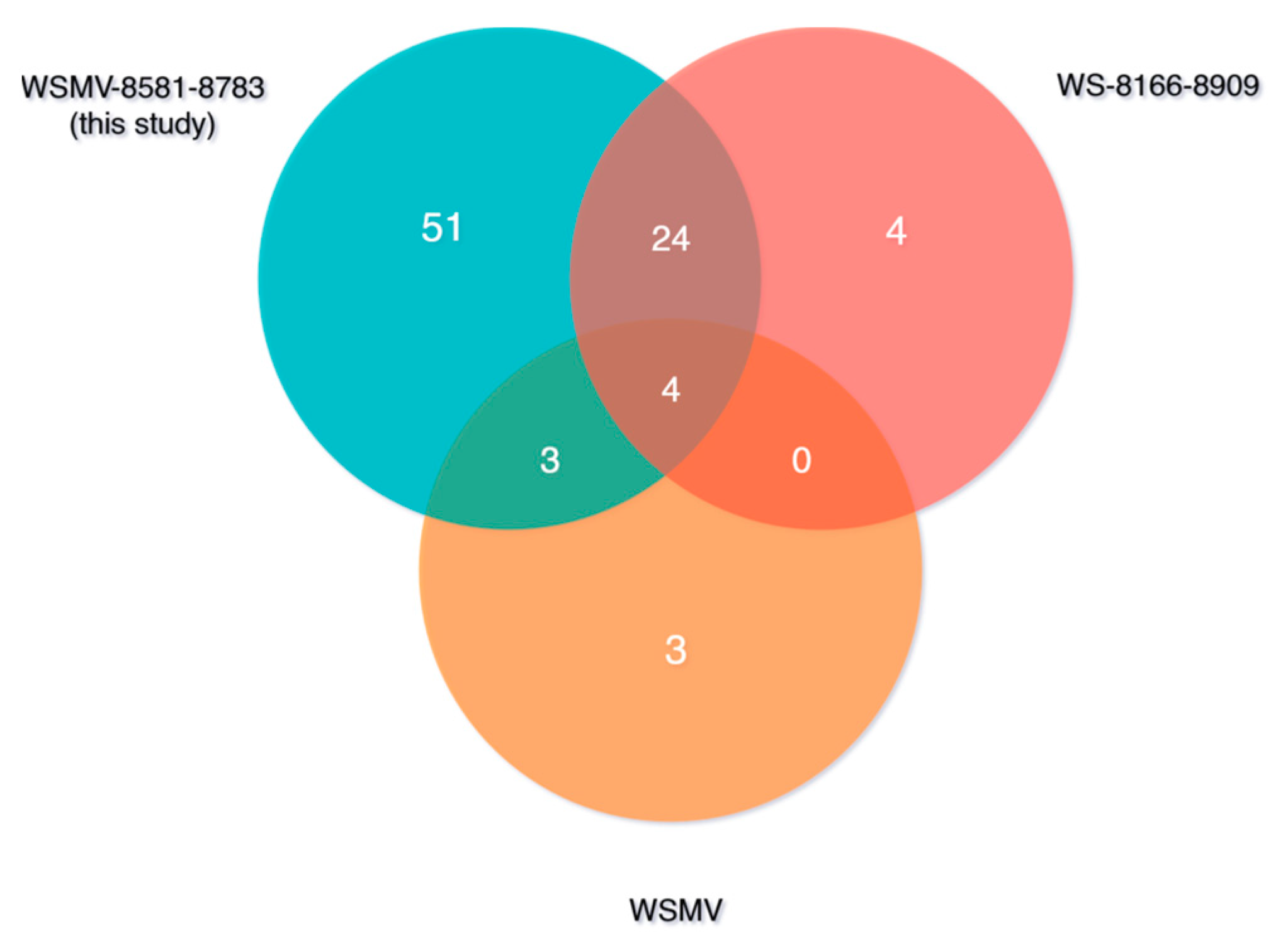
3.2. Genetic Variability of WSMV and BSMV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Location Point | Latitude | Longitude | Host Plant | Plants with Symptoms Tested | Plants without Symptoms Tested | PCR WSMV+ | PCR BSMV+ | Sequences |
|---|---|---|---|---|---|---|---|---|
| 3 | 50°11.133′ | 58°40.880′ | Wheat | 0 | 2 | 0 | 0 | |
| 4 | 50°14.383′ | 58°26.214′ | Wheat | 1 | 1 | 1 | 0 | |
| 5 | 50°14.381′ | 58°18.364′ | Wheat | 0 | 2 | 0 | 0 | |
| 7 | 50°33.891′ | 57°32.293′ | Barley | 23 | 1 | 21 | 2 | KZ90, KZ93, KZ94, KZ96, KZ99, KZ103, KZ104, KZ108, (WSMV) |
| 8 | 50°21.584′ | 57°23.847′ | Wheat | 4 | 9 | 4 | 0 | KZ48, KZ49 (WSMV) |
| 12 | 53°13.133′ | 63°45.438′ | Barley | 3 | 1 | 2 | 1 | |
| 13 | 53°12.957′ | 63°46.143′ | Wheat | 9 | 1 | 9 | 0 | KZ57, KZ58, KZ59, KZ60, KZ62, KZ63, KZ64, KZ65 (WSMV) |
| 14 | 53°13.288′ | 63°47.131′ | Wheat | 10 | 9 | 10 | 0 | KZ68, KZ72, KZ73, KZ69, KZ70 (WSMV) |
| 17 | 51°40.282′ | 71°01.126′ | Wheat | 0 | 5 | 0 | 0 | |
| 18 | 51°40.282′ | 71°01.143′ | Wheat | 1 | 2 | 1 | 0 | |
| 20 | 51°38.004′ | 71°02.413′ | Wheat | 1 | 18 | 1 | 0 | |
| 21 | 51°38.007′ | 71°02.411′ | Wheat | 3 | 1 | 3 | 0 | |
| 22 | 51°38.817′ | 71°01.820′ | Barley | 5 | 0 | 5 | 0 | |
| 23 | 51°37.804′ | 71°01.812′ | Wheat | 3 | 7 | 2 | 0 | |
| 26 | 50°49.495′ | 72°33.790′ | Wheat | 0 | 5 | 0 | 0 | |
| 28 | 52°44.708′ | 76°42.226′ | Wheat | 3 | 5 | 2 | 1 | KZ8 (BSMV) |
| 35 | 50°15.910’ | 72°40.247’ | Wheat | 0 | 11 | 0 | 0 | |
| 38 | 49°37.410’ | 72°53.835’ | Wheat | 16 | 6 | 11 | 8 | |
| K A | 43°13.598’ | 76°42.036’ | Wheat | 11 | 51 | 4 | 7 | |
| S B | 43°10.833’ | 76°19.950’ | Wheat, barley | 6 | 20 | 6 | 0 | KZ2, KZ3, KZ4, KZ6, KZ7 (BSMV) |
References
- Turuspekov, Y.; Plieske, J.; Ganal, M.; Akhunov, E.; Abugalieva, S. Phylogenetic Analysis of Wheat Cultivars in Kazakhstan Based on the Wheat 90 K Single Nucleotide Polymorphism Array. Plant Genet. Resour. 2017, 15, 29–35. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 July 2023).
- Tatineni, S.; Hein, G.L. Plant Viruses of Agricultural Importance: Current and Future Perspectives of Virus Disease Management Strategies. Phytopathology 2023, 113, 117–141. [Google Scholar] [CrossRef]
- Hodge, B.A.; Paul, P.A.; Stewart, L.R. Occurrence and High-Throughput Sequencing of Viruses in Ohio Wheat. Plant Dis. 2020, 104, 1789–1800. [Google Scholar] [CrossRef]
- Rotenberg, D.; Bockus, W.W.; Whitfield, A.E.; Hervey, K.; Baker, K.D.; Ou, Z.; Laney, A.G.; De Wolf, E.D.; Appel, J.A. Occurrence of Viruses and Associated Grain Yields of Paired Symptomatic and Nonsymptomatic Tillers in Kansas Winter Wheat Fields. Phytopathology 2016, 106, 202–210. [Google Scholar] [CrossRef]
- Singh, K.; Wegulo, S.N.; Skoracka, A.; Kundu, J.K. Wheat Streak Mosaic Virus: A Century Old Virus with Rising Importance Worldwide. Mol. Plant Pathol. 2018, 19, 2193–2206. [Google Scholar] [CrossRef]
- Tatineni, S.; Alexander, J.; Qu, F. Differential Synergistic Interactions Among Four Different Wheat-Infecting Viruses. Front. Microbiol. 2022, 12, 800318. [Google Scholar] [CrossRef]
- Almas, L.K.; Price, J.A.; Workneh, F.; Rush, C.M. Quantifying Economic Losses Associated with Levels of Wheat Streak Mosaic Incidence and Severity in the Texas High Plains. Crop Prot. 2016, 88, 155–160. [Google Scholar] [CrossRef]
- French, R.; Stenger, D.C. Evolution of Wheat Streak Mosaic Virus: Dynamics of Population Growth within Plants May Explain Limited Variation. Annu. Rev. Phytopathol. 2003, 41, 199–214. [Google Scholar] [CrossRef]
- Pfrieme, A.-K.; Will, T.; Pillen, K.; Stahl, A. The Past, Present, and Future of Wheat Dwarf Virus Management—A Review. Plants 2023, 12, 3633. [Google Scholar] [CrossRef]
- McKinney, H.H.; Greeley, L.W. Biological Characteristics of Barley Stripe-Mosaic Virus Strains and Their Evolution; U.S. Department of Agriculture: Washington, DC, USA, 1965. [Google Scholar]
- Donald, R.G.K.; Zhou, H.; Jackson, A.O. Serological Analysis of Barley Stripe Mosaic Virus-Encoded Proteins in Infected Barley. Virology 1993, 195, 659–668. [Google Scholar] [CrossRef]
- King, A.M.; Adams, M.; Carstens, E.; Lefkowitz, E.J. Family Virgaviridae. In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier Academic Press: Waltham, MA, USA, 2011; ISBN 978-0-12-384684-6. [Google Scholar]
- Donald, R.G.; Jackson, A.O. The Barley Stripe Mosaic Virus Gamma b Gene Encodes a Multifunctional Cysteine-Rich Protein That Affects Pathogenesis. Plant Cell 1994, 6, 1593–1606. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gustafson, G.; Armour, S.L. The Complete Nucleotide Sequence of RNA Beta from the Type Strain of Barley Stripe Mosaic Virus. Nucleic Acids Res. 1986, 14, 3895–3909. [Google Scholar] [CrossRef]
- Jackson, A.O.; Lim, H.-S.; Bragg, J.; Ganesan, U.; Lee, M.Y. Hordeivirus Replication, Movement, and Pathogenesis. Annu. Rev. Phytopathol. 2009, 47, 385–422. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, K.; Xu, K.; Zhang, K.; Jin, X.; Yang, M.; Zhang, Y.; Wang, X.; Han, C.; Yu, J.; et al. Barley Stripe Mosaic Virus Infection Requires PKA-Mediated Phosphorylation of Γb for Suppression of Both RNA Silencing and the Host Cell Death Response. New Phytol. 2018, 218, 1570–1585. [Google Scholar] [CrossRef]
- Yelina, N.E.; Savenkov, E.I.; Solovyev, A.G.; Morozov, S.Y.; Valkonen, J.P.T. Long-Distance Movement, Virulence, and RNA Silencing Suppression Controlled by a Single Protein in Hordei- and Potyviruses: Complementary Functions between Virus Families. J. Virol. 2002, 76, 12981–12991. [Google Scholar] [CrossRef]
- Donald, R.G.K.; Jackson, A.O. RNA-Binding Activities of Barley Stripe Mosaic Virus Γb Fusion Proteins. J. Gen. Virol. 1996, 77, 879–888. [Google Scholar] [CrossRef]
- Bragg, J.N.; Jackson, A.O. The C-Terminal Region of the Barley Stripe Mosaic Virusγb Protein Participates in Homologous Interactions and Is Required for Suppression of RNA Silencing. Mol. Plant Pathol. 2004, 5, 465–481. [Google Scholar] [CrossRef]
- Smith, O.; Clapham, A.; Rose, P.; Liu, Y.; Wang, J.; Allaby, R.G. A Complete Ancient RNA Genome: Identification, Reconstruction and Evolutionary History of Archaeological Barley Stripe Mosaic Virus. Sci. Rep. 2014, 4, 4003. [Google Scholar] [CrossRef]
- McNeil, J.E.; French, R.; Hein, G.L.; Baenziger, P.S.; Eskridge, K.M. Characterization of Genetic Variability among Natural Populations of Wheat Streak Mosaic Virus. Phytopathology 1996, 86, 1222–1227. [Google Scholar] [CrossRef]
- Kapooria, R.G.; Ndunguru, J. Occurrence of Viruses in Irrigated Wheat in Zambia. EPPO Bull. 2004, 34, 413–419. [Google Scholar] [CrossRef]
- Stenger, D.C.; Hall, J.S.; Choi, I.-R.; French, R. Phylogenetic Relationships within the Family Potyviridae: Wheat Streak Mosaic Virus and Brome Streak Mosaic Virus Are Not Members of the Genus Rymovirus. Phytopathology 1998, 88, 782–787. [Google Scholar] [CrossRef]
- Choi, I.-R.; Horken, K.M.; Stenger, D.C.; French, R. Mapping of the P1 Proteinase Cleavage Site in the Polyprotein of Wheat Streak Mosaic Virus (Genus Tritimovirus). J. Gen. Virol. 2002, 83, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.Y.-W.; Miller, W.A.; Atkins, J.F.; Firth, A.E. An Overlapping Essential Gene in the Potyviridae. Proc. Natl. Acad. Sci. USA 2008, 105, 5897–5902. [Google Scholar] [CrossRef] [PubMed]
- Tatineni, S.; Ziems, A.D.; Wegulo, S.N.; French, R. Triticum Mosaic Virus: A Distinct Member of the Family Potyviridae with an Unusually Long Leader Sequence. Phytopathology 2009, 99, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Redila, C.D.; Phipps, S.; Nouri, S. Full Genome Evolutionary Studies of Wheat Streak Mosaic-Associated Viruses Using High-Throughput Sequencing. Front. Microbiol. 2021, 12, 699078. [Google Scholar] [CrossRef] [PubMed]
- Tatineni, S.; French, R. The C-Terminus of Wheat Streak Mosaic Virus Coat Protein Is Involved in Differential Infection of Wheat and Maize through Host-Specific Long-Distance Transport. MPMI 2014, 27, 150–162. [Google Scholar] [CrossRef]
- Choi, I.-R.; Hall, J.S.; Henry, M.; Zhang, L.; Hein, G.L.; French, R.; Stenger, D.C. Contributions of Genetic Drift and Negative Selection on the Evolution of Three Strains of Wheat Streak Mosaic Tritimovirus. Arch. Virol. 2001, 146, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, H.; Henry, M.; Cárdenas-Soriano, E.; Alvizo-Villasana, H.F. Identification of Wheat Streak Mosaic Virus and Its Vector Aceria tosichella in Mexico. Plant Dis. 2001, 85, 13–17. [Google Scholar] [CrossRef]
- Navia, D.; Truol, G.; Mendonca, R.S.; Sagadin, M. Aceria tosichella Keifer (Acari: Eriophyidae) from Wheat Streak Mosaic Virus-Infected Wheat Plants in Argentina. Int. J. Acarol. 2006, 32, 189–193. [Google Scholar] [CrossRef]
- Rabenstein, F.; Seifers, D.L.; Schubert, J.; French, R.; Stenger, D.C. Phylogenetic Relationships, Strain Diversity and Biogeography of Tritimoviruses. J. Gen. Virol. 2002, 83, 895–906. [Google Scholar] [CrossRef]
- Foulad, P.; Izadpanah, K. Identification of Wheat Streak Mosaic Virus in Iran. Iran. Agric. Res. 1986, 5, 73–84. [Google Scholar]
- Hassani, M.; Lotfipour, M.; Qasemi Nejad, M.; Tabib, M.H.; Baimani, M. Occurrence of Major Viruses in Wheat in Khuzestan Province. J. Iran. Plant Prot. Res. 2022, 36, 1–8. [Google Scholar] [CrossRef]
- Dwyer, G.I.; Gibbs, M.J.; Gibbs, A.J.; Jones, R.A.C. Wheat Streak Mosaic Virus in Australia: Relationship to Isolates from the Pacific Northwest of the USA and Its Dispersion Via Seed Transmission. Plant Dis. 2007, 91, 164–170. [Google Scholar] [CrossRef]
- Ellis, M.H.; Rebetzke, G.J.; Mago, R.; Chu, P. First Report of Wheat Streak Mosaic Virus in Australia. Australas. Plant Pathol. 2003, 32, 551–553. [Google Scholar] [CrossRef]
- Fellers, J.P.; Seifers, D.; Ryba-White, M.; Joe Martin, T. The Complete Genome Sequence of Triticum Mosaic Virus, a New Wheat-Infecting Virus of the High Plains. Arch. Virol. 2009, 154, 1511–1515. [Google Scholar] [CrossRef]
- Seifers, D.L.; Martin, T.J.; Harvey, T.L.; Fellers, J.P.; Stack, J.P.; Ryba-White, M.; Haber, S.; Krokhin, O.; Spicer, V.; Lovat, N.; et al. Triticum Mosaic Virus: A New Virus Isolated from Wheat in Kansas. Plant Dis. 2008, 92, 808–817. [Google Scholar] [CrossRef]
- Young, B.A.; Stenger, D.C.; Qu, F.; Morris, T.J.; Tatineni, S.; French, R. Tritimovirus P1 Functions as a Suppressor of RNA Silencing and an Enhancer of Disease Symptoms. Virus Res. 2012, 163, 672–677. [Google Scholar] [CrossRef]
- Tatineni, S.; Alexander, J.; Gupta, A.K.; French, R. Asymmetry in Synergistic Interaction Between Wheat Streak Mosaic Virus and Triticum Mosaic Virus in Wheat. MPMI 2019, 32, 336–350. [Google Scholar] [CrossRef]
- Byamukama, E.; Wegulo, S.N.; Tatineni, S.; Hein, G.L.; Graybosch, R.A.; Baenziger, P.S.; French, R. Quantification of Yield Loss Caused by Triticum Mosaic Virus and Wheat Streak Mosaic Virus in Winter Wheat under Field Conditions. Plant Dis. 2014, 98, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Byamukama, E.; Tatineni, S.; Hein, G.; McMechan, J.; Wegulo, S.N. Incidence of Wheat Streak Mosaic Virus, Triticum Mosaic Virus, and Wheat Mosaic Virus in Wheat Curl Mites Recovered from Maturing Winter Wheat Spikes. Plant Dis. 2016, 100, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; Curtis Hendrickson, R.; et al. Recent Changes to Virus Taxonomy Ratified by the International Committee on Taxonomy of Viruses (2022). Arch. Virol. 2022, 167, 2429–2440. [Google Scholar] [CrossRef]
- Tatineni, S.; McMechan, A.J.; Wosula, E.N.; Wegulo, S.N.; Graybosch, R.A.; French, R.; Hein, G.L. An Eriophyid Mite-Transmitted Plant Virus Contains Eight Genomic RNA Segments with Unusual Heterogeneity in the Nucleocapsid Protein. J. Virol. 2014, 88, 11834–11845. [Google Scholar] [CrossRef]
- Ahn, K.K.; Kim, K.S.; Gergerich, R.C.; Jensen, S.G. High Plains Disease of Corn and Wheat: Ultrastructural and Serological Aspects. J. Submicrosc. Cytol. Pathol. 1998, 30, 563–571. [Google Scholar]
- Mielke-Ehret, N.; Mühlbach, H.-P. Emaravirus: A Novel Genus of Multipartite, Negative Strand RNA Plant Viruses. Viruses 2012, 4, 1515–1536. [Google Scholar] [CrossRef]
- Burrows, M.; Franc, G.; Rush, C.; Blunt, T.; Ito, D.; Kinzer, K.; Olson, J.; O’Mara, J.; Price, J.; Tande, C.; et al. Occurrence of Viruses in Wheat in the Great Plains Region, 2008. Plant Health Prog. 2009, 10, 14. [Google Scholar] [CrossRef]
- Pozhylov, I.; Snihur, H.; Shevchenko, T.; Budzanivska, I.; Liu, W.; Wang, X.; Shevchenko, O. Occurrence and Characterization of Wheat Streak Mosaic Virus Found in Mono- and Mixed Infection with High Plains Wheat Mosaic Virus in Winter Wheat in Ukraine. Viruses 2022, 14, 1220. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Vazquez-Iglesias, I.; McGreig, S.; Fox, A.; Gibbs, A.J. Genomic High Plains Wheat Mosaic Virus Sequences from Australia: Their Phylogenetics and Evidence for Emaravirus Recombination and Reassortment. Viruses 2023, 15, 401. [Google Scholar] [CrossRef]
- McMechan, A.J.; Tatineni, S.; French, R.; Hein, G.L. Differential Transmission of Triticum Mosaic Virus by Wheat Curl Mite Populations Collected in the Great Plains. Plant Dis. 2014, 98, 806–810. [Google Scholar] [CrossRef]
- Seifers, D.L.; Martin, T.J.; Harvey, T.L.; Fellers, J.P.; Michaud, J.P. Identification of the Wheat Curl Mite as the Vector of Triticum Mosaic Virus. Plant Dis. 2009, 93, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Byamukama, E.; Seifers, D.L.; Hein, G.L.; De Wolf, E.; Tisserat, N.A.; Langham, M.A.C.; Osborne, L.E.; Timmerman, A.; Wegulo, S.N. Occurrence and Distribution of Triticum Mosaic Virus in the Central Great Plains. Plant Dis. 2013, 97, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.C.; Coutts, B.A.; Mackie, A.E.; Dwyer, G.I. Seed Transmission of Wheat Streak Mosaic Virus Shown Unequivocally in Wheat. Plant Dis. 2005, 89, 1048–1050. [Google Scholar] [CrossRef]
- Gautam, S.; Chinnaiah, S.; Herron, B.; Workneh, F.; Rush, C.M.; Gadhave, K.R. Seed Transmission of Wheat Streak Mosaic Virus and Triticum Mosaic Virus in Differentially Resistant Wheat Cultivars. Viruses 2023, 15, 1774. [Google Scholar] [CrossRef]
- Price, J.A.; Smith, J.; Simmons, A.; Fellers, J.; Rush, C.M. Multiplex Real-Time RT-PCR for Detection of Wheat Streak Mosaic Virus and Tritcum Mosaic Virus. J. Virol. Methods 2010, 165, 198–201. [Google Scholar] [CrossRef]
- Tatineni, S.; Graybosch, R.A.; Hein, G.L.; Wegulo, S.N.; French, R. Wheat Cultivar-Specific Disease Synergism and Alteration of Virus Accumulation during Co-Infection with Wheat Streak Mosaic Virus and Triticum Mosaic Virus. Phytopathology 2010, 100, 230–238. [Google Scholar] [CrossRef]
- Bryan, B.; Paetzold, L.; Workneh, F.; Rush, C.M. Incidence of Mite-Vectored Viruses of Wheat in the Texas High Plains and Interactions with Their Host and Vector. Plant Dis. 2019, 103, 2996–3001. [Google Scholar] [CrossRef]
- Zarzyńska, A.; Jeżewska, M.; Trzmiel, K.; Hasiów-Jaroszewska, B. Development of a One-Step Immunocapture Real-Time RT-PCR Assay for the Detection of Barley Stripe Mosaic Virus Strains in Barley Seedlings. Acta Virol. 2014, 58, 81–85. [Google Scholar] [CrossRef]
- Zarzyńska-Nowak, A.; Hasiów-Jaroszewska, B.; Jeżewska, M. Molecular Analysis of Barley Stripe Mosaic Virus Isolates Differing in Their Biological Properties and the Development of Reverse Transcription Loop-Mediated Isothermal Amplification Assays for Their Detection. Arch. Virol. 2018, 163, 1163–1170. [Google Scholar] [CrossRef]
- Amraee, L.; Rahmani, F. Modified CTAB Protocol for RNA Extraction from Lemon Balm (Melissa Officinalis L.). Acta Agric. Slov. 2020, 115, 53–57. [Google Scholar] [CrossRef]
- Kúdela, O.; Kúdelová, M.; Nováková, S.; Glasa, M. First Report of Wheat Streak Mosaic Virus in Slovakia. Plant Dis. 2008, 92, 1365. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; Varlamov, A.; Vaskin, Y.; Efremov, I.; German Grehov, O.G.; Kandrov, D.; Rasputin, K.; Syabro, M.; et al. Unipro UGENE: A Unified Bioinformatics Toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.-M.; Spirason, N.; Iannello, P.; Jelley, L.; Lau, H.; Barr, I.G. A Simplified Sanger Sequencing Method for Full Genome Sequencing of Multiple Subtypes of Human Influenza A Viruses. J. Clin. Virol. 2015, 68, 43–48. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian Phylogenetic Inference under Mixed Models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian Inference of Phylogenetic Trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Martin, D.P.; Varsani, A.; Roumagnac, P.; Botha, G.; Maslamoney, S.; Schwab, T.; Kelz, Z.; Kumar, V.; Murrell, B. RDP5: A Computer Program for Analyzing Recombination in, and Removing Signals of Recombination from, Nucleotide Sequence Datasets. Virus Evol. 2021, 7, veaa087. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Rybicki, E. RDP: Detection of Recombination amongst Aligned Sequences. Bioinformatics 2000, 16, 562–563. [Google Scholar] [CrossRef] [PubMed]
- Padidam, M.; Sawyer, S.; Fauquet, C.M. Possible Emergence of New Geminiviruses by Frequent Recombination. Virology 1999, 265, 218–225. [Google Scholar] [CrossRef]
- Martin, D.P.; Posada, D.; Crandall, K.A.; Williamson, C. A Modified Bootscan Algorithm for Automated Identification of Recombinant Sequences and Recombination Breakpoints. AIDS Res. Hum. Retroviruses 2005, 21, 98–102. [Google Scholar] [CrossRef]
- Smith, J.M. Analyzing the Mosaic Structure of Genes. J. Mol. Evol. 1992, 34, 126–129. [Google Scholar] [CrossRef]
- Posada, D.; Crandall, K.A. Evaluation of Methods for Detecting Recombination from DNA Sequences: Computer Simulations. Proc. Natl. Acad. Sci. USA 2001, 98, 13757–13762. [Google Scholar] [CrossRef]
- Gibbs, M.J.; Armstrong, J.S.; Gibbs, A.J. Sister-Scanning: A Monte Carlo Procedure for Assessing Signals in Recombinant Sequences. Bioinformatics 2000, 16, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.M.; Ratmann, O.; Boni, M.F. Improved Algorithmic Complexity for the 3SEQ Recombination Detection Algorithm. Mol. Biol. Evol. 2018, 35, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Baldi, P.; La Porta, N. Molecular Approaches for Low-Cost Point-of-Care Pathogen Detection in Agriculture and Forestry. Front. Plant Sci. 2020, 11, 570862. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, L.K.; Adhab, M.; Aguirre-Rojas, L.M.; Timm, A.E. Occurrences of Wheat Curl Mite Aceria tosichella Keifer 1969 (Eriophyidae) and the Associated Viruses, (WSMV, HPWMoV, TRIMV) in Iraq. Iraqi J. Agric. Sci. 2023, 54, 837–849. [Google Scholar] [CrossRef]
- Snihur, H.; Pozhylov, I.; Budzanivska, I.; Shevchenko, O. First Report of High Plains Wheat Mosaic Virus on Different Hosts in Ukraine. J. Plant Pathol. 2020, 102, 545–546. [Google Scholar] [CrossRef]
- Nourbakhsh, F.; Massah, A.; Soorni, A.; Talaee, L. First Report of High Plains Wheat Mosaic Virus in Iran. New Dis. Rep. 2023, 47, e12188. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health (PLH); Bragard, C.; Baptista, P.; Chatzivassiliou, E.; Di Serio, F.; Gonthier, P.; Jaques Miret, J.A.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; et al. Pest Categorisation of High Plains Wheat Mosaic Virus. EFSA J. 2022, 20, e07302. [Google Scholar] [CrossRef]
- Lebas, B.S.M.; Ochoa-Corona, F.M.; Elliott, D.R.; Tang, Z.; Alexander, B.J.R. Development of an RT-PCR for High Plains Virus Indexing Scheme in New Zealand Post-Entry Quarantine. Plant Dis. 2005, 89, 1103–1108. [Google Scholar] [CrossRef]
- Robinson, M.D.; Murray, T.D. Genetic Variation of Wheat Streak Mosaic Virus in the United States Pacific Northwest. Phytopathology 2013, 103, 98–104. [Google Scholar] [CrossRef]
- Orlob, G.B. Feeding and Transmission Characteristics of Aceria tulipae Keifer as Vector of Wheat Streak Mosaic Virus1). J. Phytopathol. 1966, 55, 218–238. [Google Scholar] [CrossRef]
- State Revenue Committee of the Ministry of Finance of the Republic of Kazakhstan. Available online: https://kgd.gov.kz/en/exp_trade_files (accessed on 18 November 2023).
- Omar, S.; Easa, Z.; Abed-Alsaed, A. First Report of Barley Stripe Mosaic Virus Infecting Barley (Hordem Vulgare L.) In Al-Jabal Al-Akhdar- Libya. AlQalam J. Med. Appl. Sci. 2023, 6, 408–415. [Google Scholar]
- Oliveira-Hofman, C.; Wegulo, S.N.; Tatineni, S.; Hein, G.L. Impact of Wheat Streak Mosaic Virus and Triticum Mosaic Virus Coinfection of Wheat on Transmission Rates by Wheat Curl Mites. Plant Dis. 2015, 99, 1170–1174. [Google Scholar] [CrossRef]

| Primer Name | Target Virus | Target Region | Primer Sequence | Tm (°C) | Product Size (bp) | Source |
|---|---|---|---|---|---|---|
| WSMV-8581F | WSMV | coat protein | CGACAAGATAAAGCCTGAAT | 54 | 274 | This study |
| WSMV-8783R | AGTGCTCTGTTCTCTGG | |||||
| BSMV-2763F | BSMV | γB protein | AGAAGAAGATGCAGGAGCTGA | 58 | 134 | This study |
| BSMV-2868R | AAGAATCATCACATCCAACAG | |||||
| WS-8166F | WSMV | coat protein | GAGAGCAATACTGCGTGTACG | 45 | 750 | [62] |
| WS-8909R | GCATAATGGCTCGAAGTGATG | |||||
| WSMV | WSMV | coat protein | GTTGGGAGGCTTAATTGAAGTG | 55 | 720 | [43] |
| CAGCCATTACTCGTGTTATCCA | ||||||
| TGB2F | BSMV | γB protein | GGATGAAGACCACAGTTGGTTC | 55 | 397 | [59] |
| TGB2R | CTAGCCAATATCGCATAGTAATG | |||||
| TriMV | TriMV | PIPO region | CTTAAGCACATGTTACAATC | 45 | 1200 | [43] |
| GTCCCTGATAACTAATTCTA | ||||||
| WMoV | HPWMoV | RNA3 | GTTCCAATTCCTGTGCTTGATCTGTC | 45 | 490 | [43] |
| AACAATGACATAGCAATTACCTCAGCA |
| Set | Protein | Primer | Sequence (5′-3′) |
|---|---|---|---|
| 1 | CP | F3 | GCAGCAACTGATGCTGTCT |
| B3 | CTTGACCAGTCTTGGCTCC | ||
| FIP | GCCGTGCTTGCACTCAGACTCGCAAATGCGGAAGTGGCA | ||
| BIP | ACCATCAGGATCAGGTTCCGGATGACCGACACGTTGCTAGA | ||
| LB | GCGGGTGGATCAGGTTCAG | ||
| LF | GCGTGCTTCCACTACTCG | ||
| 2 | CP | F3 | CGAGTGAGCAGCAACTGA |
| B3 | GACACGTTGCTAGACTGTGT | ||
| FIP | GACTCGACTGCGTGCTTCCACTCTGGCAGCAGCAAATGC | ||
| BIP | CAAGCACGGCAAGCGGATCAGATCCTGAACCTGATCCACC | ||
| LB | TCACCATCAGGATCAGGTTCCG | ||
| 3 | NIb | F3 | GCATACTACAAGGACGTGTT |
| B3 | TTTCTTCCCTGAATATAACGC | ||
| FIP | TAGTTGCAACCACTGCCTTCGGTACAACAAAGATATAATTGTCGGA | ||
| BIP | GAGATAGCCGGGTTCTCAAAGGGCATCCAGATTCAAGTCGTT | ||
| LB | AAAGCACACTTCATCAGTTCC | ||
| LF | GACAATTTCAGCCCATCTTTGA |
| Target Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| WSMV coat protein | TCGAGTAGTGGAAGCACTCAGTC | CATGACAGATACGTTATTAGATTG |
| TCATGCGCGGTGCAGATGACACA | ATGACGTGAGTTGTCCTCATTAG | |
| BSMV γB | ATGATGGCTACTTTCTCTTGTGT | TTACAACTTAGAAACGGAAGAAG |
| TTCTTGTTTCAGAATATACATGC | GCTAAGCTTGAAAGTGAGGTTAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapytina, A.; Kolchenko, M.; Kerimbek, N.; Pozharskiy, A.S.; Nizamdinova, G.; Taskuzhina, A.; Adilbayeva, K.; Khusnitdinova, M.; Amidullayeva, M.; Moisseyev, R.; et al. Distribution of Wheat-Infecting Viruses and Genetic Variability of Wheat Streak Mosaic Virus and Barley Stripe Mosaic Virus in Kazakhstan. Viruses 2024, 16, 96. https://doi.org/10.3390/v16010096
Kapytina A, Kolchenko M, Kerimbek N, Pozharskiy AS, Nizamdinova G, Taskuzhina A, Adilbayeva K, Khusnitdinova M, Amidullayeva M, Moisseyev R, et al. Distribution of Wheat-Infecting Viruses and Genetic Variability of Wheat Streak Mosaic Virus and Barley Stripe Mosaic Virus in Kazakhstan. Viruses. 2024; 16(1):96. https://doi.org/10.3390/v16010096
Chicago/Turabian StyleKapytina, Anastasiya, Mariya Kolchenko, Nazym Kerimbek, Alexandr S. Pozharskiy, Gulnaz Nizamdinova, Aisha Taskuzhina, Kamila Adilbayeva, Marina Khusnitdinova, Malika Amidullayeva, Ruslan Moisseyev, and et al. 2024. "Distribution of Wheat-Infecting Viruses and Genetic Variability of Wheat Streak Mosaic Virus and Barley Stripe Mosaic Virus in Kazakhstan" Viruses 16, no. 1: 96. https://doi.org/10.3390/v16010096
APA StyleKapytina, A., Kolchenko, M., Kerimbek, N., Pozharskiy, A. S., Nizamdinova, G., Taskuzhina, A., Adilbayeva, K., Khusnitdinova, M., Amidullayeva, M., Moisseyev, R., Kachiyeva, Z., & Gritsenko, D. (2024). Distribution of Wheat-Infecting Viruses and Genetic Variability of Wheat Streak Mosaic Virus and Barley Stripe Mosaic Virus in Kazakhstan. Viruses, 16(1), 96. https://doi.org/10.3390/v16010096






