Chlorine Dioxide: Antiviral That Reduces the Spread of ToBRFV in Tomato (Solanum lycopersicum L.) Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Inoculum
Identification of ToBRFV by RT-PCR
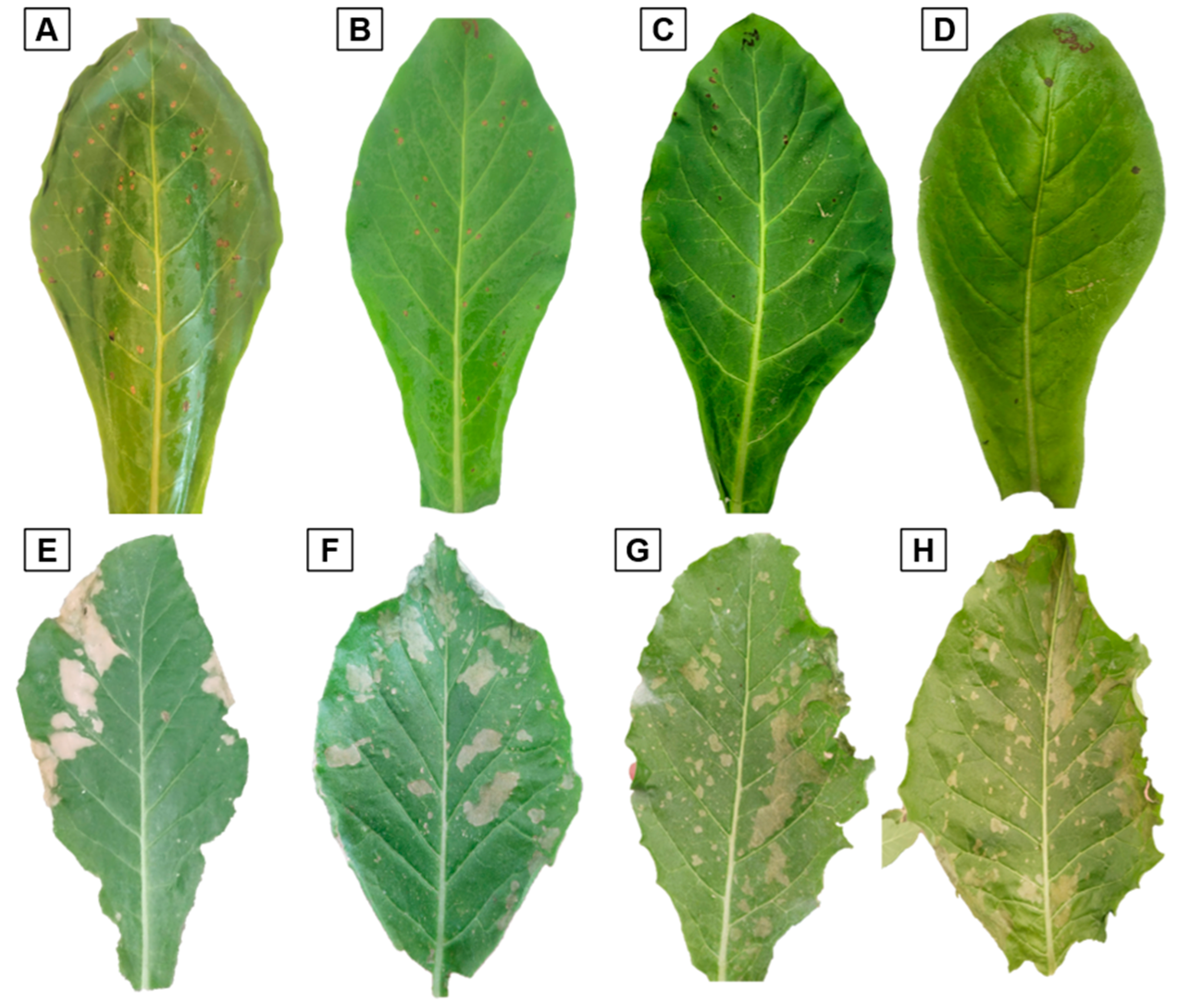
2.2. Determination of Phytotoxicity and Effectiveness of Chlorine Dioxide on Nicotiana longiflora
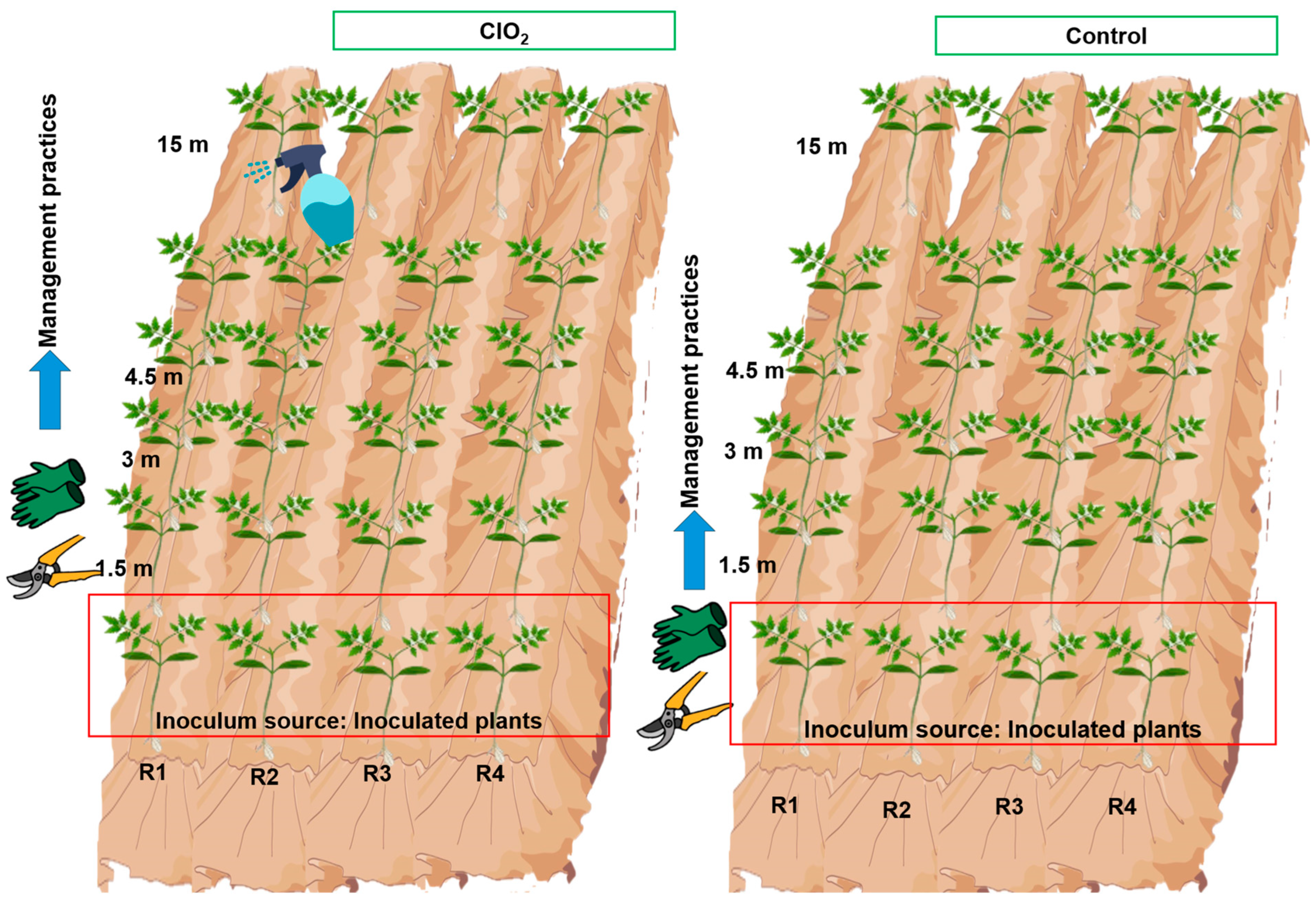
2.3. Experiment under Open-Field Conditions
2.3.1. Evaluation of the Effect of ClO2 on ToBRFV Propagation vs. Distance from the Inoculum Source
2.3.2. Estimation of the Area under the Curve of ToBRFV Disease Progression
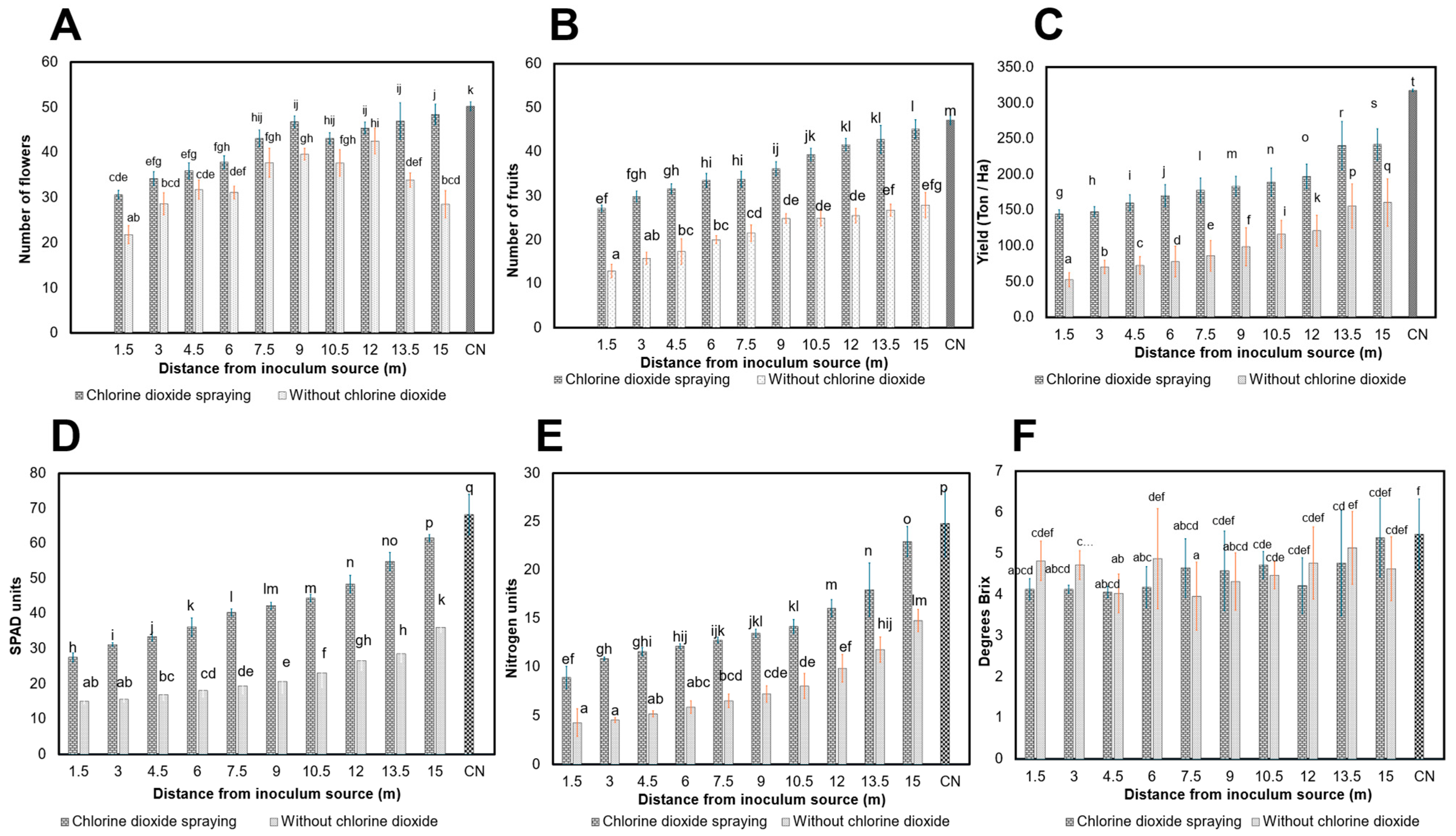
2.3.3. Agronomic Variables
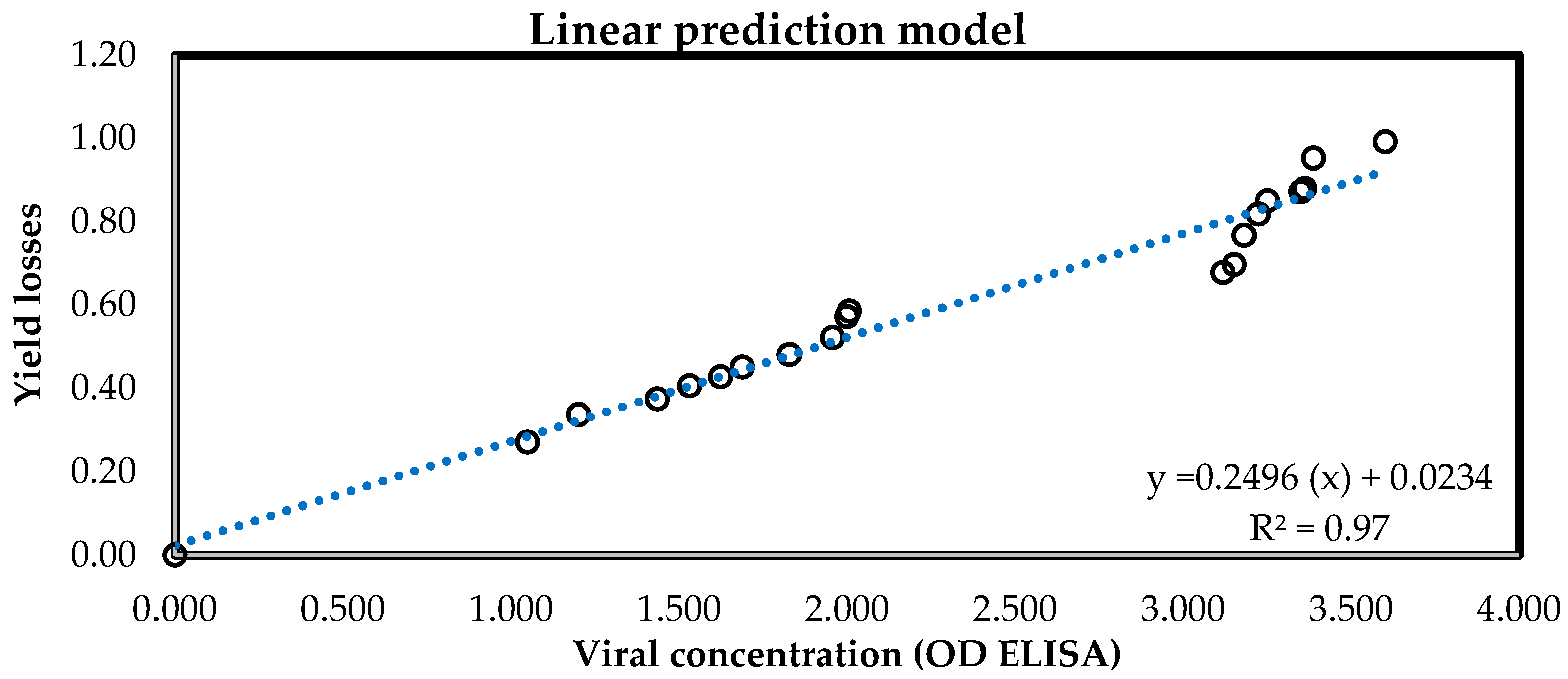
2.3.4. Predicting Tomato Yield Losses in the Open Field
2.4. Experiment under Greenhouse Conditions
2.4.1. Effect of ClO2 on the Propagation of ToBRFV vs. Viral Dilutions of the Inoculum
2.4.2. Estimation of ABCPE by ToBRFV
2.4.3. Evaluation of Agronomic Variables
3. Results
3.1. Identification of Inoculum Source ToBRFV
3.2. Phytotoxicity and Effectiveness of Chlorine Dioxide on Nicotiana longiflora
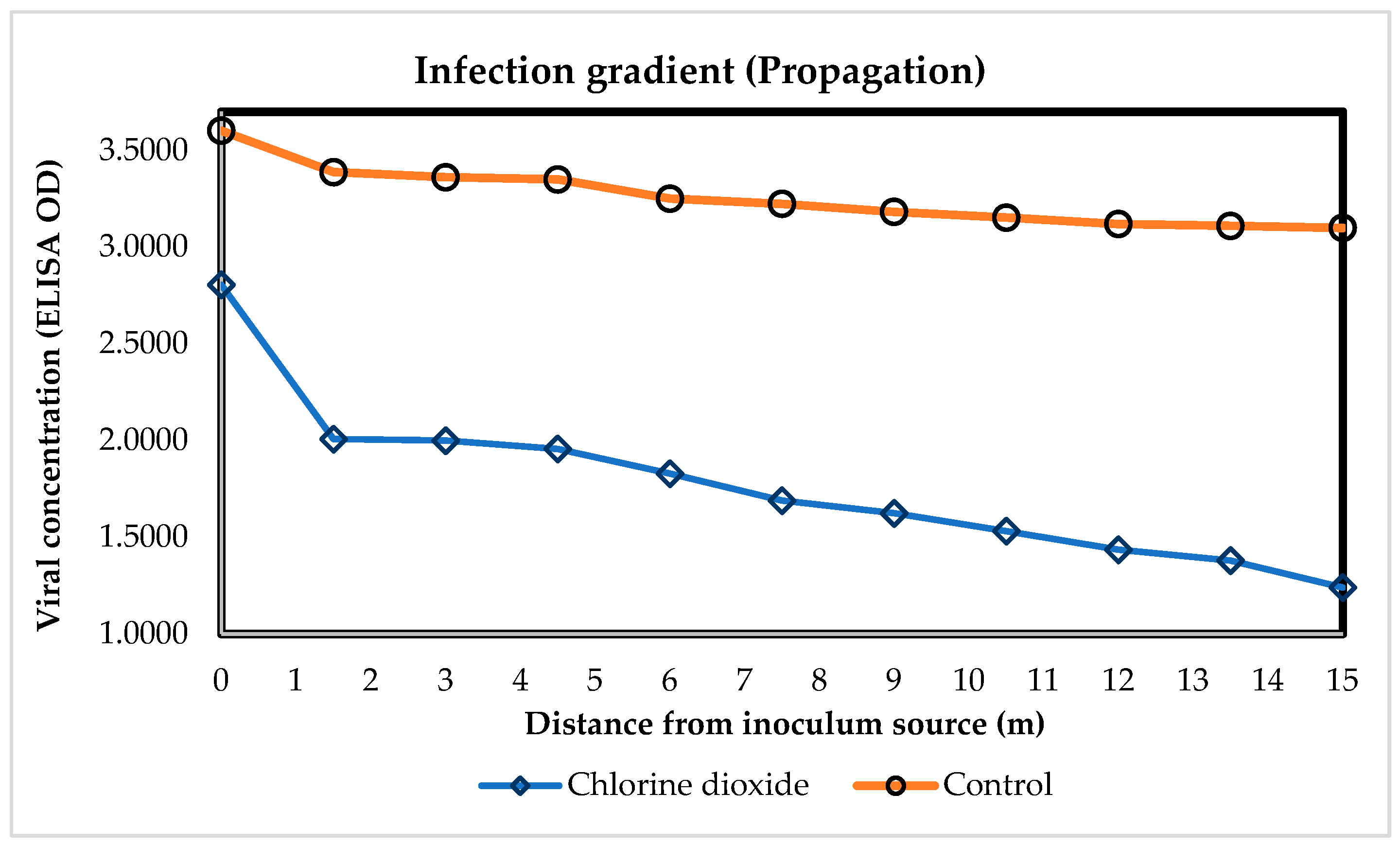
3.3. Open Field: Effect of ClO2 on the Spread of ToBRFV vs. Distance from the Inoculum Source
3.3.1. AUCDP by ToBRFV in Tomato Plants Distant from the Inoculum Source
3.3.2. Effect of ClO2 on Agronomic Variables of Tomato Plants
3.3.3. Estimation of Yield Losses of Plants Grown in the Open Air
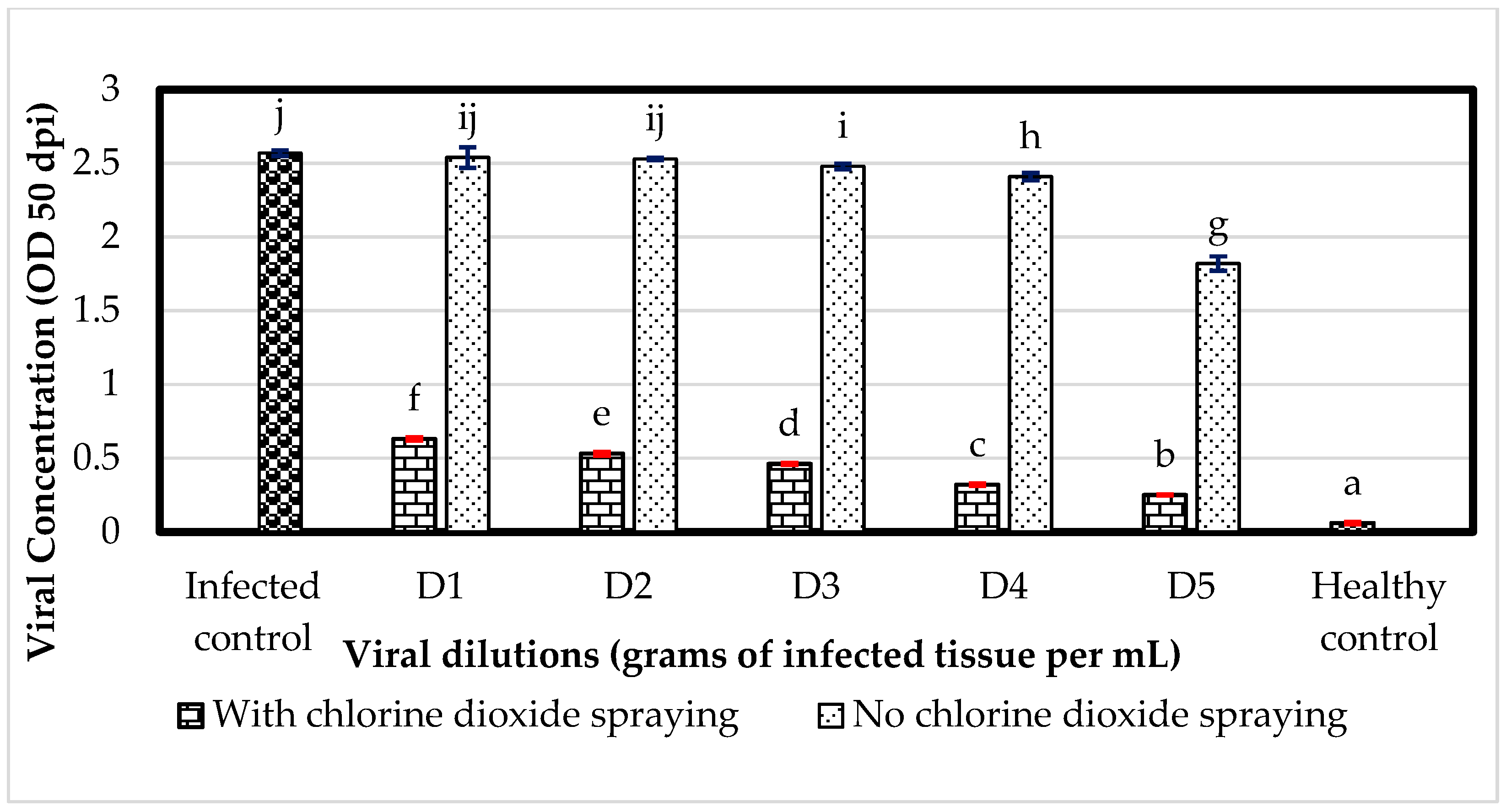
3.4. Greenhouse: Effect of ClO2 on the Spread of ToBRFV with Respect to Infection by Viral Dilutions
3.4.1. AUCDP by ToBRFV
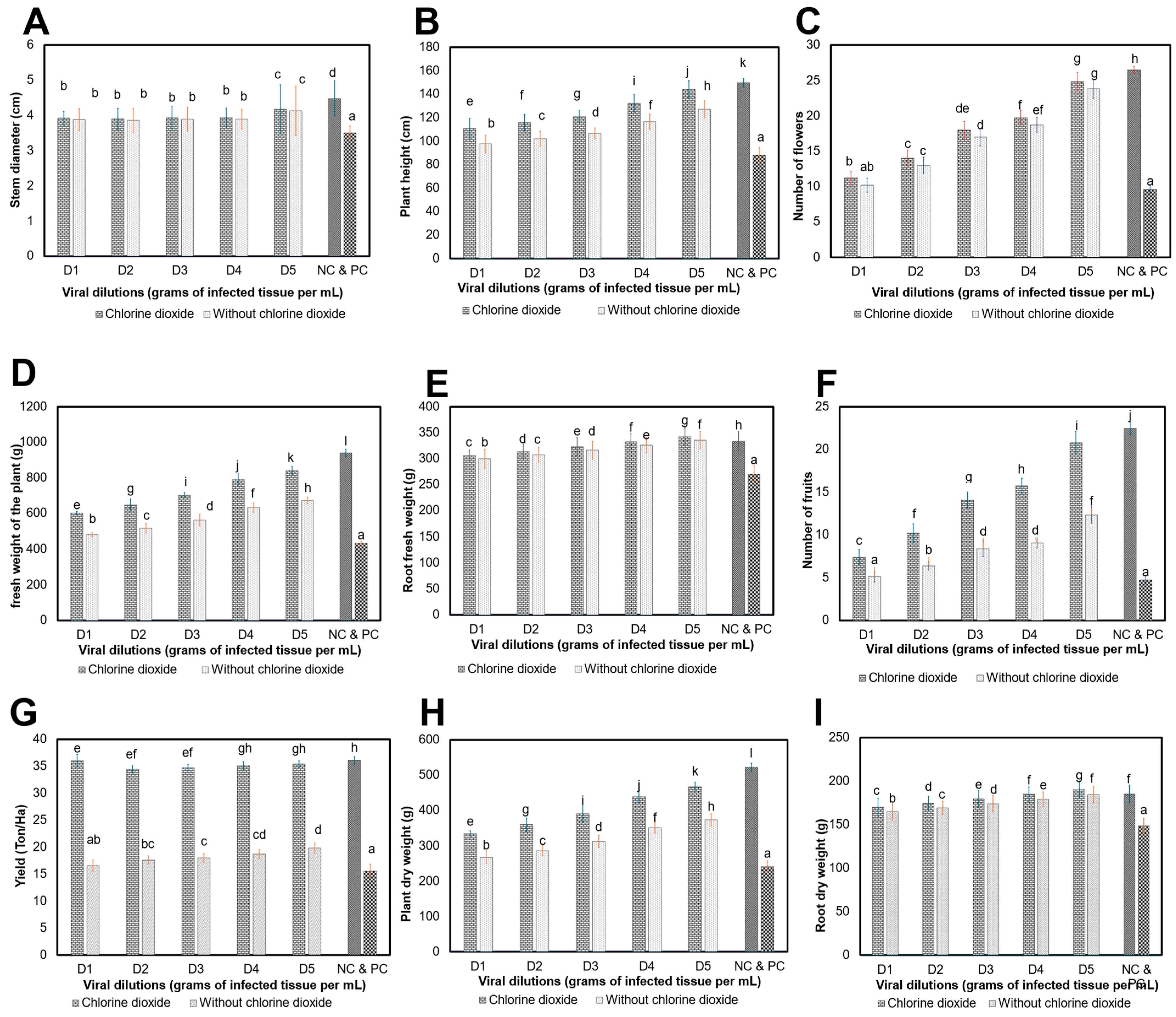
3.4.2. Effect of ClO2 on Agronomic Parameters of Plants Grown in Greenhouse
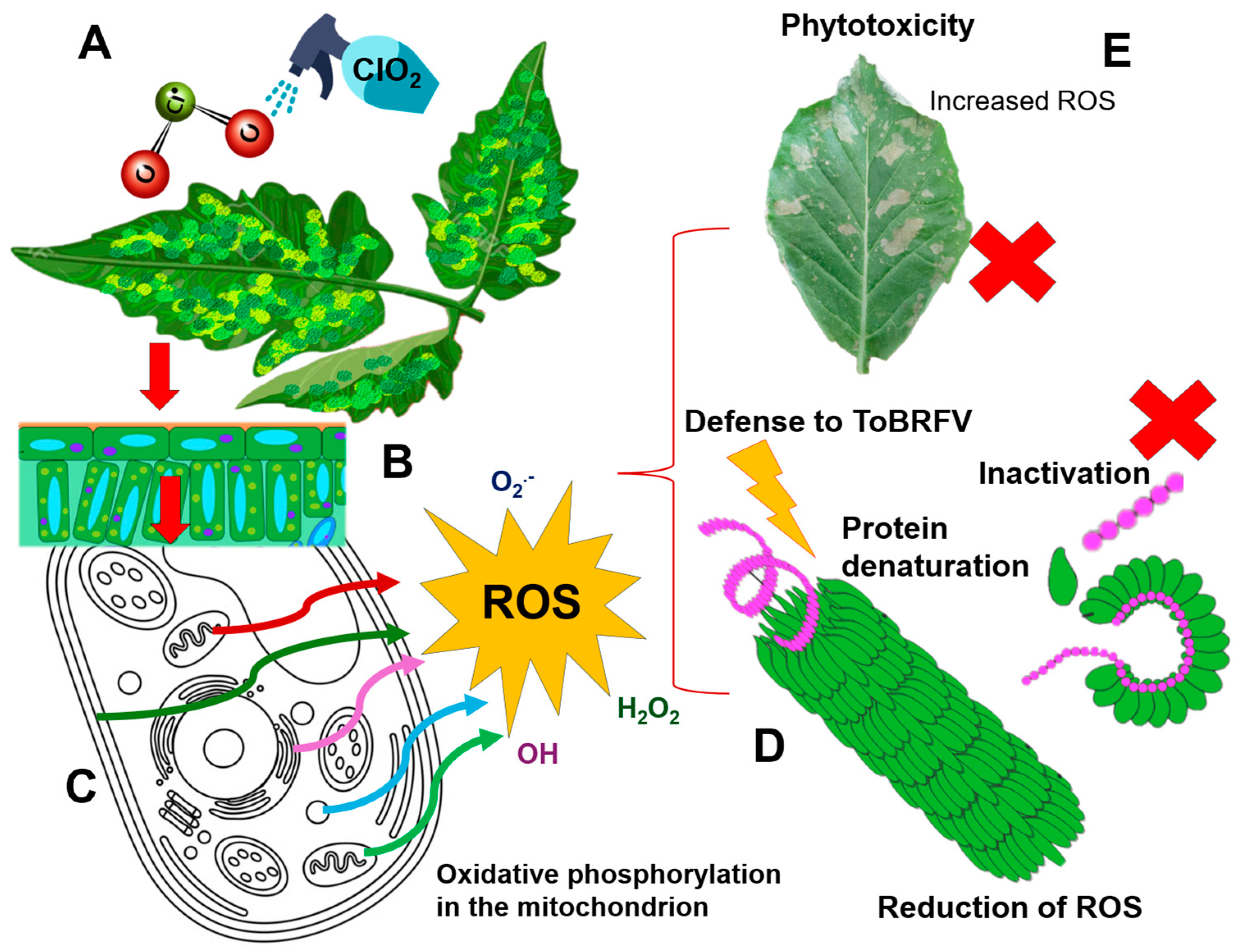
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruíz-Orona-Castillo, I.; Del-Toro-Sánchez, C.L.; Fortis-Hernández, M.; Preciado-Rangel, P.; Espinoza-Arellano, J.J.; Rueda-Puente, E.; Flores-Vázquez, M.; Cano-Ríos, P. Indicadores técnico-económicos de la producción del cultivo de tomate bajo agricultura protegida en la Comarca Lagunera, México. Biotecnia 2022, 24, 70–76. [Google Scholar] [CrossRef]
- SIAP. Anuario Estadístico de la Producción Agrícola. Servicio de Información Agroalimentaria y Pesquera. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 10 August 2024).
- González-Concha, L.F.; Ramírez-Gil, J.G.; Mora-Romero, G.A.; García-Estrada, R.S.; Carrillo-Fasio, J.A.; Tovar-Pedraza, J.M. Development of a scale for assessment of disease severity and impact of tomato brown rugose fruit virus on tomato yield. Eur. J. Plant Pathol. 2023, 165, 579–592. [Google Scholar] [CrossRef]
- Aiewsaku, P.; Katzourakis, A. Time-dependent rate phenomenon in viruses. J. Virol. 2016, 90, 7184–7195. [Google Scholar] [CrossRef]
- Zhang, S.; Griffiths, J.S.; Marchand, G.; Bernards, M.A.; Wang, A. Tomato brown rugose fruit virus: An emerging and rapidly spreading plant RNA virus that threatens tomato production worldwide. Mol. Plant Pathol. 2022, 23, 1262–1277. [Google Scholar] [CrossRef]
- Hanssen, I.M.; Lapidot, M.; Thomma, B.P.H.J. Emerging Viral Diseases of Tomato Crops. Mol. Plant-Microbe Interact. 2010, 23, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kumar, S.; Cheon, W.; Eo, H.; Kwon, H.; Jeon, Y.; Jung, J.; Kim, W. Anticancer and Antiviral Activity of Chlorine Dioxide by Its Induction of the Reactive Oxygen Species. J. Appl. Biol. Chem. 2017, 59, 31–36. [Google Scholar] [CrossRef]
- Peredo-Lovillo, A.; Romero-Luna, H.E.; Juárez-Trujillo, N.; Jiménez-Fernández, M. Antimicrobial efficiency of chlorine dioxide and its potential use as anti-SARS-CoV-2 agent: Mechanisms of action and interactions with gut microbiota. J. Appl. Microbiol. 2023, 134, lxad133. [Google Scholar] [CrossRef]
- Wang, Z.; Liao, F.; Lin, J.; Li, W.; Zhong, Y.; Tan, P.; Huang, Z. Inactivation and mechanisms of chlorine dioxide on Nosema bombycis. J. Invertebr. Pathol. 2010, 104, 134–139. [Google Scholar] [CrossRef]
- Fu, M.-R.; Zhang, X.-M.; Jin, T.; Li, B.-Q.; Zhang, Z.-Q.; Tian, S.-P. Inhibitory of grey mold on green pepper and jujube by chlorine dioxide (ClO2) fumigation and its mechanisms. LWT 2019, 100, 335–400. [Google Scholar] [CrossRef]
- Arellano-Gutiérrez, G.; Aldana-Zaragoza, E.H.; Pérez-Fabián, A. Intestinal perforation associated with chlorine dioxide ingestion: And adult chronic consumer during COVID-19 pandemic. Clin. J. Gastroenterol. 2021, 14, 1655–1660. [Google Scholar] [CrossRef]
- Insignares-Carrione, E.; Bolano Gómez, B.; Andrade, Y.; Callisperis, P.; Suxo, A.M.; Ajata-San Martin, A.B.; Ostria-Gonzalez, C. Determination of the effectiveness of chlorine dioxide in the treatment of COVID-19. J. Mol. Genet. Med. 2021, 15, 2–11. [Google Scholar]
- Davino, S.; Caruso, A.G.; Bertacca, S.; Barone, S.; Panno, S. Tomato brown rugose fruit virus: Seed transmission rate and efficacy of different seed disinfection treatments. Plants 2000, 9, 1615. [Google Scholar] [CrossRef] [PubMed]
- Fidan, H.; Ulusoy, D.; Albezirgan, H.N. Exploring Effective Strategies for ToBRFV Management in Tomato Production: Insights into Seed Transmission Dynamics and Innovative Control Approaches. Agriculture 2024, 14, 108. [Google Scholar] [CrossRef]
- Chanda, B.; Shamimuzzaman, M.; Gilliard, A.; Ling, K.S. Effectiveness of disinfectants against the spread of tobamoviruses: Tomato brown rugose fruit virus and Cucumber green mottle mosaic virus. Virol. J. 2021, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.; Zarghani, S.N.; Kroschewski, B.; Büttner, C.; Bandte, M. Cleaning of Tomato brown rugose fruit virus (ToBRFV) from Contaminated Clothing of Greenhouse Employees. Horticulturae 2022, 8, 751. [Google Scholar] [CrossRef]
- Ehlers, J.; Zarghani, S.N.; Kroschewski, B.; Büttner, C.; Bandte, M. Decontamination of Tomato Brown Rugose Fruit Virus-Contaminated Shoe Soles under Practical Conditions. Horticulturae 2022, 8, 1210. [Google Scholar] [CrossRef]
- Ling, K.S.; Gilliard, A.C.; Zia, B. Disinfectants Useful to Manage the Emerging Tomato Brown Rugose Fruit Virus in Greenhouse Tomato Production. Horticulturae 2022, 8, 1193. [Google Scholar] [CrossRef]
- Nourinejhad Zarghani, S.; Ehlers, J.; Monavari, M.; von Bargen, S.; Hamacher, J.; Büttner, C.; Bandte, M. Applicability of Different Methods for Quantifying Virucidal Efficacy Using MENNO Florades and Tomato Brown Rugose Fruit Virus as an Example. Plants 2023, 12, 894. [Google Scholar] [CrossRef]
- Mani, K.A.; Berenice, M.; Cohen, R.; Feldbaum-Amar, R.; Dombrovsky, A.; Mechrez, G. Biocompatible antiviral Pickering emulsion-based formulation for plant root protection from tobamovirus-infected soil. Polym. Adv. Technol. 2024, 35, e6293. [Google Scholar] [CrossRef]
- Nissim, M.; Lline-Vul, T.; Shoshani, S.; Jacobi, G.; Malka, E.; Dombrovsky, A.; Margel, S. Synthesis and Characterization of Durable Antibiofilm and Antiviral Silane-Phosphonium Thin Coatings for Medical and Agricultural Applications. ACS Omega 2023, 8, 39354–39365. [Google Scholar] [CrossRef]
- Tian, C.; Xie, Z.; Zhao, Y.; Zhang, Z.; Xue, T.; Sheng, W.; Zhao, F.; Duan, Y. Microgram-grade concentration of chlorine dioxide induces one-step plant regeneration in chrysanthemum. In Vitro Cell. Dev. Biol.-Plant 2022, 58, 1138–1144. [Google Scholar] [CrossRef]
- Campagna, M.V.; Faure-Kumar, E.; Treger, J.A.; Cushman, J.D.; Grogan, T.R.; Kasahara, N.; Lawson, G.W. Factors in the selection of surface disinfectants for use in laboratory animal setting. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 175–188. [Google Scholar] [PubMed]
- Carvajal, C.C. Especies Reactivas del Oxígeno: Formación, Función y Estrés Oxidativo. Med. Leg. Costa Rica 2019, 36, 91–100. Available online: http://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S1409-00152019000100091&lng=en&tlng=es (accessed on 23 July 2024).
- Medina-Avitia, E.; Tella-Vega, P.; García-Estrada, C. Acute kidney injury secondary to chlorine dioxide use for COVID-19 prevention. Hemodial. Int. 2021, 25, E40–E43. [Google Scholar] [CrossRef]
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.W.; Turina, M. A new Tobamovirus infecting tomato crops in Jordan. Arch. Virol. 2016, 161, 503–506. [Google Scholar] [CrossRef]
- Luria, N.; Smith, E.; Reingold, V.; Bekelman, I.; Lapidot, M.; Levin, I.; Elad, N.; Tam, Y.; Sela, N.; Abu-Ras, A.; et al. A New Israeli Tobamovirus Isolate Infects Tomato Plants Harboring Tm-22 Resistance Genes. PLoS ONE 2017, 12, e0170429. [Google Scholar] [CrossRef]
- Ortiz-Martínez, L.E.; Ochoa-Martínez, D.L.; Rojas-Martínez, R.I.; Aranda-Ocampo, S.; Cruz, M.Á.G. Respuesta de variedades de chile a la infección con Tomato brown rugose fruit virus. Summa Phytopathol. 2022, 47, 209–215. [Google Scholar] [CrossRef]
- Rodríguez-Mendoza, J.; García-Ávila, C.J.; López-Buenfil, J.A.; Araujo-Ruiz, K.; Quezada-Salinas, A.; Cambrón-Crisantos, J.M.; Ochoa-Martínez, D.L. Identificación de Tomato brown rugose fruit virus por RT-PCR de una región codificante de la replicasa (RdRP). Rev. Mex. Fitopatol. 2019, 37, 345–356. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- Kone, N.; Asare-Bediako, E.; Silue, S.; Kone, D.; Koita, O.; Menzel, W.; Winter, S. Influence of planting date on incidence and severity of viral disease on cucurbits under field condition. Ann. Agric. Sci. 2017, 62, 99–104. [Google Scholar] [CrossRef]
- Vásquez-Gutiérrez, U.; Frías-Treviño, G.A.; López-López, H.; Delgado-Ortiz, J.C.; Aguirre-Uribe, L.A.; Flores-Olivas, A. Evaluation of the Pathogenicity of Three Isolates of Tomato brown rugose fruit virus in tomato plants (Solanum lycopersicum L.) from Coahuila, Mexico. Rev. Bio Cienc. 2024, 11, e1576. [Google Scholar] [CrossRef]
- Shaner, G. The Effect of Nitrogen Fertilization on the Expression of Slow-Mildewing Resistance in Knox Wheat. Phytopathology 1977, 67, 1051–1056. [Google Scholar] [CrossRef]
- Berger, R.D.; Luke, H.H. Spatial and temporal spread of oat crown rust. Phytopathology 1979, 69, 1199–1201. [Google Scholar] [CrossRef]
- Berger, R.D.; Jones, J.W. A general model for disease progress with functions for variable latency and lesion expansion on growing host plants. Phytopathology 1985, 75, 792. [Google Scholar] [CrossRef]
- Minogue, K.P.; Fry, W.E. Models for the spread of disease: Model description. Phytopathology 1983, 73, 1168–1173. [Google Scholar] [CrossRef]
- Madden, L.V.; Campbell, C.L. Nonlinear Disease Progress Curves. In Epidemics of Plant Diseases. Ecological Studies; Kranz, J., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; Volume 13. [Google Scholar] [CrossRef]
- Madden, L.V.; Nutter, F.W. Modeling crop losses at the field scale. Can. J. Plant Pathol. 1995, 17, 124–137. [Google Scholar] [CrossRef]
- Ling, K.S. Recent emergence of seed-borne viruses and viroids on tomato, seed health tests and their implications in global seed trade. Acta Hortic. 2021, 1316, 127–134. [Google Scholar] [CrossRef]
- Zarghani, S.N.; Monavari, M.; Ehlers, J.; Hamacher, J.; Büttner, C.; Bandte, M. Comparison of Models for Quantification of Tomato Brown Rugose Fruit Virus Based on a Bioassay Using a Local Lesion Host. Plants 2023, 11, 3443. [Google Scholar] [CrossRef]
- Cayanan, D.F.; Zhang, P.; Liu, W.; Dixon, M.; Zheng, Y. Efficacy of chlorine in controlling five common plant pathogens. HortScience 2009, 44, 157–163. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, C.I.; Zamora-Macorra, E.J.; Ochoa Martínez, D.L.; González-Garza, R. Disinfectants effectiveness in Tomato brown rugose fruit virus (ToBRFV) transmission in tobacco plants. Mex. J. Phytopathol. 2022, 40, 240–253. [Google Scholar] [CrossRef]
- Vásquez-Gutiérrez, U.; López-López, H.; Frías-Treviño, G.A.; Delgado-Ortiz, J.C.; Flores-Olivas, A.; Aguirre-Uribe, L.A.; Hernández-Juarez, A. Biological Exploration and Physicochemical Characteristics of Tomato Brown Rugose Fruit Virus in Several Host Crops. Agronomy 2024, 14, 388. [Google Scholar] [CrossRef]
- Van der Plank, J.E. Plant Diseases: Epidemics and Control; Elsevier Science: New York, NY, USA, 1963; Volume 349, ISBN 978-0-12-711450-7. [Google Scholar] [CrossRef]
- Ogata, N. Denaturation of protein by chlorine dioxide: Oxidative modification of tryptophan and tyrosine residues. Biochemistry 2007, 46, 4898–4911. [Google Scholar] [CrossRef]
- Samarah, N.; Sulaiman, A.; Salem, N.M.; Turina, M. Disinfection treatments eliminated tomato brown rugose fruit virus in tomato seeds. Eur. J. Plant Pathol. 2021, 159, 153–162. [Google Scholar] [CrossRef]
- Dudek-Wicher, R.; Brozyna, M.; Paleczny, J.; Maczynska, B.; Dudek, B.; Migdal, P.; Junka, A. No Miracle, Just a Mineral: The Not-So-Magical Antimicrobial World of Chlorine Dioxide. bioRxiv 2024, 15. [Google Scholar] [CrossRef]
- Zhou, J.; Gilliard, A.; Ling, K.-S. Tomato Brown Rugose Fruit Virus Is Transmissible through a Greenhouse Hydroponic System but May Be Inactivated by Cold Plasma Ozone Treatment. Horticulturae 2024, 10, 416. [Google Scholar] [CrossRef]
- Molad, O.; Smith, E.; Luria, N.; Bakelman, E.; Lachman, O.; Reches, M.; Dombrovsky, A. Studying tomato brown rugose fruit virus longevity in soil and virion susceptibility to pH treatments helped improve virus control by soil disinfection. Plant Soil 2024, 1–16. [Google Scholar] [CrossRef]
- Jin, M.; Shan, J.; Chen, Z.; Guo, X.; Shen, Z.; Qiu, Z. Chlorine dioxide inactivation of enterovirus 71 in water and its impact on genomic targets. Environ. Sci. Technol. 2013, 7, 4590–4597. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Moon, J.C.; Kim, C.; Manoharan, K.; Kim, W. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011, 5, 709–725. [Google Scholar]
- Fraire-Velázquez, S.; Balderas-Hernández, V.E. Abiotic Stress in Plants and Metabolic Responses; IntechOpen: London, UK, 2013; Volume 10, pp. 25–48. [Google Scholar]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 2012, 1819, 86–96. [Google Scholar] [CrossRef]
- Boghossian, A.A.; Sen, F.; Gibbons, B.M.; Sen, S.; Faltermeier, S.M.; Giraldo, J.P.; Strano, M.S. Application of nanoparticle antioxidants to enable hyperstable chloroplasts for solar energy harvesting. Adv. Energy Mater. 2013, 3, 881–893. [Google Scholar] [CrossRef]
- López-Moreno, M.L.; Avilés, L.L.; Pérez, N.G.; Irizarry, B.Á.; Perales, O.; Cedeno-Mattei, Y.; Román, F. Effect of cobalt ferrite (CoFe2O4) nanoparticles on the growth and development of Lycopersicon lycopersicum (tomato plants). Sci. Total Environ. 2016, 550, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Shekhawat, G.S. Toxicity of ZnO engineered nanoparticles and evaluation of their effect on growth, metabolism and tissue specific accumulation in Brassica juncea. J. Environ. Chem. Eng. 2014, 2, 105–114. [Google Scholar] [CrossRef]
- Li, R.; Baysal-Gurel, F.; Abdo, Z. Evaluation of disinfectants to prevent mechanical transmission of viruses and a viroid in greenhouse tomato production. Virol. J. 2015, 12, 5. [Google Scholar] [CrossRef]
- Mink, G.I. Identification of rugose mosaic-diseased cherry trees by enzyme-linked immunosorbent assay. Plant Dis. 1980, 64, 691–694. [Google Scholar] [CrossRef][Green Version]
- Menzel, W.; Winter, S. Identification of novel and known Tobamoviruses in tomato and other solanaceous crops using a new pair of generic primers and development of a specific RT-qPCR for ToBRFV. ISHS Acta Hortic. 2019, 1316, 143–148. [Google Scholar] [CrossRef]
- Ortiz-Martínez, L.E.; Ochoa-Martínez, D.L. Elicitors and biostimulants in the production of tomato infected with Tomato brown rugose fruit virus. J. Plant Dis. Prot. 2023, 130, 351–360. [Google Scholar] [CrossRef]
- Avni, B.; Gelbart, D.; Sufrin-Ringwald, T.; Zemach, H.; Belausov, E.; Kamenetsky-Goldstein, R.; Lapidot, M. ToBRFV Infects the Reproductive Tissues of Tomato Plants but Is Not Transmitted to the Progenies by Pollination. Cells 2022, 11, 2864. [Google Scholar] [CrossRef]
- González-Concha, L.F.; Ramírez-Gil, J.G.; García-Estrada, R.S.; Rebollar-Alviter, Á.; Tovar-Pedraza, J.M. Spatiotemporal analyses of tomato brown rugose fruit virus in commercial tomato greenhouses. Agronomy 2021, 11, 1268. [Google Scholar] [CrossRef]
- Panno, S.; Caruso, A.G.; Barone, S.; Lo Bosco, G.; Rangel, E.A.; Davino, S. Spread of Tomato Brown Rugose Fruit Virus in Sicily and Evaluation of the Spatiotemporal Dispersion in Experimental Conditions. Agronomy 2020, 10, 834. [Google Scholar] [CrossRef]


| Models | Equation | Linearized Equation | Authors |
|---|---|---|---|
| Berger | [34,35] | ||
| inverted exponential | [36,37] | ||
| Exponential model | [37] | ||
| Monomolecular | |||
| Logistics | |||
| Line | - | [38] |
| Treatments | Phytotoxicity A | NLLs B |
|---|---|---|
| 100 mg L−1 ClO2 + ToBRFV | 0.00 ± 0.00 a | 29.28 ± 0.42 d |
| 250 mg L−1 ClO2 + ToBRFV | 0.00 ± 0.00 a | 19.61 ± 3.10 c |
| 500 mg L−1 ClO2 + ToBRFV | 0.00 ± 0.00 a | 9.76 ± 0.34 b |
| 760 mg L−1 ClO2 + ToBRFV | 0.00 ± 0.00 a | 1.53 ± 0.07 a |
| 1520 mg L−1 ClO2 + ToBRFV | 17.44 ± 0.82 b | 0.74 ± 0.09 a |
| 3040 mg L−1 ClO2 + ToBRFV | 26.55 ± 1.41 c | 0.36 ± 0.07 a |
| 6080 mg L−1 ClO2 + ToBRFV | 44.31 ± 0.85 d | 0.00 ± 0.00 a |
| Distilled H2O (1 × 100) | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Positive control | 0.00 ± 0.00 a | 38.13 ± 4.81 e |
| 6080 mg L−1 ClO2 | 43.68 ± 1.33 d | 0.00 ± 0.00 a |
| AUCDP | ||
|---|---|---|
| Distance | ClO2 | Control |
| IS | 310.67 ± 18.23 j | 374.09 ± 14.59 l |
| 1.5 | 102.77 ± 22.47 e | 369.09 ± 14.45 l |
| 3 | 94.54 ± 18.42 e | 358.50± 14.09 kl |
| 4.5 | 84.95 ± 18.61 de | 338.42 ± 13.61 k |
| 6 | 69.05 ± 17.42 cd | 282.15 ± 13.30 i |
| 7.5 | 55.35 ± 12.69 bc | 255.68 ± 1204 h |
| 9 | 45.58 ± 10.42 ab | 247.02 ± 11.82 h |
| 10.5 | 40.97 ± 10.08 ab | 243.57 ± 11.45 h |
| 12 | 38.62 ± 8.87 ab | 209.54 ± 11.50 g |
| 13.5 | 32.75 ± 9.69 a | 192.47 ± 10.87 fg |
| 15 | 26.62 ± 10.09 a | 181.66 ± 9.74 f |
| NC | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Models | Equation | Substituted Equation | R2 |
|---|---|---|---|
| Berger | 0.94 | ||
| Inverted exponential | 0.89 | ||
| Exponential | ] | 0.68 | |
| Monomolecular | 0.80 | ||
| Logistic | Y = | 0.81 | |
| Lineal | 0.97 |
| AUCDP | ||
|---|---|---|
| Treatments | ClO2 | Control |
| NC 1 × 100 | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| 1 × 10−3 | 105.26 ± 1.45 g | 220.91 ± 4.16 j |
| 1 × 10−3.5 | 78.80 ± 1.75 e | 167.64 ± 4.1 i |
| 1 × 10−4 | 56.93 ± 1.88 d | 122.77 ± 4.63 h |
| 1 × 10−4.5 | 43.32 ± 2.60 c | 94.92 ± 5.54 f |
| 1 × 10−5 | 34.86 ± 1.88 b | 76.35 ± 2.30 e |
| PC 1 × 101 | 309.10 ± 6.01 k | 309.10 ± 6.01 k |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, U.V.; Treviño, G.A.F.; Ortiz, J.C.D.; Uribe, L.A.A.; Olivas, A.F.; Beache, M.B.; Castillo, F.D.H. Chlorine Dioxide: Antiviral That Reduces the Spread of ToBRFV in Tomato (Solanum lycopersicum L.) Plants. Viruses 2024, 16, 1510. https://doi.org/10.3390/v16101510
Gutiérrez UV, Treviño GAF, Ortiz JCD, Uribe LAA, Olivas AF, Beache MB, Castillo FDH. Chlorine Dioxide: Antiviral That Reduces the Spread of ToBRFV in Tomato (Solanum lycopersicum L.) Plants. Viruses. 2024; 16(10):1510. https://doi.org/10.3390/v16101510
Chicago/Turabian StyleGutiérrez, Ubilfrido Vásquez, Gustavo Alberto Frías Treviño, Juan Carlos Delgado Ortiz, Luis Alberto Aguirre Uribe, Alberto Flores Olivas, Mariana Beltrán Beache, and Francisco Daniel Hernández Castillo. 2024. "Chlorine Dioxide: Antiviral That Reduces the Spread of ToBRFV in Tomato (Solanum lycopersicum L.) Plants" Viruses 16, no. 10: 1510. https://doi.org/10.3390/v16101510
APA StyleGutiérrez, U. V., Treviño, G. A. F., Ortiz, J. C. D., Uribe, L. A. A., Olivas, A. F., Beache, M. B., & Castillo, F. D. H. (2024). Chlorine Dioxide: Antiviral That Reduces the Spread of ToBRFV in Tomato (Solanum lycopersicum L.) Plants. Viruses, 16(10), 1510. https://doi.org/10.3390/v16101510








