A CRISPR-Cas13b System Degrades SARS-CoV and SARS-CoV-2 RNA In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culturing
2.2. Viral Strains
2.3. Biosafety
2.4. SARS-CoV-2 Genomes Used for gRNA Design
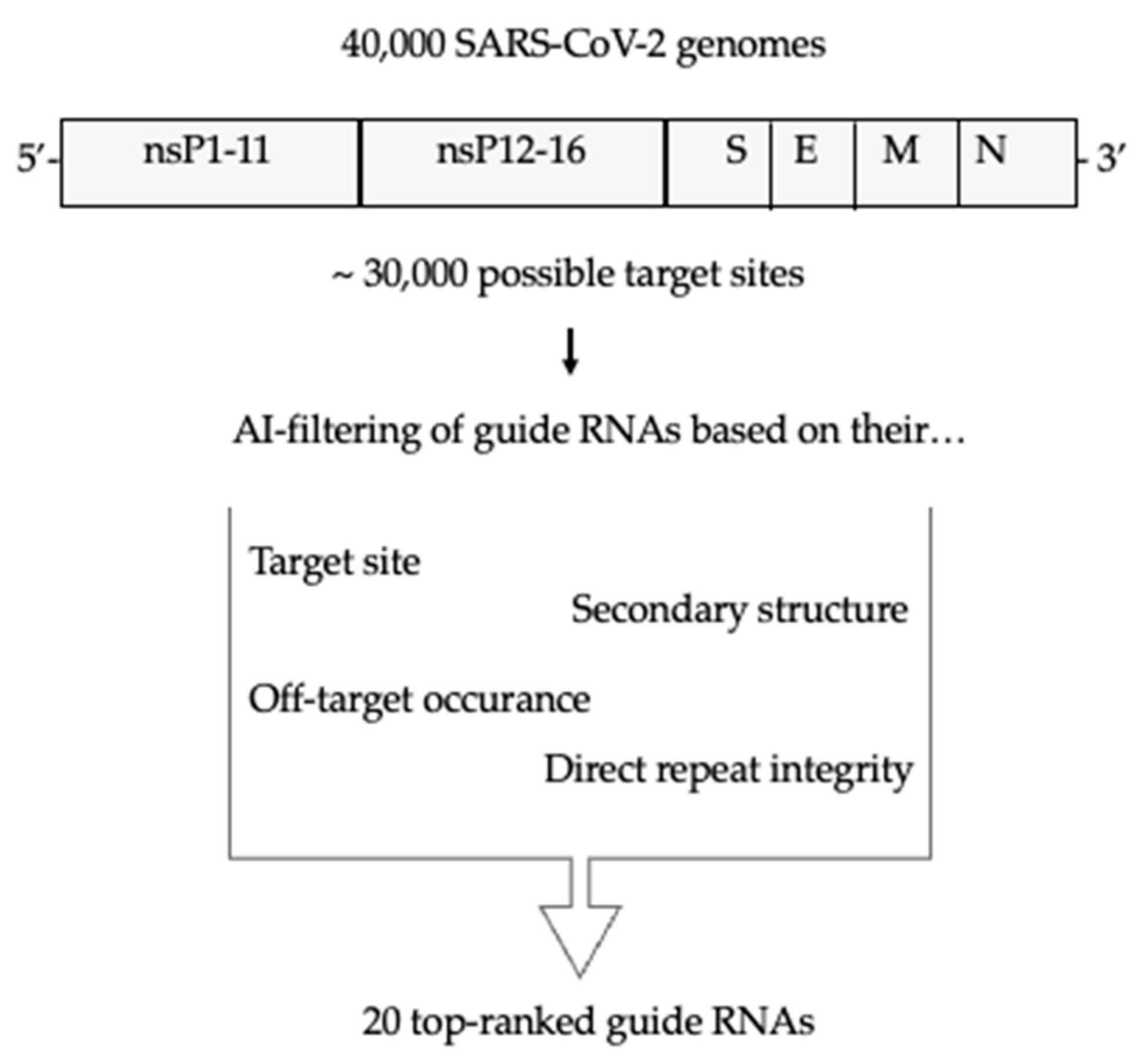
2.5. gRNA Design
2.6. Cloning of Guide RNA Plasmids
- M:
- 3′-CTGGACCTTCCACAACCCTTTTAATTGAATTAATATATGTTTTTGGGGTGTTTCGTTCCTTTCCACA-5′
- NSP3 (1):
- 3′-CTGGACCTTCCACAACCTGATAGTAGTAGATTGGTTAGAAGAAGAAGGTGTTTCGTTCCTTTCCACA-5′
- NSP3 (2):
- 3′-CTGGACCTTCCACAACGTTGAATCCCAGTTAAAGACATGTTTGTTGGGTGTTTCGTTCCTTTCCACA-5′
- NSP3 (3):
- 3′-CTGGACCTTCCACAACCCTCCCATTTTTCTTGTTATGTATACA CTTGGTGTTTCGTTCCTTTCCACA-5′
- NSP3 (4):
- 3′-CTGGACCTTCCACAACTAGGAAAGTAGTTCAAGTTTTCACTATAAGGGTGTTTCGTTCCTTTCCACA-5′
- N:
- 3′-CTGGACCTTCCACAACCCCTTAAATTCCAGAAGGAACG GTACAACTGGTGTTTCGTTCCTTTCCACA-5′
- NSP5 (1):
- 3′-CTGGACCTTCCACAACCCATTAAGGTATACCACGTACATTGTTTTTGGTGTTTCGTTCCTTTCCACA-5′
- NSP5 (2):
- 3′-CTGGACCTTCCACAACCTTGTAATGGTCGGACATGGTTCTTTAATAGGTGTTTCGTTCCTTTCCACA-5′
- NSP5 (3):
- 3′-CTGGACCTTCCACAACTTTTGCCGTTAAGGTCAAACTCGTCTTTCTGGTGTTTCGTTCCTTTCCACA-5′
- S (1):
- 3′-CTGGACCTTCCACAACCAGACTGAAGTAGTGGAGATTAATGTTTACGGTGTTTCGTTCCTTTCCACA-5′
- S (2):
- 3′-CTGGACCTTCCACAACCCACCACAAAACATTTAAACAA ACTGAACAGGTGTTTCGTTCCTTTCCACA-5′
- S (3):
- 3′-CTGGACCTTCCACAACTCCCATTATTTGTGGTGCACACTTTCTTAAGGTGTTTCGTTCCTTTCCACA-5′
- S (4):
- 3′-CTGGACCTTCCACAACCCCATTATTTGTGGTGCACACTTTCTTAATGGTGTTTCGTTCCTTTCCACA-5′
- S (5):
- 3′-CTGGACCTTCCACAACCCATTAAATATTAATATTAGTCGTTAGAAAGGTGTTTCGTTCCTTTCCACA-5′
- NSP12 (1):
- 3′-CTGGACCTTCCACAACCCGTAATTGTTACTTATTATTCTTAGATGTGGTGTTTCGTTCCTTTCCACA-5′
- NSP12 (2):
- 3′-CTGGACCTTCCACAACCTACTTTGACAGATAACCAGTATCATGATGGGTGTTTCGTTCCTTTCCACA-5′
- NSP12 (3):
- 3′-CTGGACCTTCCACAACCCATTCCTTCCATGTGTATTAGTAGTGGGAGGTGTTTCGTTCCTTTCCACA-5′
- NSP12 (4):
- 3′-CTGGACCTTCCACAACCCCTACTGTAATGCAAAACATATACGCTTTGGTGTTTCGTTCCTTTCCACA-5′
- NSP12 (5):
- 3′-CTGGACCTTCCACAACCGCTCGTTCTTGTTCACTCCGGTATTAAGAGGTGTTTCGTTCCTTTCCACA-5′
- NSP12 (6):
- 3′-CTGGACCTTCCACAACATTGCTATCATCAGTATTAGCGACTATCGTGGTGTTTCGTTCCTTTCCACA-5′
2.7. pspCas13b Plasmid
2.8. Production of gRNA and Cas13b Plasmids
2.9. Reverse Transfections of Cas13b and Single gRNA Plasmids in HEK293/ACE2 Cells
2.10. Combination of Guide RNAs in HEK293/ACE2 Cells
2.11. Infection Assays
2.12. RNA Extraction and qPCR of Infected Samples
2.13. Data Analysis
3. Results
3.1. Selection of Guide RNAs Using a Novel AI Tool
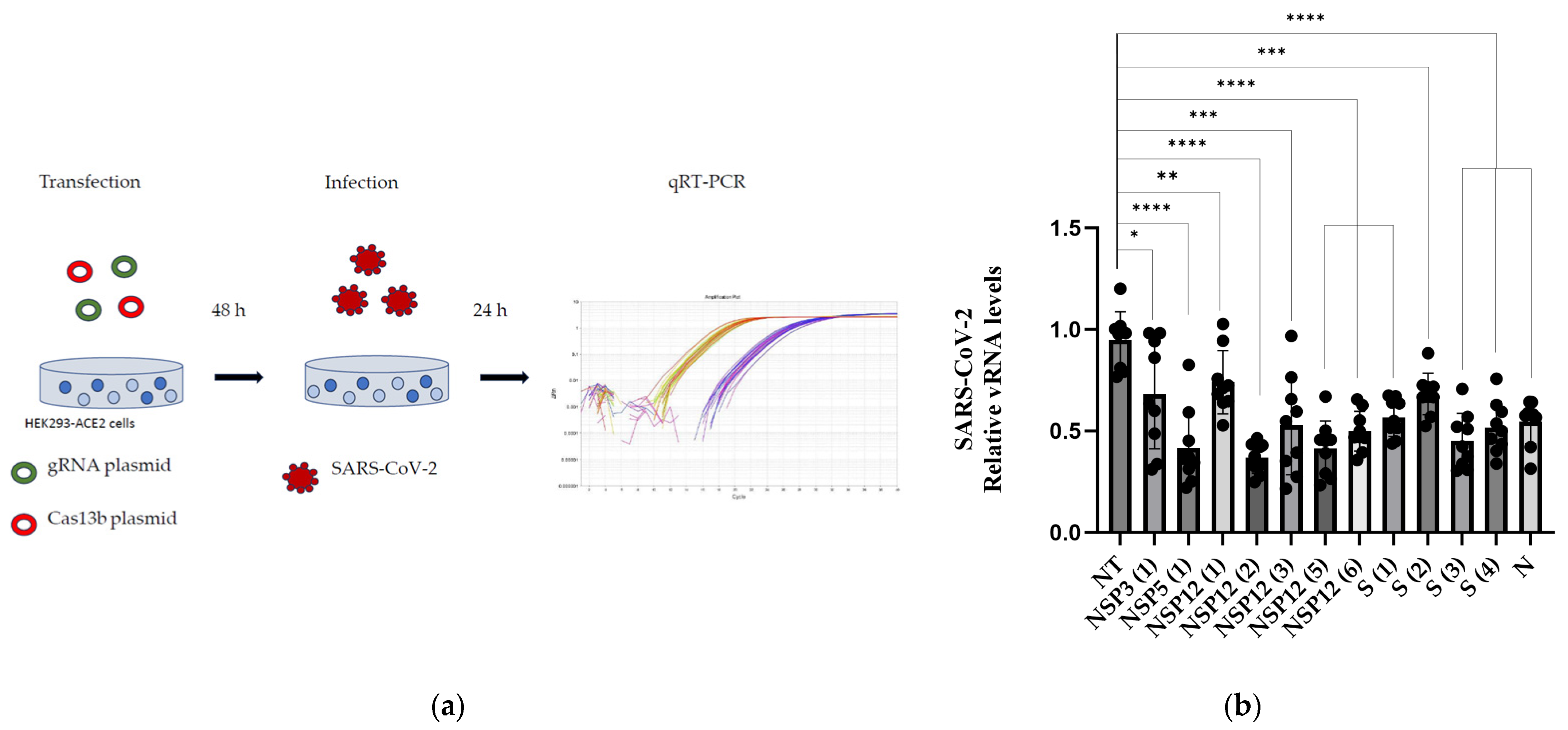
3.2. Validation of the gRNAs
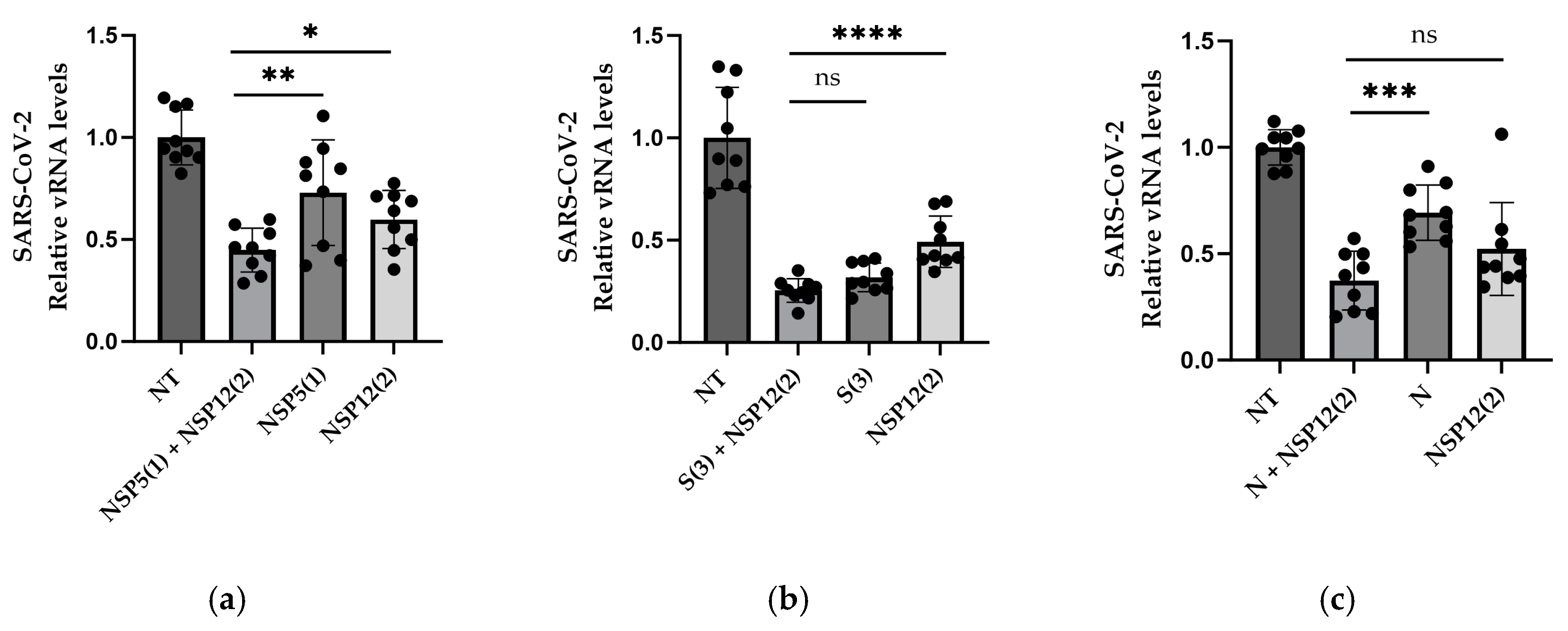
3.3. gRNAs in Combination
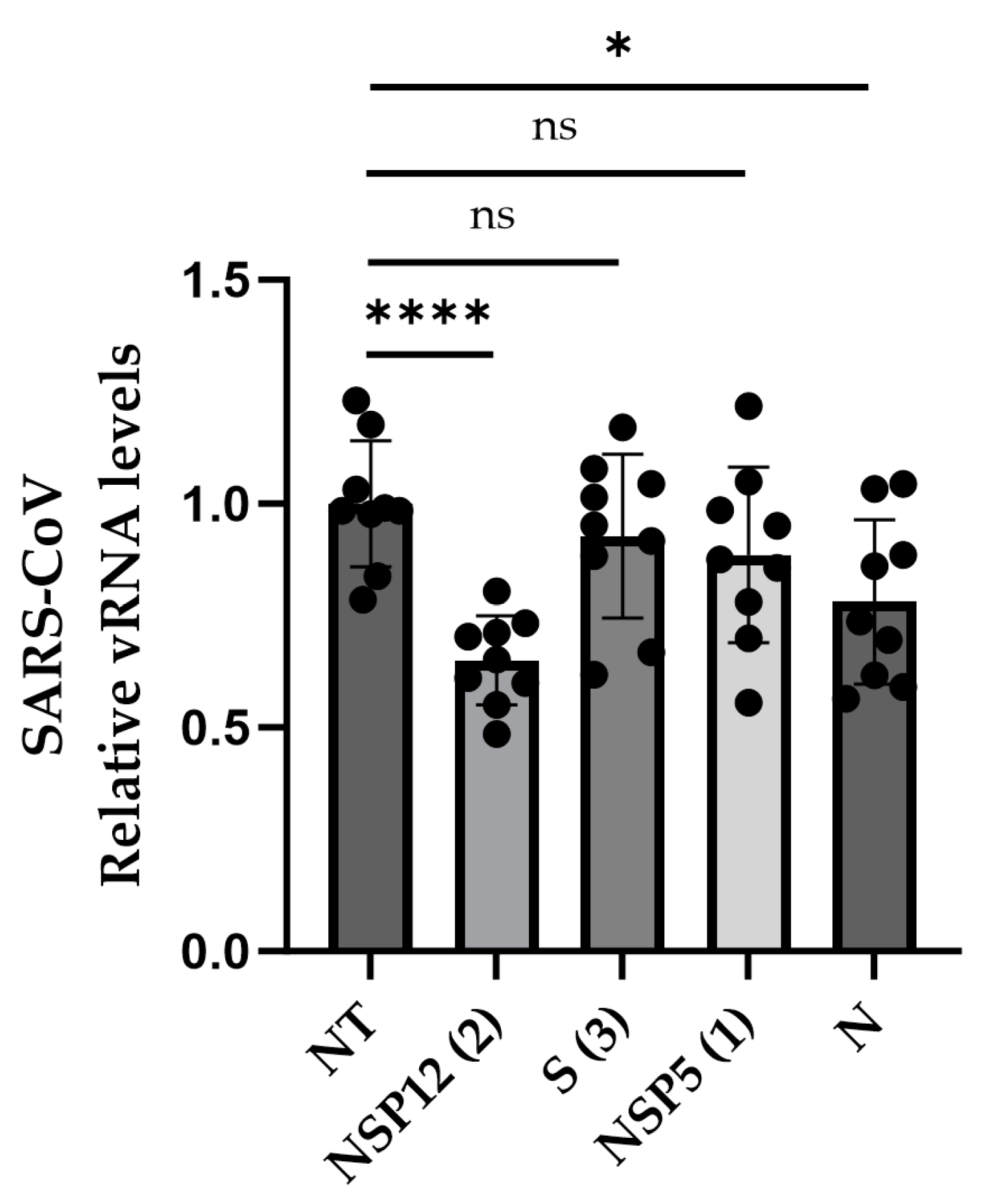
3.4. Efficiency against SARS-CoV
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carrasco-Hernandez, R.; Jácome, R.; Vidal, Y.L.; de León, S.P. Are RNA Viruses Candidate Agents for the Next Global Pandemic? A Review. ILAR J. 2017, 58, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Sun, J.; Li, D.; Wang, N.; Wang, L.; Zhang, C.; Meng, X.; Ji, X.; Suchard, M.A.; Zhang, X.; et al. Emerging viruses: Cross-species transmission of coronaviruses, filoviruses, henipaviruses, and rotaviruses from bats. Cell Rep. 2022, 39, 110969. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Liu, Z.; Chen, D. Human coronaviruses: Origin, host and receptor. J. Clin. Virol. 2022, 155, 105246. [Google Scholar] [CrossRef] [PubMed]
- McCoullough, L.C.; Fareh, M.; Hu, W.; Sozzi, V.; Makhlouf, C.; Droungas, Y.; Lee, C.L.; Takawy, M.; Fabb, S.A.; Payne, T.J.; et al. CRISPR-Cas13b-mediated suppression of HBV replication and protein expression. J. Hepatol. 2024. [Google Scholar] [CrossRef]
- Fareh, M.; Zhao, W.; Hu, W.; Casan, J.M.L.; Kumar, A.; Symons, J.; Zerbato, J.M.; Fong, D.; Voskoboinik, I.; Ekert, P.G.; et al. Reprogrammed CRISPR-Cas13b suppresses SARS-CoV-2 replication and circumvents its mutational escape through mismatch tolerance. Nat. Commun. 2021, 12, 4270. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, Y.; Nguyenla, X.H.; Abbott, T.R.; Han, M.; Zhu, Y.; Chemparathy, A.; Lin, X.; Chen, X.; Wang, H.; et al. Broad-spectrum CRISPR-mediated inhibition of SARS-CoV-2 variants and endemic coronaviruses in vitro. Nat. Commun. 2022, 13, 2766. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, X.; Kan, C.; Li, L.; Zhang, Y.; Gao, Y.; Zhang, S.; Zhou, L.; Zhao, H.; Li, M.; et al. CRISPR-Cas13d effectively targets SARS-CoV-2 variants, including Delta and Omicron, and inhibits viral infection. Medcomm 2023, 4, e208. [Google Scholar] [CrossRef]
- Nguyen, H.; Wilson, H.; Jayakumar, S.; Kulkarni, V.; Kulkarni, S. Efficient Inhibition of HIV Using CRISPR/Cas13d Nuclease System. Viruses 2021, 13, 1850. [Google Scholar] [CrossRef]
- Tong, H.; Huang, J.; Xiao, Q.; He, B.; Dong, X.; Liu, Y.; Yang, X.; Han, D.; Wang, Z.; Wang, X.; et al. High-fidelity Cas13 variants for targeted RNA degradation with minimal collateral effects. Nat. Biotechnol. 2022, 41, 108–119. [Google Scholar] [CrossRef]
- Abudayyeh, O.O.; Gootenberg, J.S.; Essletzbichler, P.; Han, S.; Joung, J.; Belanto, J.J.; Verdine, V.; Cox, D.B.T.; Kellner, M.J.; Regev, A.; et al. RNA targeting with CRISPR–Cas13. Nature 2017, 550, 280–284. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, P.; Zhou, Y.; Zhang, J.; Shi, J.; Zhang, M.; Yang, H.; Fei, J. The effects of length and sequence of gRNA on Cas13b and Cas13d activity in vitro and in vivo. Biotechnol. J. 2023, 18, e2300002. [Google Scholar] [CrossRef]
- Méndez-Mancilla, A.; Wessels, H.-H.; Legut, M.; Kadina, A.; Mabuchi, M.; Walker, J.; Robb, G.B.; Holden, K.; Sanjana, N.E. Chemically modified guide RNAs enhance CRISPR-Cas13 knockdown in human cells. Cell Chem. Biol. 2021, 29, 321–327.e4. [Google Scholar] [CrossRef]
- Wessels, H.H.; Méndez-Mancilla, A.; Hao, Y.; Papalexi, E.; Mauck, W.M., III; Lu, L.; Morris, J.A.; Mimitou, E.P.; Smibert, P.; Sanjana, N.E.; et al. Efficient combinatorial targeting of RNA tran-scripts in single cells with Cas13 RNA Perturb-seq. Nat. Methods 2023, 20, 86–94. [Google Scholar] [CrossRef]
- Kang, B.; Zhang, J.; Schwoerer, M.P.; Nelson, A.N.; Schoeman, E.; Guo, A.; Ploss, A.; Myhrvold, C. Highly multiplexed mRNA quantitation with CRISPR-Cas13. bioRxiv 2023. [Google Scholar] [CrossRef]
- Wessels, H.-H.; Méndez-Mancilla, A.; Guo, X.; Legut, M.; Daniloski, Z.; Sanjana, N.E. Massively parallel Cas13 screens reveal principles for guide RNA design. Nat. Biotechnol. 2020, 38, 722–727. [Google Scholar] [CrossRef]
- Abbott, T.R.; Dhamdhere, G.; Liu, Y.; Lin, X.; Goudy, L.; Zeng, L.; Chemparathy, A.; Chmura, S.; Heaton, N.S.; Debs, R.; et al. Development of CRISPR as an Antiviral Strategy to Combat SARS-CoV-2 and Influenza. Cell 2020, 181, 865–876.e12. [Google Scholar] [CrossRef]
- Cui, J.; Techakriengkrai, N.; Nedumpun, T.; Suradhat, S. Abrogation of PRRSV infectivity by CRISPR-Cas13b-mediated viral RNA cleavage in mammalian cells. Sci. Rep. 2020, 10, 9617. [Google Scholar] [CrossRef]
- Cox, D.B.T.; Gootenberg, J.S.; Abudayyeh, O.O.; Franklin, B.; Kellner, M.J.; Joung, J.; Zhang, F. RNA editing with CRISPR-Cas13. Science 2017, 358, 1019–1027. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Cheng, L.; Luo, Y. An overview and metanalysis of machine and deep learning-based CRISPR gRNA design tools. RNA Biol. 2019, 17, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, S.; Tsuji, M.H.; Shimizu, Y.; Usami, K.; Lee, S.; Takei, N.K.; Yoshitome, K.; Nishimura, Y.; Otsuki, T.; Ito, T. Structure-based design of gRNA for Cas13. Sci. Rep. 2020, 10, 11610. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, R.; Tinker-Kulberg, R.; Salehin, M.; Supakar, T.; Chamberlain, S.; Ligaba-Osena, A.; Josephs, E.A. Polyvalent guide RNAs for CRISPR antivirals. iScience 2022, 25, 105333. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, Y.; Chemparathy, A.; Pande, T.; La Russa, M.; Qi, L.S. A comprehensive analysis and resource to use CRISPR-Cas13 for broad-spectrum targeting of RNA viruses. Cell Rep. Med. 2021, 2, 100245. [Google Scholar] [CrossRef]
- Guo, X.; Rahman, J.A.; Wessels, H.-H.; Méndez-Mancilla, A.; Haro, D.; Chen, X.; Sanjana, N.E. Transcriptome-wide Cas13 guide RNA design for model organisms and viral RNA pathogens. Cell Genom. 2021, 1, 100001. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Han, H.-J.; Yu, J.; Kim, J.; Lee, G.-H.; Yang, J.-H.; Song, B.-M.; Tark, D.; Choi, B.-S.; Kang, S.-M.; et al. Pseudoknot-targeting Cas13b combats SARS-CoV-2 infection by suppressing viral replication. Mol. Ther. 2023, 31, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; Kok, K.-H.; Zhu, Z.; Chu, H.; To, K.K.-W.; Yuan, S.; Yuen, K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef]
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol. 2020, 11, 552909. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, S.; Kumar, R.; Tripathi, B.N.; Barua, S.; Ly, H.; Rouse, B.T. Host-Directed Antiviral Therapy. Clin. Microbiol. Rev. 2020, 33, e00168-19. [Google Scholar] [CrossRef]
- Geraghty, R.J.; Aliota, M.T.; Bonnac, L.F. Broad-Spectrum Antiviral Strategies and Nucleoside Analogues. Viruses 2021, 13, 667. [Google Scholar] [CrossRef]
- Kamzeeva, P.N.; Aralov, A.V.; Alferova, V.A.; Korshun, V.A. Recent Advances in Molecular Mechanisms of Nucleoside Antivirals. Curr. Issues Mol. Biol. 2023, 45, 6851–6879. [Google Scholar] [CrossRef]
- Freije, C.A.; Myhrvold, C.; Boehm, C.K.; Lin, A.E.; Welch, N.L.; Carter, A.; Metsky, H.C.; Luo, C.Y.; Abudayyeh, O.O.; Gootenberg, J.S.; et al. Programmable Inhibition and Detection of RNA Viruses Using Cas13. Mol. Cell 2019, 76, 826–837.e11. [Google Scholar] [CrossRef]
- Chaves, L.C.S.; Orr-Burks, N.; Vanover, D.; Mosur, V.V.; Hosking, S.R.; Kumar E. K., P.; Jeong, H.; Jung, Y.; Assumpção, J.A.F.; Peck, H.E.; et al. mRNA-encoded Cas13 treatment of Influenza via site-specific degradation of genomic RNA. PLoS Pathog. 2024, 20, e1012345. [Google Scholar] [CrossRef]
- Blanchard, E.L.; Vanover, D.; Bawage, S.S.; Tiwari, P.M.; Rotolo, L.; Beyersdorf, J.; Peck, H.E.; Bruno, N.C.; Hincapie, R.; Michel, F.; et al. Treatment of influenza and SARS-CoV-2 infections via mRNA-encoded Cas13a in rodents. Nat. Biotechnol. 2021, 39, 717–726. [Google Scholar] [CrossRef]
- Konermann, S.; Lotfy, P.; Brideau, N.J.; Oki, J.; Shokhirev, M.N.; Hsu, P.D. Transcriptome Engineering with RNA-Targeting Type VI-D CRISPR Effectors. Cell 2018, 173, 665–676.e14. [Google Scholar] [CrossRef]
- Feuillet, V.; Canard, B.; Trautmann, A. Combining Antivirals and Immunomodulators to Fight COVID-19. Trends Immunol. 2020, 42, 31–44. [Google Scholar] [CrossRef]
- Hu, W.; Kumar, A.; Ahmed, S.F.; Qi, S.; Ma, D.K.G.; Chen, H.; Singh, G.J.; Casan, J.M.L.; Haber, M.; Voskoboinik, I.; et al. Single-base tiled screen unveils design principles of PspCas13b for potent and off-target-free RNA silencing. Nat. Struct. Mol. Biol. 2024. [Google Scholar] [CrossRef]
- Yan, W.; Zheng, Y.; Zeng, X.; He, B.; Cheng, W. Structural biology of SARS-CoV-2: Open the door for novel therapies. Signal Transduct. Target. Ther. 2022, 7, 26. [Google Scholar] [CrossRef]
- Islam, J.; Islam, N.N.; Alom, S.; Kabir, M.; Halim, M.A. A review on structural, non-structural, and accessory proteins of SARS-CoV-2: Highlighting drug target sites. Immunobiology 2022, 228, 152302. [Google Scholar] [CrossRef]
- Cannalire, R.; Cerchia, C.; Beccari, A.R.; Di Leva, F.S.; Summa, V. Targeting SARS-CoV-2 Proteases and Polymerase for COVID-19 Treatment: State of the Art and Future Opportunities. J. Med. Chem. 2020, 65, 2716–2746. [Google Scholar] [CrossRef]
- Báez-Santos, Y.M.; John, S.E.; Mesecar, A.D. The SARS-coronavirus papain-like protease: Structure, function and inhibition by designed antiviral compounds. Antiviral. Res. 2015, 115, 21–38. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; Ismail, M.I.; Bauer, M.R.; Bekhit, A.A.; Boeckler, F.M. Supporting SARS-CoV-2 Papain-Like Protease Drug Discovery: In silico Methods and Benchmarking. Front. Chem. 2020, 8, 592289. [Google Scholar] [CrossRef]
- Tam, D.; Lorenzo-Leal, A.C.; Hernández, L.R.; Bach, H. Targeting SARS-CoV-2 Non-Structural Proteins. Int. J. Mol. Sci. 2023, 24, 13002. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Luo, R.; Ren, S.; Liang, L.; Huang, Q.; Tang, Y.; Fan, H.; Ren, H.; Zhang, W.; et al. SARS-CoV-2 NSP12 utilizes various host splicing factors for replication and splicing regulation. J. Med Virol. 2024, 96, e29396. [Google Scholar] [CrossRef]
- Aljuaid, A.; Salam, A.; Almehmadi, M.; Baammi, S.; Alshabrmi, F.M.; Allahyani, M.; Al-Zaydi, K.M.; Izmirly, A.M.; Almaghrabi, S.; Baothman, B.K.; et al. Structural Homology-Based Drug Repurposing Approach for Targeting NSP12 SARS-CoV-2. Molecules 2022, 27, 7732. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: First Regulatory Approvals for CRISPR-Cas9 Therapeutic Gene Editing for Sickle Cell Disease and Transfusion-Dependent β-Thalassemia. Med Sci. Monit. 2024, 30, e944204. [Google Scholar] [CrossRef]
- Sheridan, C. The world’s first CRISPR therapy is approved: Who will receive it? Nat. Biotechnol. 2023, 42, 3–4. [Google Scholar] [CrossRef]
| gRNA ID | Target | Sequence 5′ -> 3′ |
|---|---|---|
| NSP3 (1) | NSP3 | GACUAUCAUCAUCUAACCAAUCUUCUUCUU |
| NSP3 (2) | NSP3 | CAACUUAGGGUCAAUUUCUGUACAAACAAC |
| NSP3 (3) | NSP3 | GGAGGGUAAAAAGAACAAUACAUAUGUGAA |
| NSP3 (4) | NSP3 | AUCCUUUCAUCAAGUUCAAAAGUGAUAUUC |
| NSP5 (1) | NSP5ab | GGUAAUUCCAUAUGGUGCAUGUAACAAAAA |
| NSP5 (2) | NSP5ab | GAACAUUACCAGCCUGUACCAAGAAAUUAU |
| NSP5 (3) | NSP5ab | AAAACGGCAAUUCCAGUUUGAGCAGAAAGA |
| NSP12 (1) | NSP12 | GGCAUUAACAAUGAAUAAUAAGAAUCUACA |
| NSP12 (2) | NSP12 | GAUGAAACUGUCUAUUGGUCAUAGUACUAC |
| NSP12 (3) | NSP12 | GGUAAGGAAGGUACACAUAAUCAUCACCCU |
| NSP12 (4) | NSP12 | GGGAUGACAUUACGUUUUGUAUAUGCGAAA |
| NSP12 (5) | NSP12 | GCGAGCAAGAACAAGUGAGGCCAUAAUUCU |
| NSP12 (6) | NSP12 | UAACGAUAGUAGUCAUAAUCGCUGAUAGCA |
| S (1) | Spike | GUCUGACUUCAUCACCUCUAAUUACAAAUG |
| S (2) | Spike | GGUGGUGUUUUGUAAAUUUGUUUGACUUGU |
| S (3) | Spike | AGGGUAAUAAACACCACGUGUGAAAGAAUU |
| S (4) | Spike | GGGUAAUAAACACCACGUGUGAAAGAAUUA |
| S (5) | Spike | GGUAAUUUAUAAUUAUAAUCAGCAAUCUUU |
| M | Membrane | GGAAAAUUAACUUAAUUAUAUACAAAAACC |
| N | Nucleocapsid | GGGAAUUUAAGGUCUUCCUUGCCAUGUUGA |
| Guide RNA ID | Relative vRNA Levels | RNA Knockdown (%) |
|---|---|---|
| NSP12 (2) | 0.37 | 63 |
| NSP12 (5) | 0.41 | 59 |
| NSP5 (1) | 0.41 | 59 |
| S (3) | 0.45 | 55 |
| NSP12 (6) | 0.5 | 50 |
| S (4) | 0.52 | 48 |
| NSP12 (3) | 0.53 | 47 |
| N | 0.54 | 46 |
| S (1) | 0.57 | 43 |
| NSP3 (1) | 0.68 | 32 |
| S (2) | 0.68 | 32 |
| NSP12 (1) | 0.74 | 26 |
| NSP3 (2) | ns | ns |
| NSP3 (3) | ns | ns |
| NSP3 (4) | ns | ns |
| NSP5ab (2) | ns | ns |
| NSP5ab (3) | ns | ns |
| NSP12 (4) | ns | ns |
| S (5) | ns | ns |
| Guide RNA ID | RNA Knockdown (%) | Number of Mismatches to SARS-CoV |
|---|---|---|
| NSP12 (2) | 63 | 1 |
| NSP5 (1) | 59 | 7 |
| S (3) | 55 | 12 |
| N | 46 | 4 |
| gRNA ID | Knockdown SARS-CoV-2 (%) | Number of Mismatches to SARS-CoV | Knockdown SARS-CoV (%) |
|---|---|---|---|
| NSP12 (2) | 63 | 1 | 35 |
| NSP5 (1) | 59 | 7 | ns |
| S (3) | 55 | 12 | ns |
| N | 46 | 4 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersson, K.; Azatyan, A.; Ekenberg, M.; Güçlüler, G.; Sardon Puig, L.; Puumalainen, M.; Pramer, T.; Monteil, V.M.; Mirazimi, A. A CRISPR-Cas13b System Degrades SARS-CoV and SARS-CoV-2 RNA In Vitro. Viruses 2024, 16, 1539. https://doi.org/10.3390/v16101539
Andersson K, Azatyan A, Ekenberg M, Güçlüler G, Sardon Puig L, Puumalainen M, Pramer T, Monteil VM, Mirazimi A. A CRISPR-Cas13b System Degrades SARS-CoV and SARS-CoV-2 RNA In Vitro. Viruses. 2024; 16(10):1539. https://doi.org/10.3390/v16101539
Chicago/Turabian StyleAndersson, Klara, Ani Azatyan, Martin Ekenberg, Gözde Güçlüler, Laura Sardon Puig, Marjo Puumalainen, Theodor Pramer, Vanessa M. Monteil, and Ali Mirazimi. 2024. "A CRISPR-Cas13b System Degrades SARS-CoV and SARS-CoV-2 RNA In Vitro" Viruses 16, no. 10: 1539. https://doi.org/10.3390/v16101539
APA StyleAndersson, K., Azatyan, A., Ekenberg, M., Güçlüler, G., Sardon Puig, L., Puumalainen, M., Pramer, T., Monteil, V. M., & Mirazimi, A. (2024). A CRISPR-Cas13b System Degrades SARS-CoV and SARS-CoV-2 RNA In Vitro. Viruses, 16(10), 1539. https://doi.org/10.3390/v16101539






