The Role of Macrophages in Airway Disease Focusing on Porcine Reproductive and Respiratory Syndrome Virus and the Treatment with Antioxidant Nanoparticles
Abstract
:1. Background
2. Lung Epithelial Cells and the Status of Common Lung Diseases Associated with M1/M2
Alveolar Macrophages and Interstitial Macrophages
3. Sequence–Structure–Function Theory of Antigens and the Application of Macrophage Epitopes in Various Diseases, Including Lung Disease
4. Lung Disease: Focusing on PRRSV
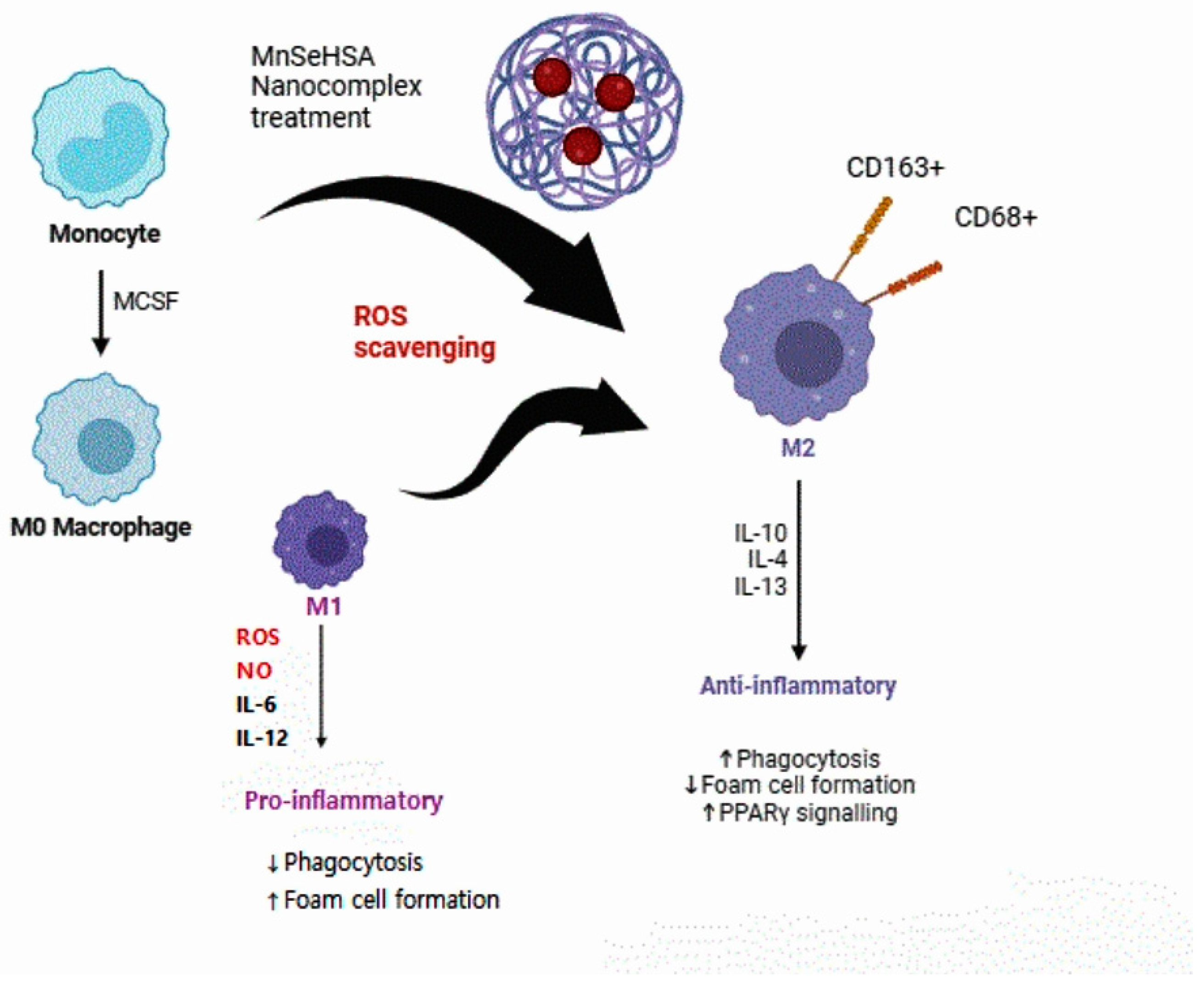
5. Therapeutic Effects of Changes in Macrophage Status through ROS Scavenging through Nanoparticles
Effects of Nanoparticle Treatment on the Human Body
6. Macrophages and Lung Diseases in the Veterinary Field
7. Conclusions and Perspectives
Funding
Conflicts of Interest
Abbreviations
References
- Schluger, N.W.; Koppaka, R. Lung disease in a global context. A call for public health action. Ann. Am. Thorac. Soc. 2014, 11, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Deem, S.L.; Spelman, L.H.; Yates, R.A.; Montali, R.J. Canine distemper in terrestrial carnivores: A review. J. Zoo Wildl. Med. 2000, 31, 441–451. [Google Scholar] [PubMed]
- Chae, C. Commercial PRRS modified-live virus vaccines. Vaccines 2021, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Reinero, C.R. Advances in the understanding of pathogenesis, and diagnostics and therapeutics for feline allergic asthma. Vet. J. 2011, 190, 28–33. [Google Scholar] [CrossRef]
- Arora, S.; Dev, K.; Agarwal, B.; Das, P.; Syed, M.A. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 2018, 223, 383–396. [Google Scholar] [CrossRef]
- Byrne, A.J.; Mathie, S.A.; Gregory, L.G.; Lloyd, C.M. Pulmonary macrophages: Key players in the innate defence of the airways. Thorax 2015, 70, 1189–1196. [Google Scholar] [CrossRef]
- Shi, T.; Denney, L.; An, H.; Ho, L.-P.; Zheng, Y. Alveolar and lung interstitial macrophages: Definitions, functions, and roles in lung fibrosis. J. Leukoc. Biol. 2021, 110, 107–114. [Google Scholar] [CrossRef]
- Liegeois, M.; Legrand, C.; Desmet, C.J.; Marichal, T.; Bureau, F. The interstitial macrophage: A long-neglected piece in the puzzle of lung immunity. Cell Immunol. 2018, 330, 91–96. [Google Scholar] [CrossRef]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Barnes, P.J. Alveolar macrophages as orchestrators of COPD. COPD J. Chronic Obstr. Pulm. Dis. 2004, 1, 59–70. [Google Scholar] [CrossRef]
- Fricker, M.; Gibson, P.G. Macrophage dysfunction in the pathogenesis and treatment of asthma. Eur. Respir. J. 2017, 50, 1700196. [Google Scholar] [CrossRef]
- Strzelak, A.; Ratajczak, A.; Adamiec, A.; Feleszko, W. Tobacco Smoke Induces and Alters Immune Responses in the Lung Triggering Inflammation, Allergy, Asthma and Other Lung Diseases: A Mechanistic Review. Int. J. Environ. Res. Public Health 2018, 15, 1033. [Google Scholar] [CrossRef] [PubMed]
- Saradna, A.; Do, D.C.; Kumar, S.; Fu, Q.-L.; Gao, P. Macrophage polarization and allergic asthma. Transl. Res. 2018, 191, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-L.; Huang, X.-Y.; Luo, Y.-F.; Tan, W.-P.; Chen, P.-F.; Guo, Y.-B. Activation of M1 macrophages plays a critical role in the initiation of acute lung injury. Biosci. Rep. 2018, 38, BSR20171555. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Covarrubias, A.; Byles, V.; Horng, T. ROS sets the stage for macrophage differentiation. Cell Res. 2013, 23, 984–985. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Choi, S.H.; Byeon, H.J.; Choi, J.S.; Thao, L.; Kim, I.; Lee, E.S.; Shin, B.S.; Lee, K.C.; Youn, Y.S. Inhalable self-assembled albumin nanoparticles for treating drug-resistant lung cancer. J. Control. Release 2015, 197, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-F.; Chen, G.-W.; Chen, Y.-C.; Shen, C.-K.; Lu, D.-Y.; Yang, L.-Y.; Chen, J.-H.; Yeh, W.-L. Regulatory effects of quercetin on M1/M2 macrophage polarization and oxidative/antioxidative balance. Nutrients 2021, 14, 67. [Google Scholar] [CrossRef]
- Kwon, D.H.; Lee, H.; Park, C.; Hong, S.-H.; Hong, S.H.; Kim, G.-Y.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Hwang, H.-J. Glutathione induced immune-stimulatory activity by promoting M1-like macrophages polarization via potential ROS scavenging capacity. Antioxidants 2019, 8, 413. [Google Scholar] [CrossRef]
- Griess, B.; Mir, S.; Datta, K.; Teoh-Fitzgerald, M. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic. Biol. Med. 2020, 147, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Jiang, H.; Thapa, P.; Ding, N.; Alshahrani, A.; Fujii, J.; Toledano, M.B.; Wei, Q. Critical Role of the Sulfiredoxin-Peroxiredoxin IV Axis in Urethane-Induced Non-Small Cell Lung Cancer. Antioxidants 2023, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Johansson, A.; Lundborg, M.; Orre, L.; Sköld, C.M.; Camner, P. Functional and morphological differences between human alveolar and interstitial macrophages. Exp. Mol. Pathol. 2001, 70, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Whisstock, J.C.; Lesk, A.M. Prediction of protein function from protein sequence and structure. Q. Rev. Biophys. 2003, 36, 307–340. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Choi, K.; Han, M.; Kim, S.J. A systematic review of chromogranin A (CgA) and its biomedical applications, unveiling its structure-related functions. J. Korean Phys. Soc. 2021, 78, 427–441. [Google Scholar] [CrossRef]
- Kaku, Y.; Imaoka, H.; Morimatsu, Y.; Komohara, Y.; Ohnishi, K.; Oda, H.; Takenaka, S.; Matsuoka, M.; Kawayama, T.; Takeya, M. Overexpression of CD163, CD204 and CD206 on alveolar macrophages in the lungs of patients with severe chronic obstructive pulmonary disease. PLoS ONE 2014, 9, e87400. [Google Scholar] [CrossRef]
- Etzerodt, A.; Rasmussen, M.R.; Svendsen, P.; Chalaris, A.; Schwarz, J.; Galea, I.; Møller, H.J.; Moestrup, S.K. Structural basis for inflammation-driven shedding of CD163 ectodomain and tumor necrosis factor-α in macrophages. J. Biol. Chem. 2014, 289, 778–788. [Google Scholar] [CrossRef]
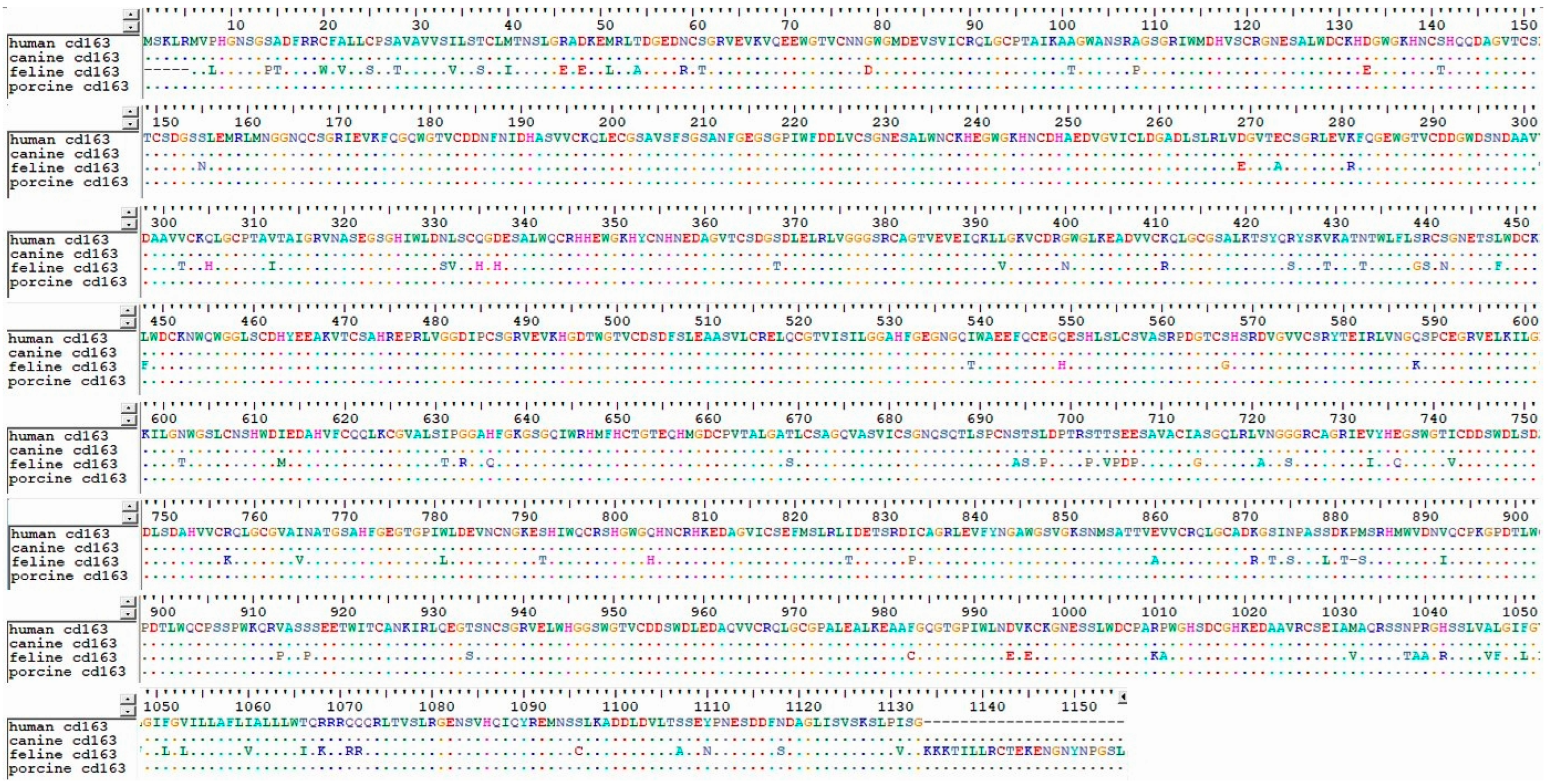
- Ma, H.; Jiang, L.; Qiao, S.; Zhi, Y.; Chen, X.-X.; Yang, Y.; Huang, X.; Huang, M.; Li, R.; Zhang, G.-P. The crystal structure of the fifth scavenger receptor cysteine-rich domain of porcine CD163 reveals an important residue involved in porcine reproductive and respiratory syndrome virus infection. J. Virol. 2019, 93, e02185-18. [Google Scholar] [CrossRef]
- Kweon, C.-H.; Kwon, B.-J.; Lee, H.-J.; Cho, J.-J.; Hwang, E.-K.; Shin, J.-H.; Yoon, Y.-D.; Kang, Y.-B.; An, S.-H.; Kim, Y.-H. Isolation of porcine reproductive and respiratory syndrome virus (PRRSV) in Korea. Korean J. Vet. Res. 1994, 34, 77–83. [Google Scholar]
- Meulenberg, J. PRRSV, the virus. Vet. Res. 2000, 31, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Wensvoort, G.; Terpstra, C.; Pol, J.; Laak, E.T.; Bloemraad, M.; Kluyver, E.D.; Kragten, C.; Buiten, L.V.; Besten, A.D.; Wagenaar, F. Mystery swine disease in the Netherlands: The isolation of Lelystad virus. Veter. Q. 1991, 13, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.E.; Benfield, D.A.; Christianson, W.T.; Harris, L.; Hennings, J.C.; Shaw, D.P.; Goyal, S.M.; McCullough, S.; Morrison, R.B.; Joo, H.S. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Investig. 1992, 4, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Dokland, T. The structural biology of PRRSV. Virus Res. 2010, 154, 86–97. [Google Scholar] [CrossRef]
- Su, C.-M.; Rowland, R.R.R.; Yoo, D. Recent advances in PRRS virus receptors and the targeting of receptor–ligand for control. Vaccines 2021, 9, 354. [Google Scholar] [CrossRef]
- Wills, R.W.; Zimmerman, J.J.; Yoon, K.-J.; Swenson, S.L.; Hoffman, L.J.; McGinley, M.J.; Hill, H.T.; Platt, K.B. Porcine reproductive and respiratory syndrome virus: Routes of excretion. Vet. Microbiol. 1997, 57, 69–81. [Google Scholar] [CrossRef]
- Prieto, C.; Castro, J.M. Porcine reproductive and respiratory syndrome virus infection in the boar: A review. Theriogenology 2005, 63, 1–16. [Google Scholar] [CrossRef]
- Beyer, J.; Fichtner, D.; Schirrmeir, H.; Polster, U.; Weiland, E.; Wege, H. Porcine reproductive and respiratory syndrome virus (PRRSV): Kinetics of infection in lymphatic organs and lung. J. Vet. Med. Ser. B 2000, 47, 9–25. [Google Scholar] [CrossRef]
- Bochev, I. Porcine respiratory disease complex (PRDC): A review. I. Etiology, epidemiology, clinical forms and pathoanatomical features. Bulg. J. Vet. Med. 2007, 10, 131–146. [Google Scholar]
- Pejsak, Z.; Stadejek, T.; Markowska-Daniel, I. Clinical signs and economic losses caused by porcine reproductive and respiratory syndrome virus in a large breeding farm. Vet. Microbiol. 1997, 55, 317–322. [Google Scholar] [CrossRef]
- Prieto, C.; Suarez, P.; Bautista, J.; Sanchez, R.; Rillo, S.; Simarro, I.; Solana, A.; Castro, J.M. Semen changes in boars after experimental infection with porcine reproductive and respiratory syndrome (PRRS) virus. Theriogenology 1996, 45, 383–395. [Google Scholar] [CrossRef]
- Ruedas-Torres, I.; Rodríguez-Gómez, I.; Sánchez-Carvajal, J.M.; Larenas-Muñoz, F.; Pallarés, F.; Carrasco, L.; Gomez-Laguna, J. The jigsaw of PRRSV virulence. Vet. Microbiol. 2021, 260, 109168. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Lager, K.M.; Schlink, S.N.; Kehrli, M.E., Jr.; Brockmeier, S.L.; Miller, L.C.; Swenson, S.L.; Faaberg, K.S. Chinese and Vietnamese strains of HP-PRRSV cause different pathogenic outcomes in United States high health swine. Virology 2013, 446, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Balka, G.; Podgórska, K.; Brar, M.S.; Bálint, Á.; Cadar, D.; Celer, V.; Dénes, L.; Dirbakova, Z.; Jedryczko, A.; Márton, L. Genetic diversity of PRRSV 1 in Central Eastern Europe in 1994–2014: Origin and evolution of the virus in the region. Sci. Rep. 2018, 8, 7811. [Google Scholar] [CrossRef] [PubMed]
- Jakab, S.; Kaszab, E.; Marton, S.; Bányai, K.; Bálint, Á.; Nemes, I.; Szabó, I. Genetic diversity of imported PRRSV-2 strains, 2005–2020, Hungary. Front. Vet. Sci. 2022, 9, 986850. [Google Scholar]
- Pileri, E.; Mateu, E. Review on the transmission porcine reproductive and respiratory syndrome virus between pigs and farms and impact on vaccination. Vet. Res. 2016, 47, 108. [Google Scholar] [CrossRef]
- Haynes, J.; Halbur, P.; Sirinarumitr, T.; Paul, P.; Meng, X.-J.; Huffman, E. Temporal and morphologic characterization of the distribution of porcine reproductive and respiratory syndrome virus (PRRSV) by in situ hybridization in pigs infected with isolates of PRRSV that differ in virulence. Vet. Pathol. 1997, 34, 39–43. [Google Scholar] [CrossRef]
- Han, K.; Seo, H.W.; Park, C.; Oh, Y.; Kang, I.; Chae, C. Comparative pathogenesis of type 1 (European genotype) and type 2 (North American genotype) porcine reproductive and respiratory syndrome virus in infected boar. Virol. J. 2013, 10, 156. [Google Scholar] [CrossRef]
- Piras, F.; Bollard, S.; Laval, F.; Joisel, F.; Reynaud, G.; Charreyre, C.; Andreoni, C.; Juillard, V. Porcine Reproductive and Respiratory Syndrome (PRRS) virus-specific interferon-γ+ T-cell responses after PRRS virus infection or vaccination with an inactivated PRRS vaccine. Viral Immunol. 2005, 18, 381–389. [Google Scholar] [CrossRef]
- Silva, G.S.; Corbellini, L.G.; Linhares, D.L.; Baker, K.L.; Holtkamp, D.J. Development and validation of a scoring system to assess the relative vulnerability of swine breeding herds to the introduction of PRRS virus. Prev. Vet. Med. 2018, 160, 116–122. [Google Scholar] [CrossRef]
- Beyer, J.; Fichtner, D.; Schirrmeier, H.; Granzow, H.; Polster, U.; Weiland, E.; Berndt, A.; Wege, H. Arterivirus PRRSV: Experimental studies on the pathogenesis of respiratory disease. Adv. Exp. Med. Biol. 1998, 440, 593–599. [Google Scholar] [PubMed]
- Van Gorp, H.; Van Breedam, W.; Delputte, P.L.; Nauwynck, H.J. Sialoadhesin and CD163 join forces during entry of the porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2008, 89, 2943–2953. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Chae, C. Expression of tumour necrosis factor-α is associated with apoptosis in lungs of pigs experimentally infected with porcine reproductive and respiratory syndrome virus. Res. Vet. Sci. 2002, 72, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Mateu, E.; Díaz, I. The challenge of PRRS immunology. Vet. J. 2008, 177, 345–351. [Google Scholar] [CrossRef]
- Tong, J.; Yu, Y.; Zheng, L.; Zhang, C.; Tu, Y.; Liu, Y.; Wu, J.; Li, H.; Wang, S.; Jiang, C. Hypothalamus-pituitary-adrenal axis involves in anti-viral ability through regulation of immune response in piglets infected by highly pathogenic porcine reproductive and respiratory syndrome virus. BMC Vet. Res. 2018, 14, 92. [Google Scholar] [CrossRef]
- Wahyuningtyas, R.; Lai, Y.-S.; Wu, M.-L.; Chen, H.-W.; Chung, W.-B.; Chaung, H.-C.; Chang, K.-T. Recombinant antigen of type 2 porcine reproductive and respiratory syndrome virus (PRRSV-2) promotes M1 repolarization of porcine alveolar macrophages and Th1 type response. Vaccines 2021, 9, 1009. [Google Scholar] [CrossRef]
- Gong, X.; Ma, T.; Zhang, Q.; Wang, Y.; Song, C.; Lai, M.; Zhang, C.; Fang, X.; Chen, X. Porcine reproductive and respiratory syndrome virus modulates the switch of macrophage polarization from M1 to M2 by upregulating moDC-released sCD83. Viruses 2023, 15, 773. [Google Scholar] [CrossRef]
- Sharpe, E.; Andreescu, D.; Andreescu, S. Artificial nanoparticle antioxidants. In Oxidative Stress: Diagnostics, Prevention, and Therapy; ACS Publications: Washington, DC, USA, 2011; pp. 235–253. [Google Scholar]
- Malhotra, S.; Welling, M.; Mantri, S.; Desai, K. In vitro and in vivo antioxidant, cytotoxic, and anti-chronic inflammatory arthritic effect of selenium nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 993–1003. [Google Scholar] [CrossRef]
- Battin, E.E.; Brumaghim, J.L. Antioxidant activity of sulfur and selenium: A review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem. Biophys. 2009, 55, 1–23. [Google Scholar] [CrossRef]
- Medhe, S.; Bansal, P.; Srivastava, M.M. Enhanced antioxidant activity of gold nanoparticle embedded 3, 6-dihydroxyflavone: A combinational study. Appl. Nanosci. 2014, 4, 153–161. [Google Scholar] [CrossRef]
- Okamoto, S.; Matsuura, M.; Akagi, T.; Akashi, M.; Tanimoto, T.; Ishikawa, T.; Takahashi, M.; Yamanishi, K.; Mori, Y. Poly (γ-glutamic acid) nano-particles combined with mucosal influenza virus hemagglutinin vaccine protects against influenza virus infection in mice. Vaccine 2009, 27, 5896–5905. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Pratama, A.C.; Qiu, H.; Liu, Z.; He, F. Toward innovative veterinary nanoparticle vaccines. Anim. Dis. 2024, 4, 14. [Google Scholar] [CrossRef]
- Yang, J.; Bai, Y.; Shen, S.; Tao, X.; Ma, C.; Fu, B.; Dai, Q.; Wu, J.; Meng, Z.; Sun, Q. An oral nano-antioxidant for targeted treatment of inflammatory bowel disease by regulating macrophage polarization and inhibiting ferroptosis of intestinal cells. Chem. Eng. J. 2023, 465, 142940. [Google Scholar] [CrossRef]
- Soh, M.; Kang, D.W.; Jeong, H.G.; Kim, D.; Kim, D.Y.; Yang, W.; Song, C.; Baik, S.; Choi, I.Y.; Ki, S.K. Ceria–Zirconia nanoparticles as an enhanced multi-antioxidant for sepsis treatment. Angew. Chem. 2017, 129, 11557–11561. [Google Scholar] [CrossRef]
- Fang, H.; Sha, Y.; Yang, L.; Jiang, J.; Yin, L.; Li, J.; Li, B.; Klumperman, B.; Zhong, Z.; Meng, F. Macrophage-targeted hydroxychloroquine nanotherapeutics for rheumatoid arthritis therapy. ACS Appl. Mater. Interfaces 2022, 14, 8824–8837. [Google Scholar] [CrossRef]
- Eapen, M.S.; Hansbro, P.M.; McAlinden, K.; Kim, R.Y.; Ward, C.; Hackett, T.-L.; Walters, E.H.; Sohal, S.S. Abnormal M1/M2 macrophage phenotype profiles in the small airway wall and lumen in smokers and chronic obstructive pulmonary disease (COPD). Sci. Rep. 2017, 7, 13392. [Google Scholar] [CrossRef]
- Jackute, J.; Zemaitis, M.; Pranys, D.; Sitkauskiene, B.; Miliauskas, S.; Vaitkiene, S.; Sakalauskas, R. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol. 2018, 19, 3. [Google Scholar] [CrossRef]
- Lee, J.-W.; Chun, W.; Lee, H.J.; Min, J.-H.; Kim, S.-M.; Seo, J.-Y.; Ahn, K.-S.; Oh, S.-R. The role of macrophages in the development of acute and chronic inflammatory lung diseases. Cells 2021, 10, 897. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; McDonald, C.F.; Darby, I.A.; Pouniotis, D.S. Characterization of M1/M2 tumour-associated macrophages (TAMs) and Th1/Th2 cytokine profiles in patients with NSCLC. Cancer Microenviron. 2016, 9, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Sun, L.; Gao, W.; Xiong, Y.; Ma, A.; Liu, X.; Shen, L.; Li, Q.; Yang, H. Manipulation of macrophage polarization by peptide-coated gold nanoparticles and its protective effects on acute lung injury. J. Nanobiotechnology 2020, 18, 38. [Google Scholar] [CrossRef]
- Schweer, D.; McAtee, A.; Neupane, K.; Richards, C.; Ueland, F.; Kolesar, J. Tumor-associated macrophages and ovarian cancer: Implications for therapy. Cancers 2022, 14, 2220. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Luo, S.; Zeng, Y.; Shi, C.; Li, R. Albumin-mediated biomineralization of shape-controllable and biocompatible ceria nanomaterials. ACS Appl. Mater. Interfaces 2017, 9, 6839–6848. [Google Scholar] [CrossRef] [PubMed]
- Girodet, P.-O.; Nguyen, D.; Mancini, J.D.; Hundal, M.; Zhou, X.; Israel, E.; Cernadas, M. Alternative macrophage activation is increased in asthma. Am. J. Respir. Cell Mol. Biol. 2016, 55, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, R.; Bozinovski, S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front. Immunol. 2014, 5, 435. [Google Scholar] [CrossRef]
- Braga, T.T.; Agudelo, J.S.H.; Camara, N.O.S. Macrophages during the fibrotic process: M2 as friend and foe. Front. Immunol. 2015, 6, 602. [Google Scholar] [CrossRef]
- Gibaud, S.; Andreux, J.; Weingarten, C.; Renard, M.; Couvreur, P. Increased bone marrow toxicity of doxorubicin bound to nanoparticles. Eur. J. Cancer 1994, 30, 820–826. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Gupta, V.; Zern, B.J.; Pan, D.; Zakrewsky, M.; Muzykantov, V.; Mitragotri, S. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano 2013, 7, 11129–11137. [Google Scholar] [CrossRef]
- Drozdov, A.S.; Nikitin, P.I.; Rozenberg, J.M. Systematic review of cancer targeting by nanoparticles revealed a global association between accumulation in tumors and spleen. Int. J. Mol. Sci. 2021, 22, 13011. [Google Scholar] [CrossRef]
- Demoy, M.; Andreux, J.-P.; Weingarten, C.; Gouritin, B.; Guilloux, V.; Couvreur, P. Spleen capture of nanoparticles: Influence of animal species and surface characteristics. Pharm. Res. 1999, 16, 37–41. [Google Scholar] [CrossRef]
- Den Haan, J.M.; Kraal, G. Innate immune functions of macrophage subpopulations in the spleen. J. Innate Immun. 2012, 4, 437–445. [Google Scholar] [CrossRef]
- Mulder, R.; Banete, A.; Basta, S. Spleen-derived macrophages are readily polarized into classically activated (M1) or alternatively activated (M2) states. Immunobiology 2014, 219, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Venosa, A.; Malaviya, R.; Gow, A.J.; Hall, L.; Laskin, J.D.; Laskin, D.L. Protective role of spleen-derived macrophages in lung inflammation, injury, and fibrosis induced by nitrogen mustard. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 309, L1487–L1498. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.; Meek, B.; van de Garde, E.M.; van Moorsel, C.H.; Vugts, D.J.; Keijsers, R.G.; Grutters, J.C. Altered splenic [89Zr] Zr-rituximab uptake in patients with interstitial lung disease not responding to rituximab: Could this indicate a splenic immune-mediated mechanism? Am. J. Nucl. Med. Mol. Imaging 2020, 10, 168. [Google Scholar] [PubMed]
- Newairy, A.; El-Sharaky, A.; Badreldeen, M.; Eweda, S.; Sheweita, S. The hepatoprotective effects of selenium against cadmium toxicity in rats. Toxicology 2007, 242, 23–30. [Google Scholar] [CrossRef]
- Messarah, M.; Klibet, F.; Boumendjel, A.; Abdennour, C.; Bouzerna, N.; Boulakoud, M.S.; El Feki, A. Hepatoprotective role and antioxidant capacity of selenium on arsenic-induced liver injury in rats. Exp. Toxicol. Pathol. 2012, 64, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Holm, B.; Notter, R.; Finkelstein, J. Surface property changes from interactions of albumin with natural lung surfactant and extracted lung lipids. Chem. Phys. Lipids 1985, 38, 287–298. [Google Scholar] [CrossRef]
- Ma, H.; Li, R.; Jiang, L.; Qiao, S.; Chen, X.-X.; Wang, A.; Zhang, G. Structural comparison of CD163 SRCR5 from different species sheds some light on its involvement in porcine reproductive and respiratory syndrome virus-2 infection in vitro. Vet. Res. 2021, 52, 97. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, K. The Role of Macrophages in Airway Disease Focusing on Porcine Reproductive and Respiratory Syndrome Virus and the Treatment with Antioxidant Nanoparticles. Viruses 2024, 16, 1563. https://doi.org/10.3390/v16101563
Choi K. The Role of Macrophages in Airway Disease Focusing on Porcine Reproductive and Respiratory Syndrome Virus and the Treatment with Antioxidant Nanoparticles. Viruses. 2024; 16(10):1563. https://doi.org/10.3390/v16101563
Chicago/Turabian StyleChoi, Kyuhyung. 2024. "The Role of Macrophages in Airway Disease Focusing on Porcine Reproductive and Respiratory Syndrome Virus and the Treatment with Antioxidant Nanoparticles" Viruses 16, no. 10: 1563. https://doi.org/10.3390/v16101563





