Minimally Modified HIV-1 Infection of Macaques: Development, Utility, and Limitations of Current Models
Abstract
1. Introduction
2. Rationale for the Development of Minimally Modified HIV-1-Based NHP Models
3. Obstacles to HIV-1 Infection of Macaques
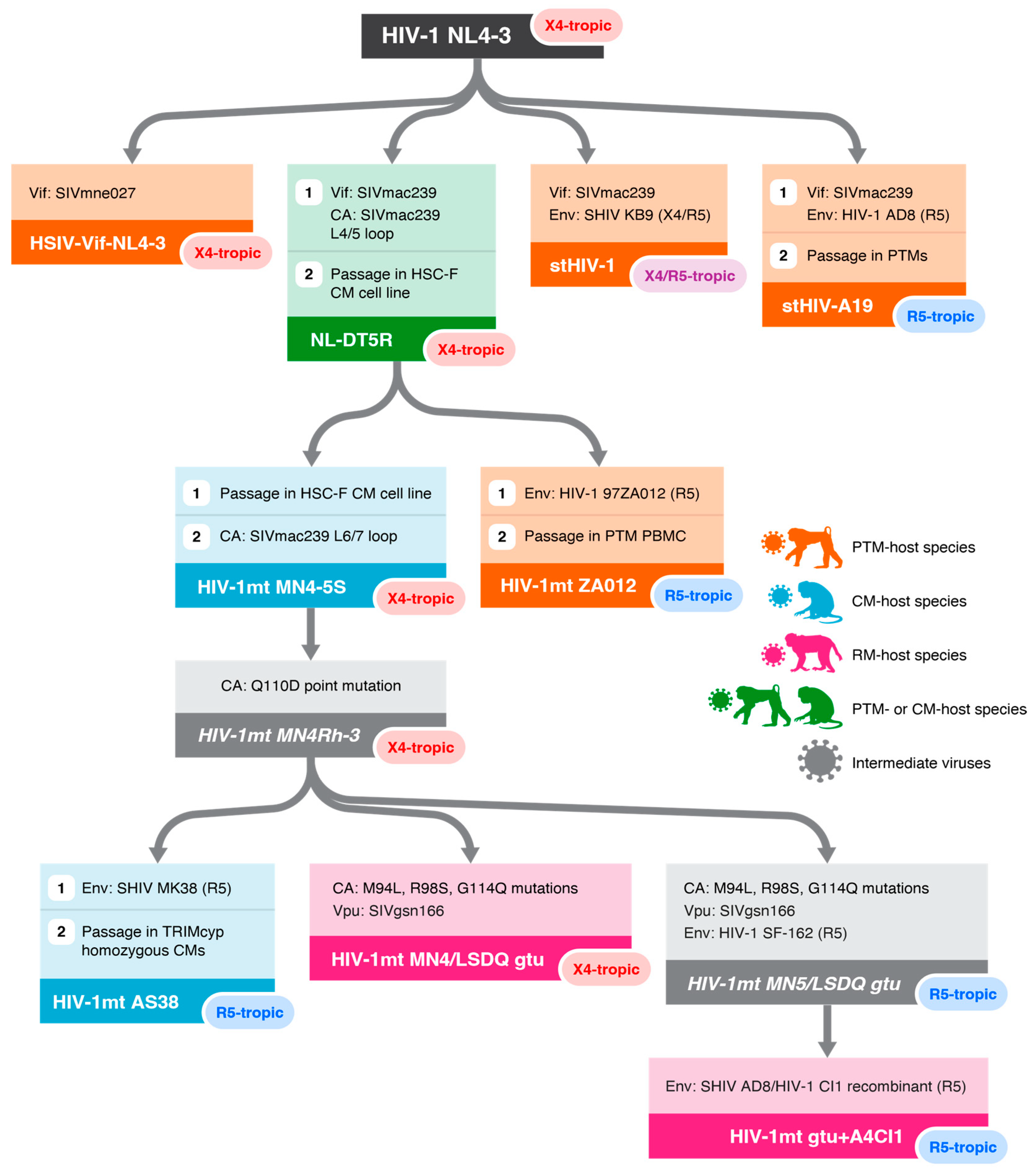
| Virus | Backbone | Vif | CA Modifications | Env | Coreceptor Tropism | Host NHP Species | n * | Peak PVL (vRNA Copies/mL) | Duration of Detected PVL | Pathogenic? | Notes | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NL-DT5R and derivatives | ||||||||||||
| NL-DT5R | HIV-1 NL4-3 | SIVmac239 | SIVmac239 L4/5 swap | HIV-1 NL4-3 | CXCR4 | PTM | 4 | ~104 | 5–16 weeks | No | 2 of 4 animals: anti-CD8α on days 1, 4, 7 | [45] |
| NL-DT5R | HIV-1 NL4-3 | SIVmac239 | SIVmac239 L4/5 swap | HIV-1 NL4-3 | CXCR4 | CM | 2 | ~103 | 4 weeks | No | [46] | |
| HIV-1mt ZA012 | HIV-1 NL4-3 | SIVmac239 | NL-DT5R | HIV-1 97ZA012 | CCR5 | PTM | 2 | ~106 | 8–16 weeks | No | Serially passaged in PTM PBMC | [47] |
| HIV-1mt AS38 | HIV-1 NL4-3 | SIVmac239 | NL-DT5R + SIVmac239 L6/7 swap + Q110D | SHIV MK38 | CCR5 | CM | 2 | ~106 | 10–20 weeks | No | Serially passaged in TRIMcyp homozygous CMs | [48] |
| HIV-1rmt MN4/ LSDQgtu | HIV-1 NL4-3 | SIVmac239 | HIV-1mt AS38 + M94L, R98S, G114Q | HIV-1 NL4-3 | CXCR4 | RM | 2 | ~105 | 6 weeks | No | Additional changes in pol, vpu | [49,50] |
| HIV-1rmt gtu+A4CI1 | HIV-1 NL4-3 | SIVmac239 | HIV-1mtAS38 + M94L, R98S, G114Q | SHIV AD8/CI1 Recombinant | CCR5 | RM | 1 | ~104 | 5 weeks | No | Additional changes in pol, vpu | [49,50] |
| HSIV | ||||||||||||
| HSIV-vif-NL4-3 | HIV-1 NL4-3 | SIVmne027 | N/A | HIV-1 NL4-3 | CXCR4 | PTM | 4 | 104–105 | 8–20 weeks | No | Tested in juvenile (n = 2) and newborn (n = 2) PTMs | [51] |
| stHIV | ||||||||||||
| stHIV-1 | HIV-1 NL4-3 | SIVmac239 | N/A | SHIV KB9 | CXCR4/CCR5 | PTM | 4 | 105–106 | 24 weeks | No | [52] | |
| stHIV-A19 | HIV-1 NL4-3 | SIVmac239 | N/A | HIV-1 AD8 | CCR5 | PTM | 3 | ~106 | >100 weeks | No | [53,54] | |
| stHIV-A19 (+CD8α depletion) | HIV-1 NL4-3 | SIVmac239 | N/A | HIV-1 AD8 | CCR5 | PTM | 3 | 106–107 | Until euthanasia | Yes | [53,54] | |
4. First-Generation Minimally Modified HIV-1s for Infection of PTMs
5. Further Development of PTM-Based Models
6. Strengths and Utility of PTM-Based Minimally Modified HIV-1 Models
6.1. Genetic Similarities between HIV-1 and mmHIV-1
6.2. Evaluations of Pre-Exposure Prophylaxis (PrEP) Agents
6.3. Utility of PTMs as a Host Species for Modeling for HIV Infection
7. Limitations of PTM-Based Models
8. Efforts to Develop Minimally Modified HIV-1 for Infection of CM and RM
9. Minimally Modified HIV-1 Models: Future Directions for the Field
9.1. Development of a Pathogenic mmHIV-1
9.2. Demonstration of mmHIV-1 Mucosal Transmissibility
9.3. Demonstration of the Establishment of a Rebound-Competent Viral Reservoir in mmHIV-1-Infected Macaques
9.4. Characterization of Residual Infected Cells That Persist in mmHIV-1 Models
10. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fultz, P.N. Nonhuman primate models for AIDS. Clin. Infect. Dis. 1993, 17 (Suppl. S1), S230–S235. [Google Scholar] [CrossRef] [PubMed]
- Lusso, P.; Markham, P.D.; Ranki, A.; Earl, P.; Moss, B.; Dorner, F.; Gallo, R.C.; Krohn, K.J. Cell-mediated immune response toward viral envelope and core antigens in gibbon apes (Hylobates lar) chronically infected with human immunodeficiency virus-1. J. Immunol. 1988, 141, 2467–2473. [Google Scholar] [CrossRef] [PubMed]
- Barre-Sinoussi, F.; Chermann, J.C.; Rey, F.; Nugeyre, M.T.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, C.; Vezinet-Brun, F.; Rouzioux, C.; et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef]
- Levy, J.A. HIV pathogenesis: 25 years of progress and persistent challenges. AIDS 2009, 23, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Schuler, T.; Zupancic, M.; Wietgrefe, S.; Staskus, K.A.; Reimann, K.A.; Reinhart, T.A.; Rogan, M.; Cavert, W.; Miller, C.J.; et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 1999, 286, 1353–1357. [Google Scholar] [CrossRef]
- Veazey, R.S.; Tham, I.C.; Mansfield, K.G.; DeMaria, M.; Forand, A.E.; Shvetz, D.E.; Chalifoux, L.V.; Sehgal, P.K.; Lackner, A.A. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: Highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 2000, 74, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Veazey, R.S.; DeMaria, M.; Chalifoux, L.V.; Shvetz, D.E.; Pauley, D.R.; Knight, H.L.; Rosenzweig, M.; Johnson, R.P.; Desrosiers, R.C.; Lackner, A.A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 1998, 280, 427–431. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef]
- Estes, J.D.; Harris, L.D.; Klatt, N.R.; Tabb, B.; Pittaluga, S.; Paiardini, M.; Barclay, G.R.; Smedley, J.; Pung, R.; Oliveira, K.M.; et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010, 6, e1001052. [Google Scholar] [CrossRef]
- Silvestri, G.; Sodora, D.L.; Koup, R.A.; Paiardini, M.; O’Neil, S.P.; McClure, H.M.; Staprans, S.I.; Feinberg, M.B. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 2003, 18, 441–452. [Google Scholar] [CrossRef]
- Silvestri, G.; Fedanov, A.; Germon, S.; Kozyr, N.; Kaiser, W.J.; Garber, D.A.; McClure, H.; Feinberg, M.B.; Staprans, S.I. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J. Virol. 2005, 79, 4043–4054. [Google Scholar] [CrossRef] [PubMed]
- Sumpter, B.; Dunham, R.; Gordon, S.; Engram, J.; Hennessy, M.; Kinter, A.; Paiardini, M.; Cervasi, B.; Klatt, N.; McClure, H.; et al. Correlates of preserved CD4+ T cell homeostasis during natural, nonpathogenic simian immunodeficiency virus infection of sooty mangabeys: Implications for AIDS pathogenesis. J. Immunol. 2007, 178, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.D.; Gordon, S.N.; Zeng, M.; Chahroudi, A.M.; Dunham, R.M.; Staprans, S.I.; Reilly, C.S.; Silvestri, G.; Haase, A.T. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J. Immunol. 2008, 180, 6798–6807. [Google Scholar] [CrossRef]
- Horiike, M.; Iwami, S.; Kodama, M.; Sato, A.; Watanabe, Y.; Yasui, M.; Ishida, Y.; Kobayashi, T.; Miura, T.; Igarashi, T. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology 2012, 423, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Whitney, J.B.; Hill, A.L.; Sanisetty, S.; Penaloza-MacMaster, P.; Liu, J.; Shetty, M.; Parenteau, L.; Cabral, C.; Shields, J.; Blackmore, S.; et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 2014, 512, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Keele, B.F.; Okoye, A.A.; Fennessey, C.M.; Varco-Merth, B.; Immonen, T.T.; Kose, E.; Conchas, A.; Pinkevych, M.; Lipkey, L.; Newman, L.; et al. Early antiretroviral therapy in SIV-infected rhesus macaques reveals a multiphasic, saturable dynamic accumulation of the rebound competent viral reservoir. PLoS Pathog. 2024, 20, e1012135. [Google Scholar] [CrossRef]
- Daniel, M.D.; Letvin, N.L.; King, N.W.; Kannagi, M.; Sehgal, P.K.; Hunt, R.D.; Kanki, P.J.; Essex, M.; Desrosiers, R.C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science 1985, 228, 1201–1204. [Google Scholar] [CrossRef]
- Simon, M.A.; Brodie, S.J.; Sasseville, V.G.; Chalifoux, L.V.; Desrosiers, R.C.; Ringler, D.J. Immunopathogenesis of SIVmac. Virus Res. 1994, 32, 227–251. [Google Scholar] [CrossRef]
- Pollom, E.; Dang, K.K.; Potter, E.L.; Gorelick, R.J.; Burch, C.L.; Weeks, K.M.; Swanstrom, R. Comparison of SIV and HIV-1 genomic RNA structures reveals impact of sequence evolution on conserved and non-conserved structural motifs. PLoS Pathog. 2013, 9, e1003294. [Google Scholar] [CrossRef]
- Ambrose, Z.; KewalRamani, V.N.; Bieniasz, P.D.; Hatziioannou, T. HIV/AIDS: In search of an animal model. Trends Biotechnol. 2007, 25, 333–337. [Google Scholar] [CrossRef]
- O’Brien, S.P.; Swanstrom, A.E.; Pegu, A.; Ko, S.Y.; Immonen, T.T.; Del Prete, G.Q.; Fennessey, C.M.; Gorman, J.; Foulds, K.E.; Schmidt, S.D.; et al. Rational design and in vivo selection of SHIVs encoding transmitted/founder subtype C HIV-1 envelopes. PLoS Pathog. 2019, 15, e1007632. [Google Scholar] [CrossRef] [PubMed]
- Mosier, D.E. How HIV changes its tropism: Evolution and adaptation? Curr. Opin. HIV AIDS 2009, 4, 125–130. [Google Scholar] [CrossRef]
- Boyd, D.F.; Peterson, D.; Haggarty, B.S.; Jordan, A.P.; Hogan, M.J.; Goo, L.; Hoxie, J.A.; Overbaugh, J. Mutations in HIV-1 envelope that enhance entry with the macaque CD4 receptor alter antibody recognition by disrupting quaternary interactions within the trimer. J. Virol. 2015, 89, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Siddappa, N.B.; Song, R.; Kramer, V.G.; Chenine, A.L.; Velu, V.; Ong, H.; Rasmussen, R.A.; Grisson, R.D.; Wood, C.; Zhang, H.; et al. Neutralization-sensitive R5-tropic simian-human immunodeficiency virus SHIV-2873Nip, which carries env isolated from an infant with a recent HIV clade C infection. J. Virol. 2009, 83, 1422–1432. [Google Scholar] [CrossRef]
- Cheng-Mayer, C.; Brown, A.; Harouse, J.; Luciw, P.A.; Mayer, A.J. Selection for neutralization resistance of the simian/human immunodeficiency virus SHIVSF33A variant in vivo by virtue of sequence changes in the extracellular envelope glycoprotein that modify N-linked glycosylation. J. Virol. 1999, 73, 5294–5300. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, S.; Lee, F.H.; Roark, R.S.; Murphy, A.I.; Smith, J.; Zhao, C.; Rando, J.; Chohan, N.; Ding, Y.; et al. New SHIVs and Improved Design Strategy for Modeling HIV-1 Transmission, Immunopathogenesis, Prevention and Cure. J. Virol. 2021, 95, e00071-21. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.M.; Ziani, W.; Lindemuth, E.; Kuri-Cervantes, L.; Li, H.; Lee, F.H.; Watkins, M.; Ding, W.; Xu, H.; Veazey, R.; et al. Novel Transmitted/Founder Simian-Human Immunodeficiency Viruses for Human Immunodeficiency Virus Latency and Cure Research. J. Virol. 2020, 94, e01659-19. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, G.Q.; Keele, B.F.; Fode, J.; Thummar, K.; Swanstrom, A.E.; Rodriguez, A.; Raymond, A.; Estes, J.D.; LaBranche, C.C.; Montefiori, D.C.; et al. A single gp120 residue can affect HIV-1 tropism in macaques. PLoS Pathog. 2017, 13, e1006572. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, S.; Kong, R.; Ding, W.; Lee, F.H.; Parker, Z.; Kim, E.; Learn, G.H.; Hahn, P.; Policicchio, B.; et al. Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques. Proc. Natl. Acad. Sci. USA 2016, 113, E3413–E3422. [Google Scholar] [CrossRef]
- Balzarini, J.; Weeger, M.; Camarasa, M.J.; De Clercq, E.; Uberla, K. Sensitivity/resistance profile of a simian immunodeficiency virus containing the reverse transcriptase gene of human immunodeficiency virus type 1 (HIV-1) toward the HIV-1-specific non-nucleoside reverse transcriptase inhibitors. Biochem. Biophys. Res. Commun. 1995, 211, 850–856. [Google Scholar] [CrossRef]
- Ishimatsu, M.; Suzuki, H.; Akiyama, H.; Miura, T.; Hayami, M.; Ido, E. Construction of a novel SHIV having an HIV-1-derived protease gene and its infection to rhesus macaques: A useful tool for in vivo efficacy tests of protease inhibitors. Microbes Infect. 2007, 9, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Dauner, A.; Li, B.; Srinivasan, P.; Mitchell, J.; Hendry, M.; Ellenberger, D.; Butera, S.; Otten, R.A. Generation of a dual RT Env SHIV that is infectious in rhesus macaques. J. Med. Primatol. 2010, 39, 213–223. [Google Scholar] [CrossRef]
- Agy, M.B.; Frumkin, L.R.; Corey, L.; Coombs, R.W.; Wolinsky, S.M.; Koehler, J.; Morton, W.R.; Katze, M.G. Infection of Macaca nemestrina by human immunodeficiency virus type-1. Science 1992, 257, 103–106. [Google Scholar] [CrossRef]
- Cowan, S.; Hatziioannou, T.; Cunningham, T.; Muesing, M.A.; Gottlinger, H.G.; Bieniasz, P.D. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc. Natl. Acad. Sci. USA 2002, 99, 11914–11919. [Google Scholar] [CrossRef] [PubMed]
- Munk, C.; Brandt, S.M.; Lucero, G.; Landau, N.R. A dominant block to HIV-1 replication at reverse transcription in simian cells. Proc. Natl. Acad. Sci. USA 2002, 99, 13843–13848. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.B.; Luciw, P.A. Animal models of AIDS. FASEB J. 1989, 3, 2593–2606. [Google Scholar] [CrossRef] [PubMed]
- Saxinger, C.; Alter, H.J.; Eichberg, J.W.; Fauci, A.S.; Robey, W.G.; Gallo, R.C. Stages in the progression of HIV infection in chimpanzees. AIDS Res. Hum. Retroviruses 1987, 3, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Stremlau, M.; Owens, C.M.; Perron, M.J.; Kiessling, M.; Autissier, P.; Sodroski, J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 2004, 427, 848–853. [Google Scholar] [CrossRef]
- Gaba, A.; Flath, B.; Chelico, L. Examination of the APOBEC3 Barrier to Cross Species Transmission of Primate Lentiviruses. Viruses 2021, 13, 1084. [Google Scholar] [CrossRef]
- Mariani, R.; Chen, D.; Schrofelbauer, B.; Navarro, F.; Konig, R.; Bollman, B.; Munk, C.; Nymark-McMahon, H.; Landau, N.R. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell 2003, 114, 21–31. [Google Scholar] [CrossRef]
- Neil, S.J.; Zang, T.; Bieniasz, P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 2008, 451, 425–430. [Google Scholar] [CrossRef]
- Goffinet, C.; Allespach, I.; Homann, S.; Tervo, H.M.; Habermann, A.; Rupp, D.; Oberbremer, L.; Kern, C.; Tibroni, N.; Welsch, S.; et al. HIV-1 antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe 2009, 5, 285–297. [Google Scholar] [CrossRef]
- McNatt, M.W.; Zang, T.; Hatziioannou, T.; Bartlett, M.; Fofana, I.B.; Johnson, W.E.; Neil, S.J.; Bieniasz, P.D. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 2009, 5, e1000300. [Google Scholar] [CrossRef]
- Jia, B.; Serra-Moreno, R.; Neidermyer, W.; Rahmberg, A.; Mackey, J.; Fofana, I.B.; Johnson, W.E.; Westmoreland, S.; Evans, D.T. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 2009, 5, e1000429. [Google Scholar] [CrossRef]
- Igarashi, T.; Iyengar, R.; Byrum, R.A.; Buckler-White, A.; Dewar, R.L.; Buckler, C.E.; Lane, H.C.; Kamada, K.; Adachi, A.; Martin, M.A. Human immunodeficiency virus type 1 derivative with 7% simian immunodeficiency virus genetic content is able to establish infections in pig-tailed macaques. J. Virol. 2007, 81, 11549–11552. [Google Scholar] [CrossRef]
- Saito, A.; Nomaguchi, M.; Iijima, S.; Kuroishi, A.; Yoshida, T.; Lee, Y.J.; Hayakawa, T.; Kono, K.; Nakayama, E.E.; Shioda, T.; et al. Improved capacity of a monkey-tropic HIV-1 derivative to replicate in cynomolgus monkeys with minimal modifications. Microbes Infect. 2011, 13, 58–64. [Google Scholar] [CrossRef]
- Otsuki, H.; Yoneda, M.; Igarashi, T.; Miura, T. Generation of a monkey-tropic human immunodeficiency virus type 1 carrying env from a CCR5-tropic subtype C clinical isolate. Virology 2014, 460-461, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ode, H.; Saito, A.; Washizaki, A.; Seki, Y.; Yoshida, T.; Harada, S.; Ishii, H.; Shioda, T.; Yasutomi, Y.; Matano, T.; et al. Development of a novel Macaque-Tropic HIV-1 adapted to cynomolgus macaques. J. Gen. Virol. 2022, 103, 001790. [Google Scholar] [CrossRef]
- Doi, N.; Miura, T.; Mori, H.; Sakawaki, H.; Koma, T.; Adachi, A.; Nomaguchi, M. CXCR4- and CCR5-Tropic HIV-1 Clones Are Both Tractable to Grow in Rhesus Macaques. Front. Microbiol. 2018, 9, 2510. [Google Scholar] [CrossRef]
- Nomaguchi, M.; Yokoyama, M.; Kono, K.; Nakayama, E.E.; Shioda, T.; Doi, N.; Fujiwara, S.; Saito, A.; Akari, H.; Miyakawa, K.; et al. Generation of rhesus macaque-tropic HIV-1 clones that are resistant to major anti-HIV-1 restriction factors. J. Virol. 2013, 87, 11447–11461. [Google Scholar] [CrossRef] [PubMed]
- Thippeshappa, R.; Ruan, H.; Wang, W.; Zhou, P.; Kimata, J.T. A variant macaque-tropic human immunodeficiency virus type 1 is resistant to alpha interferon-induced restriction in pig-tailed macaque CD4+ T cells. J. Virol. 2013, 87, 6678–6692. [Google Scholar] [CrossRef] [PubMed]
- Hatziioannou, T.; Ambrose, Z.; Chung, N.P.; Piatak, M., Jr.; Yuan, F.; Trubey, C.M.; Coalter, V.; Kiser, R.; Schneider, D.; Smedley, J.; et al. A macaque model of HIV-1 infection. Proc. Natl. Acad. Sci. USA 2009, 106, 4425–4429. [Google Scholar] [CrossRef] [PubMed]
- Hatziioannou, T.; Del Prete, G.Q.; Keele, B.F.; Estes, J.D.; McNatt, M.W.; Bitzegeio, J.; Raymond, A.; Rodriguez, A.; Schmidt, F.; Mac Trubey, C.; et al. HIV-1-induced AIDS in monkeys. Science 2014, 344, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Keele, B.F.; Del Prete, G.Q.; Voronin, D.; Fennessey, C.M.; Soll, S.; Kane, M.; Raymond, A.; Gifford, R.J.; KewalRamani, V.; et al. Derivation of simian tropic HIV-1 infectious clone reveals virus adaptation to a new host. Proc. Natl. Acad. Sci. USA 2019, 116, 10504–10509. [Google Scholar] [CrossRef] [PubMed]
- Agy, M.B.; Schmidt, A.; Florey, M.J.; Kennedy, B.J.; Schaefer, G.; Katze, M.G.; Corey, L.; Morton, W.R.; Bosch, M.L. Serial in vivo passage of HIV-1 infection in Macaca nemestrina. Virology 1997, 238, 336–343. [Google Scholar] [CrossRef]
- Brennan, G.; Kozyrev, Y.; Hu, S.L. TRIMCyp expression in Old World primates Macaca nemestrina and Macaca fascicularis. Proc. Natl. Acad. Sci. USA 2008, 105, 3569–3574. [Google Scholar] [CrossRef] [PubMed]
- Kimata, J.T.; Kuller, L.; Anderson, D.B.; Dailey, P.; Overbaugh, J. Emerging cytopathic and antigenic simian immunodeficiency virus variants influence AIDS progression. Nat. Med. 1999, 5, 535–541. [Google Scholar] [CrossRef]
- Thippeshappa, R.; Polacino, P.; Yu Kimata, M.T.; Siwak, E.B.; Anderson, D.; Wang, W.; Sherwood, L.; Arora, R.; Wen, M.; Zhou, P.; et al. Vif substitution enables persistent infection of pig-tailed macaques by human immunodeficiency virus type 1. J. Virol. 2011, 85, 3767–3779. [Google Scholar] [CrossRef]
- Karlsson, G.B.; Halloran, M.; Li, J.; Park, I.W.; Gomila, R.; Reimann, K.A.; Axthelm, M.K.; Iliff, S.A.; Letvin, N.L.; Sodroski, J. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J. Virol. 1997, 71, 4218–4225. [Google Scholar] [CrossRef]
- Humes, D.; Overbaugh, J. Adaptation of subtype a human immunodeficiency virus type 1 envelope to pig-tailed macaque cells. J. Virol. 2011, 85, 4409–4420. [Google Scholar] [CrossRef]
- Humes, D.; Emery, S.; Laws, E.; Overbaugh, J. A species-specific amino acid difference in the macaque CD4 receptor restricts replication by global circulating HIV-1 variants representing viruses from recent infection. J. Virol. 2012, 86, 12472–12483. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Warren, C.J.; Meyerson, N.R.; Dirasantha, O.; Feldman, E.R.; Wilkerson, G.K.; Sawyer, S.L. Selective use of primate CD4 receptors by HIV-1. PLoS Biol. 2019, 17, e3000304. [Google Scholar] [CrossRef]
- Meyerson, N.R.; Sharma, A.; Wilkerson, G.K.; Overbaugh, J.; Sawyer, S.L. Identification of Owl Monkey CD4 Receptors Broadly Compatible with Early-Stage HIV-1 Isolates. J. Virol. 2015, 89, 8611–8622. [Google Scholar] [CrossRef]
- Pang, W.; Zhang, G.H.; Jiang, J.; Zheng, H.Y.; Zhang, L.T.; Zhang, X.L.; Song, J.H.; Zhang, M.X.; Zhu, J.W.; Lei, A.H.; et al. HIV-1 can infect northern pig-tailed macaques (Macaca leonina) and form viral reservoirs in vivo. Sci. Bull. 2017, 62, 1315–1324. [Google Scholar] [CrossRef]
- Hrecka, K.; Hao, C.; Gierszewska, M.; Swanson, S.K.; Kesik-Brodacka, M.; Srivastava, S.; Florens, L.; Washburn, M.P.; Skowronski, J. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 2011, 474, 658–661. [Google Scholar] [CrossRef]
- Laguette, N.; Rahm, N.; Sobhian, B.; Chable-Bessia, C.; Munch, J.; Snoeck, J.; Sauter, D.; Switzer, W.M.; Heneine, W.; Kirchhoff, F.; et al. Evolutionary and functional analyses of the interaction between the myeloid restriction factor SAMHD1 and the lentiviral Vpx protein. Cell Host Microbe 2012, 11, 205–217. [Google Scholar] [CrossRef]
- Laguette, N.; Sobhian, B.; Casartelli, N.; Ringeard, M.; Chable-Bessia, C.; Segeral, E.; Yatim, A.; Emiliani, S.; Schwartz, O.; Benkirane, M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 2011, 474, 654–657. [Google Scholar] [CrossRef]
- Thippeshappa, R.; Polacino, P.; Chandrasekar, S.S.; Truong, K.; Misra, A.; Aulicino, P.C.; Hu, S.L.; Kaushal, D.; Kimata, J.T. In vivo Serial Passaging of Human-Simian Immunodeficiency Virus Clones Identifies Characteristics for Persistent Viral Replication. Front. Microbiol. 2021, 12, 779460. [Google Scholar] [CrossRef]
- Connor, R.I.; Sheridan, K.E.; Ceradini, D.; Choe, S.; Landau, N.R. Change in coreceptor use correlates with disease progression in HIV-1—Infected individuals. J. Exp. Med. 1997, 185, 621–628. [Google Scholar] [CrossRef]
- Schuitemaker, H.; Koot, M.; Kootstra, N.A.; Dercksen, M.W.; de Goede, R.E.; van Steenwijk, R.P.; Lange, J.M.; Schattenkerk, J.K.; Miedema, F.; Tersmette, M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: Progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J. Virol. 1992, 66, 1354–1360. [Google Scholar] [CrossRef]
- Tersmette, M.; Gruters, R.A.; de Wolf, F.; de Goede, R.E.; Lange, J.M.; Schellekens, P.T.; Goudsmit, J.; Huisman, H.G.; Miedema, F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: Studies on sequential HIV isolates. J. Virol. 1989, 63, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Connor, R.I.; Ho, D.D. Human immunodeficiency virus type 1 variants with increased replicative capacity develop during the asymptomatic stage before disease progression. J. Virol. 1994, 68, 4400–4408. [Google Scholar] [CrossRef] [PubMed]
- Tersmette, M.; de Goede, R.E.; Al, B.J.; Winkel, I.N.; Gruters, R.A.; Cuypers, H.T.; Huisman, H.G.; Miedema, F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: Frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J. Virol. 1988, 62, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Swanstrom, A.E.; Gorelick, R.J.; Welker, J.L.; Schmidt, F.; Lu, B.; Wang, K.; Rowe, W.; Breed, M.W.; Killoran, K.E.; Kramer, J.A.; et al. Long-acting lenacapavir protects macaques against intravenous challenge with simian-tropic HIV. EBioMedicine 2023, 95, 104764. [Google Scholar] [CrossRef]
- Link, J.O.; Rhee, M.S.; Tse, W.C.; Zheng, J.; Somoza, J.R.; Rowe, W.; Begley, R.; Chiu, A.; Mulato, A.; Hansen, D.; et al. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature 2020, 584, 614–618. [Google Scholar] [CrossRef]
- Witvrouw, M.; Pannecouque, C.; Van Laethem, K.; Desmyter, J.; De Clercq, E.; Vandamme, A.M. Activity of non-nucleoside reverse transcriptase inhibitors against HIV-2 and SIV. AIDS 1999, 13, 1477–1483. [Google Scholar] [CrossRef]
- Kamada, K.; Igarashi, T.; Martin, M.A.; Khamsri, B.; Hatcho, K.; Yamashita, T.; Fujita, M.; Uchiyama, T.; Adachi, A. Generation of HIV-1 derivatives that productively infect macaque monkey lymphoid cells. Proc. Natl. Acad. Sci. USA 2006, 103, 16959–16964. [Google Scholar] [CrossRef]
- Klatt, N.R.; Harris, L.D.; Vinton, C.L.; Sung, H.; Briant, J.A.; Tabb, B.; Morcock, D.; McGinty, J.W.; Lifson, J.D.; Lafont, B.A.; et al. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol. 2010, 3, 387–398. [Google Scholar] [CrossRef]
- Blakley, G.B.; Beamer, T.W.; Dukelow, W.R. Characteristics of the menstrual cycle in nonhuman primates. IV. Timed mating in Macaca nemestrina. Lab. Anim. 1981, 15, 351–353. [Google Scholar] [CrossRef]
- Kersh, E.N.; Henning, T.; Vishwanathan, S.A.; Morris, M.; Butler, K.; Adams, D.R.; Guenthner, P.; Srinivasan, P.; Smith, J.; Radzio, J.; et al. SHIV susceptibility changes during the menstrual cycle of pigtail macaques. J. Med. Primatol. 2014, 43, 310–316. [Google Scholar] [CrossRef]
- Vishwanathan, S.A.; Guenthner, P.C.; Lin, C.Y.; Dobard, C.; Sharma, S.; Adams, D.R.; Otten, R.A.; Heneine, W.; Hendry, R.M.; McNicholl, J.M.; et al. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J. Acquir. Immune Defic. Syndr. 2011, 57, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Kuroishi, A.; Saito, A.; Shingai, Y.; Shioda, T.; Nomaguchi, M.; Adachi, A.; Akari, H.; Nakayama, E.E. Modification of a loop sequence between alpha-helices 6 and 7 of virus capsid (CA) protein in a human immunodeficiency virus type 1 (HIV-1) derivative that has simian immunodeficiency virus (SIVmac239) vif and CA alpha-helices 4 and 5 loop improves replication in cynomolgus monkey cells. Retrovirology 2009, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Nomaguchi, M.; Kono, K.; Iwatani, Y.; Yokoyama, M.; Yasutomi, Y.; Sato, H.; Shioda, T.; Sugiura, W.; Matano, T.; et al. TRIM5 genotypes in cynomolgus monkeys primarily influence inter-individual diversity in susceptibility to monkey-tropic human immunodeficiency virus type 1. J. Gen. Virol. 2013, 94, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.D.; Hartshorn, K.L.; Rota, T.R.; Andrews, C.A.; Kaplan, J.C.; Schooley, R.T.; Hirsch, M.S. Recombinant human interferon alfa-A suppresses HTLV-III replication in vitro. Lancet 1985, 1, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Kornbluth, R.S.; Oh, P.S.; Munis, J.R.; Cleveland, P.H.; Richman, D.D. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J. Exp. Med. 1989, 169, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, Y.; Pitha, P.M. Alpha interferon inhibits early stages of the human immunodeficiency virus type 1 replication cycle. J. Virol. 1992, 66, 1321–1328. [Google Scholar] [CrossRef]
- Cheney, K.M.; McKnight, A. Interferon-alpha mediates restriction of human immunodeficiency virus type-1 replication in primary human macrophages at an early stage of replication. PLoS ONE 2010, 5, e13521. [Google Scholar] [CrossRef]
- Cocchi, F.; DeVico, A.L.; Garzino-Demo, A.; Arya, S.K.; Gallo, R.C.; Lusso, P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995, 270, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Mackewicz, C.E.; Blackbourn, D.J.; Levy, J.A. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc. Natl. Acad. Sci. USA 1995, 92, 2308–2312. [Google Scholar] [CrossRef]
- Meas, H.Z.; Haug, M.; Beckwith, M.S.; Louet, C.; Ryan, L.; Hu, Z.; Landskron, J.; Nordbo, S.A.; Tasken, K.; Yin, H.; et al. Sensing of HIV-1 by TLR8 activates human T cells and reverses latency. Nat. Commun. 2020, 11, 147. [Google Scholar] [CrossRef]
- Pang, W.; Shao, Y.; Zhuang, X.L.; Lu, Y.; He, W.Q.; Zheng, H.Y.; Xin, R.; Zhang, M.X.; Zhang, X.L.; Song, J.H.; et al. Genomic Evidence for the Nonpathogenic State in HIV-1-Infected Northern Pig-Tailed Macaques. Mol. Biol. Evol. 2023, 40, msad101. [Google Scholar] [CrossRef] [PubMed]
- Palesch, D.; Bosinger, S.E.; Tharp, G.K.; Vanderford, T.H.; Paiardini, M.; Chahroudi, A.; Johnson, Z.P.; Kirchhoff, F.; Hahn, B.H.; Norgren, R.B.; et al. Sooty mangabey genome sequence provides insight into AIDS resistance in a natural SIV host. Nature 2018, 553, 77–81. [Google Scholar] [CrossRef]
- He, W.Q.; He, X.Y.; Lu, Y.; Zhang, S.; Zhang, M.X.; Zheng, Y.T.; Pang, W. HIV-1 but not SIV(mac239) induces higher interferon-alpha antiviral state in chronic infected northern pig-tailed macaques (Macaca leonina). Microbes Infect. 2022, 24, 104970. [Google Scholar] [CrossRef] [PubMed]
- Arien, K.K.; Jespers, V.; Vanham, G. HIV sexual transmission and microbicides. Rev. Med. Virol. 2011, 21, 110–133. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Jansson, J.; Law, M.; Prestage, G.P.; Zablotska, I.; Imrie, J.C.; Kippax, S.C.; Kaldor, J.M.; Grulich, A.E.; Wilson, D.P. Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART. AIDS 2010, 24, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Boily, M.C.; Baggaley, R.F.; Wang, L.; Masse, B.; White, R.G.; Hayes, R.J.; Alary, M. Heterosexual risk of HIV-1 infection per sexual act: Systematic review and meta-analysis of observational studies. Lancet Infect. Dis. 2009, 9, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Vajdy, M. Mucosal HIV transmission and vaccination strategies through oral compared with vaginal and rectal routes. Expert Opin. Biol. Ther. 2010, 10, 1181–1195. [Google Scholar] [CrossRef]
- Varghese, B.; Maher, J.E.; Peterman, T.A.; Branson, B.M.; Steketee, R.W. Reducing the risk of sexual HIV transmission: Quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex. Transm. Dis. 2002, 29, 38–43. [Google Scholar] [CrossRef]
- Nduati, R.; John, G.; Mbori-Ngacha, D.; Richardson, B.; Overbaugh, J.; Mwatha, A.; Ndinya-Achola, J.; Bwayo, J.; Onyango, F.E.; Hughes, J.; et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: A randomized clinical trial. JAMA 2000, 283, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- De Cock, K.M.; Fowler, M.G.; Mercier, E.; de Vincenzi, I.; Saba, J.; Hoff, E.; Alnwick, D.J.; Rogers, M.; Shaffer, N. Prevention of mother-to-child HIV transmission in resource-poor countries: Translating research into policy and practice. JAMA 2000, 283, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, M.; Nag, M.; Del Prete, G.Q. Minimally Modified HIV-1 Infection of Macaques: Development, Utility, and Limitations of Current Models. Viruses 2024, 16, 1618. https://doi.org/10.3390/v16101618
Sharma M, Nag M, Del Prete GQ. Minimally Modified HIV-1 Infection of Macaques: Development, Utility, and Limitations of Current Models. Viruses. 2024; 16(10):1618. https://doi.org/10.3390/v16101618
Chicago/Turabian StyleSharma, Manish, Mukta Nag, and Gregory Q. Del Prete. 2024. "Minimally Modified HIV-1 Infection of Macaques: Development, Utility, and Limitations of Current Models" Viruses 16, no. 10: 1618. https://doi.org/10.3390/v16101618
APA StyleSharma, M., Nag, M., & Del Prete, G. Q. (2024). Minimally Modified HIV-1 Infection of Macaques: Development, Utility, and Limitations of Current Models. Viruses, 16(10), 1618. https://doi.org/10.3390/v16101618





