Mass Mortality in Terns and Gulls Associated with Highly Pathogenic Avian Influenza Viruses in Caspian Sea, Kazakhstan
Abstract
:1. Introduction
2. Materials and Methods
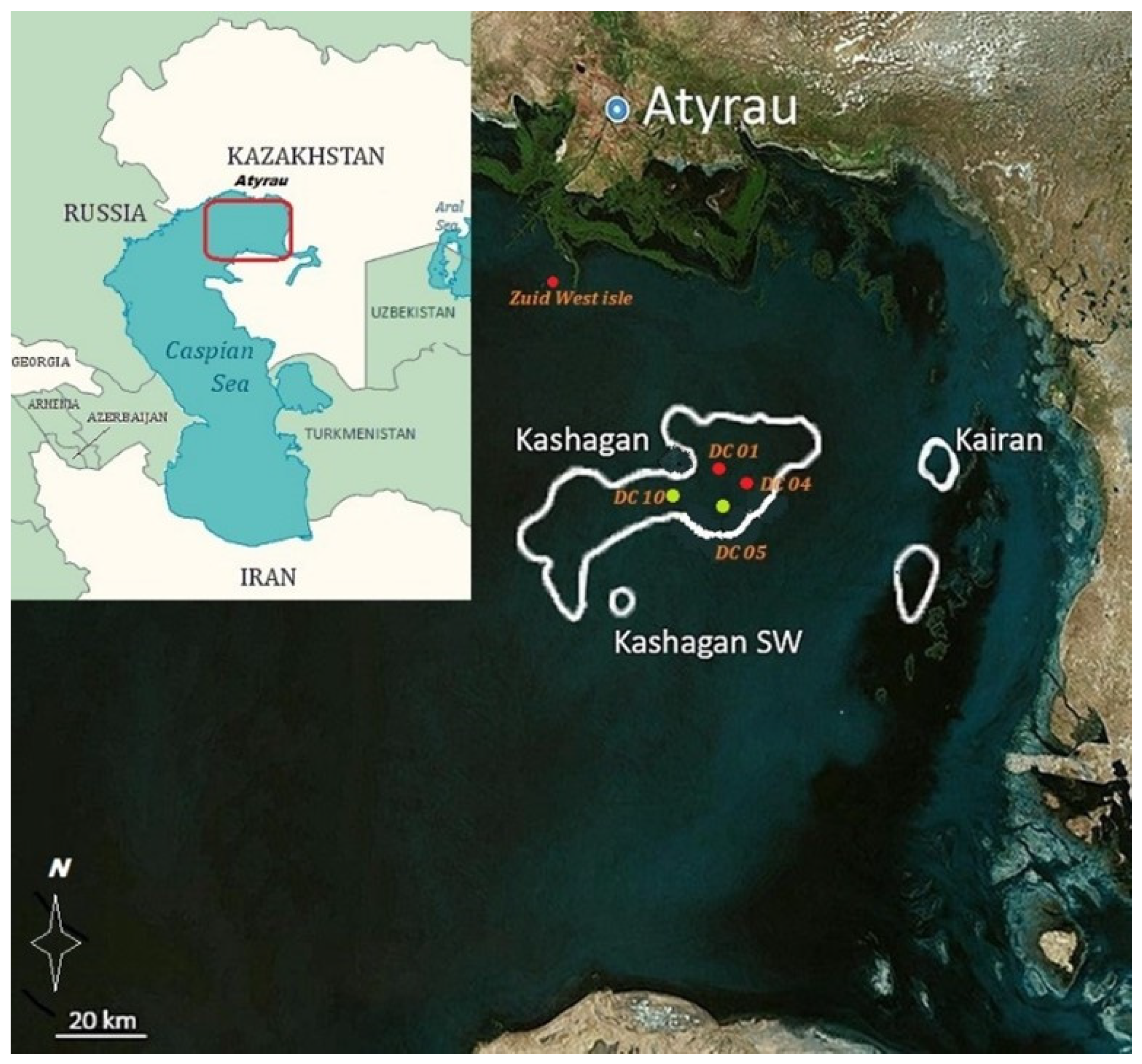
2.1. Field Study
2.2. Sample Preparation and Nucleic Acid Extraction
2.3. Diagnostic Procedure
2.4. Sequencing and Phylogenetic Analysis
3. Results
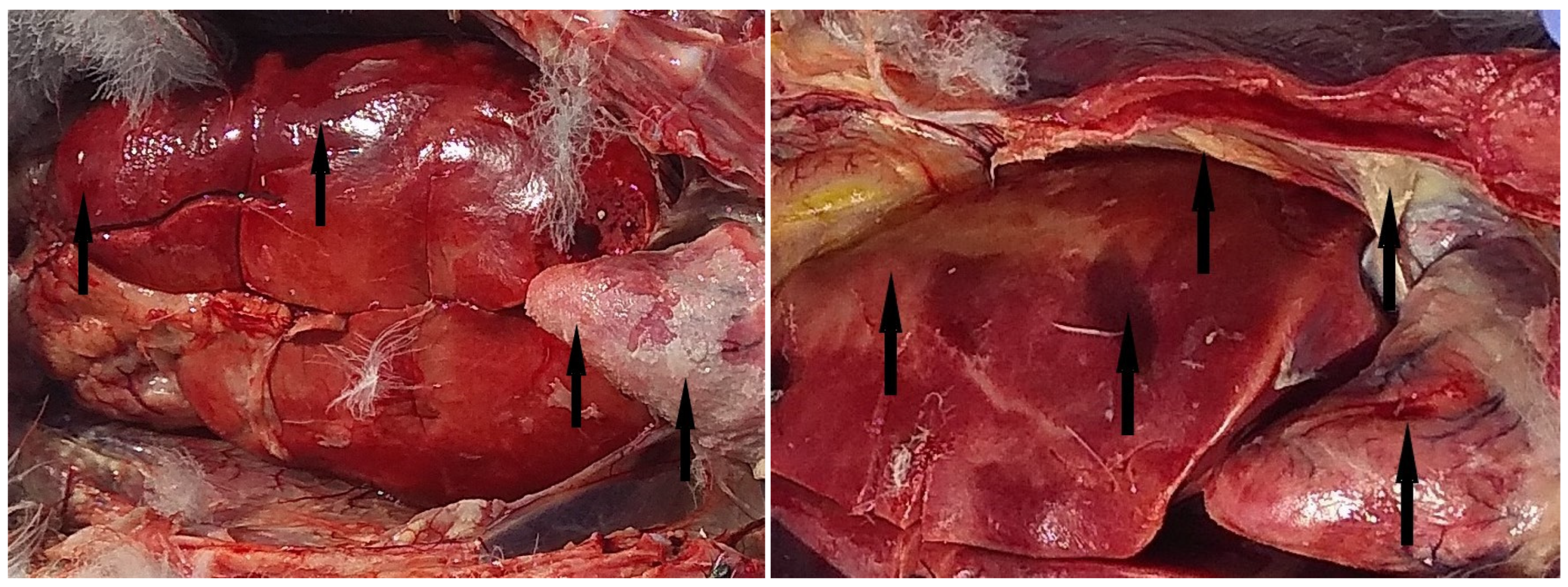
3.1. Pathological Findings
3.2. Molecular Diagnostic Findings
3.3. Phylogenetic Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fereidouni, S. Ecology of avian influenza viruses in wild birds. In Ecology of Wild Bird Diseases; CRC Press: Boca Raton, FL, USA, 2024; pp. 108–129. [Google Scholar] [CrossRef]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Fereidouni, S.; Starick, E.; Karamendin, K.; Di Genova, C.; Scott, S.D.; Khan, Y.; Harder, T.; Kydyrmanov, A. Genetic characterization of a new candidate hemagglutinin subtype of influenza A viruses. Emerg. Microbes Infect. 2023, 12, 2225645. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Bertran, K.; Kwon, J.-H.; Swayne, D.E. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. J. Vet. Sci. 2017, 18, 269–280. [Google Scholar] [CrossRef]
- Olsen, B.; Munster, V.J.; Wallensten, A.; Waldenström, J.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Global Patterns of Influenza a Virus in Wild Birds. Science 2006, 312, 384–388. [Google Scholar] [CrossRef]
- Alexander, D.J.; Brown, I.H. History of highly pathogenic avian influenza. Rev. Sci. Tech. Int. Off. Epizoot. 2009, 28, 19–38. [Google Scholar] [CrossRef]
- King, J.; Harder, T.; Conraths, F.J.; Beer, M.; Pohlmann, A. The genetics of highly pathogenic avian influenza viruses of subtype H5 in Germany, 2006–2020. Transbound. Emerg. Dis. 2020, 68, 1136–1150. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; et al. Avian influenza overview December 2021–March 2022. EFSA J. 2022, 20, e07289. [Google Scholar] [CrossRef]
- Banyard, A.C.; Lean, F.Z.X.; Robinson, C.; Howie, F.; Tyler, G.; Nisbet, C.; Seekings, J.; Meyer, S.; Whittard, E.; Ashpitel, H.F.; et al. Detection of Highly Pathogenic Avian Influenza Virus H5N1 Clade 2.3.4.4b in Great Skuas: A Species of Conservation Concern in Great Britain. Viruses 2022, 14, 212. [Google Scholar] [CrossRef]
- Lane, J.V.; Jeglinski, J.W.; Avery-Gomm, S.; Ballstaedt, E.; Banyard, A.C.; Barychka, T.; Brown, I.H.; Brugger, B.; Burt, T.V.; Careen, N.; et al. High pathogenicity avian influenza (H5N1) in Northern Gannets: Global spread, clinical signs, and demographic consequences. bioRxiv 2023, 166, 633–650. [Google Scholar] [CrossRef]
- Graham, L. Bird Flu Has Killed Nearly 1,500 Threatened Caspian Terns on Lake Michigan Islands. Michigan Radio, Published June 29, 2022 at 5:00 AM EDT. Available online: https://www.michiganradio.org/environment-climate-change/2022-06-29/bird-flu-has-killed-nearly-1-500-threatened-caspian-terns-on-lake-michigan-islands (accessed on 14 October 2024).
- Wildlife Health Information Sharing Partnership. Event Reporting System. WHISPers. 2023. Available online: https://whispers.usgs.gov/home (accessed on 14 October 2024).
- Harvey, J.A.; Mullinax, J.M.; Runge, M.C.; Prosser, D.J. The changing dynamics of highly pathogenic avian influenza H5N1: Next steps for management & science in North America. Biol. Conserv. 2023, 282, 110041. [Google Scholar] [CrossRef]
- Rijks, J.M.; Leopold, M.F.; Kühn, S.; Schenk, F.; Brenninkmeijer, A.; Lilipaly, S.J.; Ballmann, M.Z.; Kelder, L.; de Jong, J.W.; Courtens, W.; et al. Mass Mortality Caused by Highly Pathogenic Influenza A(H5N1) Virus in Sandwich Terns, the Netherlands, 2022. Emerg. Infect. Dis. 2022, 28, 2538–2542. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.; Stejskal, O.; King, J.; Bouwhuis, S.; Packmor, F.; Ballstaedt, E.; Hälterlein, B.; Hennig, V.; Stacker, L.; Graaf, A.; et al. Mass mortality among colony-breeding seabirds in the German Wadden Sea in 2022 due to distinct genotypes of HPAIV H5N1 clade 2.3.4.4b. J. Gen. Virol. 2023, 104, 001834. [Google Scholar] [CrossRef]
- Sueur, F. Impact of Highly Pathogenic H5N1 Avian Influenza on Some Gull Populations on the Coast of Hauts-de-France (France). In Birds-Conservation, Research and Ecology; Mikkola, H.J., Ed.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Karamendin, K.; Kydyrmanov, A.; Fereidouni, S. High Mortality in Terns and Gulls Associated with Infection with the Novel Gull Adenovirus. J. Wildl. Dis. 2021, 57, 662–666. [Google Scholar] [CrossRef]
- WOAH (World Organisation for Animal Health). Online. Immediate Notification/Highly Pathogenic Influenza A viruses (Inf. with)(non-poultry including wild birds)(2017-), Kazakhstan. Available online: https://wahis.woah.org/#/in-event/4503/dashboard (accessed on 14 October 2024).
- Kenzhegaliev, A.; Zhumagaliev, S.; Kenzhegalieva, D.; Orazbayev, B. Gas chromatographic-mass spectrometric investigation of n-alkanes and carboxylic acids in bottom sediments of the Northern Caspian Sea. Geologos 2018, 24, 69–78. [Google Scholar] [CrossRef]
- Payungporn, S.; Phakdeewirot, P.; Chutinimitkul, S.; Theamboonlers, A.; Keawcharoen, J.; Oraveerakul, K.; Amonsin, A.; Poovorawan, Y. Single-step multiplex reverse transcription-polymerase chain reaction (RT-PCR) for influenza A virus subtype H5N1 detection. Viral Immunol. 2004, 17, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An Ultra-Fast Single-Node Solution for Large and Complex Metagenomics Assembly via Succinct de Bruijn Graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Pohlmann, A.; King, J.; Fusaro, A.; Zecchin, B.; Banyard, A.C.; Brown, I.H.; Byrne, A.M.P.; Beerens, N.; Liang, Y.; Heutink, R.; et al. Has Epizootic Become Enzootic? Evidence for a Fundamental Change in the Infection Dynamics of Highly Pathogenic Avian Influenza in Europe, 2021. mBio 2022, 13, e0060922. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, I.; Gadzhiev, A.; Sharshov, K.; Ohlopkova, O.; Stolbunova, K.; Fadeev, A.; Dubovitskiy, N.; Glushchenko, A.; Irza, V.; Perkovsky, M.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus-Induced Mass Death of Wild Birds, Caspian Sea, Russia, 2022. Emerg. Infect. Dis. 2023, 29, 2528–2532. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Alexakis, L.; Fusaro, A.; Kuiken, T.; Mirinavičiūtė, G.; Ståhl, K.; Staubach, C.; Svartström, O.; et al. Scientific report: Avian influenza overview March–June 2024. EFSA J. 2024, 22, 8930. [Google Scholar] [CrossRef]
- E40 Environmental Monitoring of the North-East Caspian Sea during Development of NCOC N.V. Oil Fields in the Period 2006–2016; NCOC N.V., KAPE: Almaty, Kazakhstan, 2018; ISBN 978-601-332-146-2. 400p, Available online: https://www.ncoc.kz/public/publications/ncoc/NCOC_full_eng.pdf (accessed on 14 October 2024).
- Tosh, C.; Murugkar, H.V.; Nagarajan, S.; Tripathi, S.; Katare, M.; Jain, R.; Khandia, R.; Syed, Z.; Behera, P.; Patil, S.; et al. Emergence of amantadine-resistant avian influenza H5N1 virus in India. Virus Genes. 2011, 42, 10–15. [Google Scholar] [CrossRef]
- Sun, T.; Guo, Y.; Zhao, L.; Fan, M.; Huang, N.; Tian, M.; Liu, Q.; Huang, J.; Liu, Z.; Zhao, Y.; et al. Evolution of the PB1 gene of human influenza A (H3N2) viruses circulating between 1968 and 2019. Transbound. Emerg. Dis. 2022, 69, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chu, H.; Zhang, K.; Singh, K.; Li, C.; Yuan, S.; Chow, B.K.C.; Song, W.; Zhou, J.; Zheng, B.-J. Amino acid substitutions V63I or A37S/I61T/V63I/V100A in the PA N-terminal domain increase the virulence of H7N7 influenza A virus. Sci. Rep. 2016, 6, 37800. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Hu, Z.; Mo, Y.; Wu, Q.; Cui, Z.; Duan, Z.; Huang, J.; Chen, H.; Chen, Y.; Gu, M.; et al. The PA and HA gene-mediated high viral load and intense innate immune response in the brain contribute to the high pathogenicity of H5N1 avian influenza virus in mallard ducks. J. Virol. 2013, 87, 11063–11075. [Google Scholar] [CrossRef]
- Kawaoka, Y.; Yamnikova, S.; Chambers, T.M.; Lvov, D.K.; Webster, R.G. Molecular characterization of a new hemagglutinin, subtype H14, of influenza A virus. Virology 1990, 179, 759–767. [Google Scholar] [CrossRef] [PubMed]
- BirdLife International. Response to the Outbreak of Avian Flu in Senegal, Gambia, and Guinea Bissau. 24 May 2023. Available online: https://www.birdlife.org/news/2023/05/24/response-to-the-outbreak-of-avian-flu-in-senegal-gambia-and-guinea-bissau/ (accessed on 1 October 2024).
- Byholm, P.; Beal, M.; Isaksson, N.; Lötberg, U.; Åkesson, S. Paternal transmission of migration knowledge in a long-distance bird migrant. Nat. Commun. 2022, 13, 1566. [Google Scholar] [CrossRef]


| Affected Bird Species | Estimated Population on Island (2022) | Number of Found Dead Birds in a Colony | Percentage (%) of Found Dead Birds in the Colony | Mortality Rate (per 1000 Individuals) | ||||
|---|---|---|---|---|---|---|---|---|
| D1 and D4 | Zuid-West | D1 and D4 | Zuid-West | D1 and D4 | Zuid-West | D1 and D4 | Zuid-West | |
| Caspian tern | 2400 | 2500 | 2198 | 1000 | 91.6 | 40.0 | 916 | 400 |
| Caspian gull | 600 | 500 | 530 | 300 | 88.3 | 60.0 | 883 | 600 |
| Pallas’s gull | 700 | 5000 | 27 | 1200 | 3.9 | 24.0 | 39 | 240 |
| Protein | H5 AIVs | Atyrau/9184/22 | Phenotype | ||
|---|---|---|---|---|---|
| Residue (AA) | Avian-Like Motif | Mammalian-Like Motif | |||
| PB1 | 13 | L | P | P | Enhanced virulence in mammalian species [26] |
| 619 | A | T | A | Increased polymerase activity in human cells [27] | |
| PA | 61 | I | T | I | Playing a vital role in the adaptation new host species [28] |
| 237 | K | E | E | Increased polymerase activity [29] | |
| 615 | N | K/R | K | Enhanced virulence in mammalian species [26] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kydyrmanov, A.; Karamendin, K.; Kassymbekov, Y.; Daulbayeva, K.; Sabyrzhan, T.; Khan, Y.; Nuralibekov, S.; Baikara, B.; Fereidouni, S. Mass Mortality in Terns and Gulls Associated with Highly Pathogenic Avian Influenza Viruses in Caspian Sea, Kazakhstan. Viruses 2024, 16, 1661. https://doi.org/10.3390/v16111661
Kydyrmanov A, Karamendin K, Kassymbekov Y, Daulbayeva K, Sabyrzhan T, Khan Y, Nuralibekov S, Baikara B, Fereidouni S. Mass Mortality in Terns and Gulls Associated with Highly Pathogenic Avian Influenza Viruses in Caspian Sea, Kazakhstan. Viruses. 2024; 16(11):1661. https://doi.org/10.3390/v16111661
Chicago/Turabian StyleKydyrmanov, Aidyn, Kobey Karamendin, Yermukhammet Kassymbekov, Klara Daulbayeva, Temirlan Sabyrzhan, Yelizaveta Khan, Sardor Nuralibekov, Barshagul Baikara, and Sasan Fereidouni. 2024. "Mass Mortality in Terns and Gulls Associated with Highly Pathogenic Avian Influenza Viruses in Caspian Sea, Kazakhstan" Viruses 16, no. 11: 1661. https://doi.org/10.3390/v16111661
APA StyleKydyrmanov, A., Karamendin, K., Kassymbekov, Y., Daulbayeva, K., Sabyrzhan, T., Khan, Y., Nuralibekov, S., Baikara, B., & Fereidouni, S. (2024). Mass Mortality in Terns and Gulls Associated with Highly Pathogenic Avian Influenza Viruses in Caspian Sea, Kazakhstan. Viruses, 16(11), 1661. https://doi.org/10.3390/v16111661







