Prolonged Visual Evoked Potential Latencies in Dogs Naturally Infected with Canine Distemper Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Evoked Potential Recordings
2.3. Statistical Methods
3. Results
3.1. Population
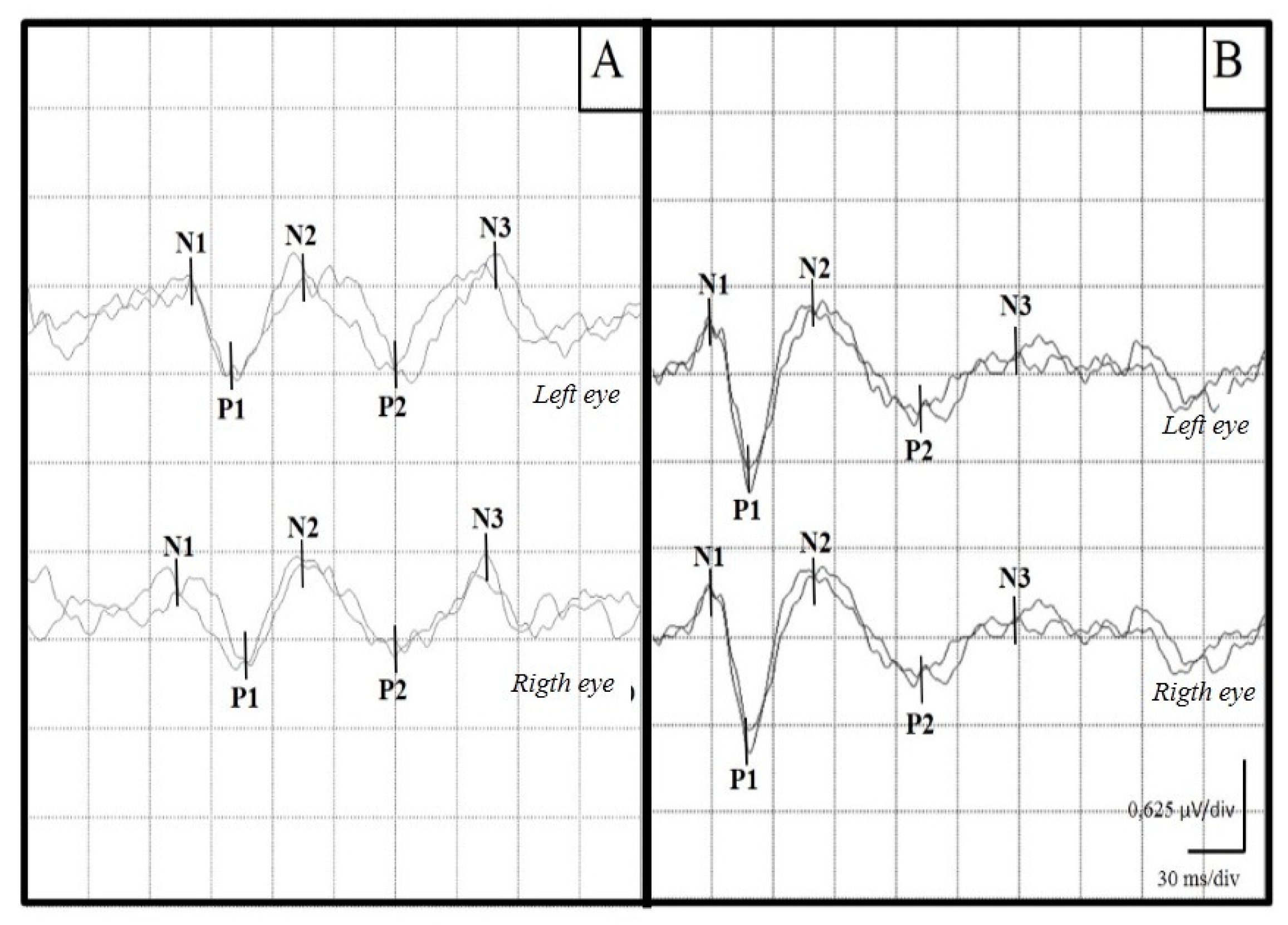
3.2. Visual Evoked Potentials
3.3. Electroretinograms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Case nº | Age (Months) | Clinical Signs |
| 1 | 9 | myoclonus, encephalitis |
| 2 | 144 | encephalitis, quadriplegia |
| 3 | 42 | nystagmus, encephalitis |
| 4 | 12 | myoclonus, encephalitis |
| 5 | 5 | blindness, encephalitis |
| 6 | 10 | myoclonus, encephalitis |
| 7 | 6 | respiratory signs |
| 8 | 18 | seizures |
| 9 | 60 | circling, encephalitis |
| 10 | 6 | myoclonus, encephalitis |
| 11 | 12 | respiratory signs |
| 12 | 10 | paresis |
| 13 | 12 | blindness, encephalitis |
| 14 | 3 | myoclonus |
| 15 | 13 | encephalitis, quadriplegia |
| 16 | 24 | myoclonus |
| 17 | 36 | encephalitis |
| 18 | 12 | digestive signs |
| 19 | 12 | digestive signs |
| 20 | 36 | quadriplegia |
| 21 | 8 | myoclonus |
| 22 | 4 | myoclonus |
| 23 | 6 | encephalitis |
| 24 | 5 | myoclonus |
| 25 | 6 | paresis |
| 26 | 24 | encephalitis |
| 27 | 24 | myoclonus |
| 28 | 3 | seizures |
| 29 | 10 | myoclonus |
| 30 | 3 | myoclonus |
| 31 | 12 | myoclonus |
| 32 | 18 | blindness, encephalitis |
| 33 | 60 | myoclonus |
| 34 | 12 | myoclonus |
| 35 | 120 | encephalitis |
References
- Martínez-Gutiérrez, M.; Ruiz-Sáenz, J. Diversity of susceptible hosts in canine distemper virus infection: A systematic review and data synthesis. BMC Vet. Res. 2016, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Beineke, A.; Puff, C.; Seehusen, F.; Baumgärtner, W. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet. Immunol. Immunopathol. 2009, 127, 1–18. [Google Scholar] [CrossRef] [PubMed]
- De Vries, R.D.; Duprex, W.P.; de Swart, R.L. Morbillivirus infections: An introduction. Viruses 2015, 7, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Rendon-Marin, S.; da Fontoura Budaszewski, R.; Canal, C.W.; Ruiz-Saenz, J. Tropism and molecular pathogenesis of canine distemper virus. Virol. J. 2019, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Panzera, Y.; Sarute, N.; Iraola, G.; Hernández, M.; Pérez, R. Molecular phylogeography of canine distemper virus: Geographic origin and global spreading. Mol. Phylogenetics Evol. 2015, 92, 147–154. [Google Scholar] [CrossRef]
- Pinotti, M.; Gollan, A.; Canavesio, M.; Passeggi, C.; Larrateguy, M.; Paz, M.; Formentini, E. Virus de distemper canino: Detección molecular de diferentes aislamientos provenientes de perros de la provincia de Santa Fé, Argentina, ente los años 2000 y 2010. InVet 2016, 18, 349–355. [Google Scholar]
- Amude, A.; Alfieri, A.; Alfieri, A. The nervous form of canine distemper. Veterinária E Zootec. 2006, 13, 125–136. [Google Scholar]
- Amude, A.; Alfieri, A.; Alfieri, A. Canine distemper virus and multiple sclerosis: A real or an anecdotal association. Appl. Microbiol. 2010, 1, 737–745. [Google Scholar]
- Galán, A.; Gamito, A.; Carletti, B.E.; Guisado, A.; de las Mulas, J.M.; Pérez, J.; Martín, E.M. Uncommon acute neurologic presentation of canine distemper in 4 adult dogs. Can. Vet. J. 2014, 55, 373–378. [Google Scholar]
- Nell, B. Optic neuritis in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 403–415. [Google Scholar] [CrossRef]
- Tipold, A.; Vandevelde, M.; Jaggy, A. Neurological manifestations of canine distemper virus infection. J. Small Anim. Pr. 1992, 33, 466–470. [Google Scholar] [CrossRef]
- Vandevelde, M.; Zurbriggen, A. The neurobiology of canine distemper virus infection. Vet. Microbiol. 1995, 44, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.; Puff, C.; Wewetzer, K.; Kalkuhl, A.; Deschl, U.; Baumgärtner, W. Transcriptional changes in canine distemper virus-induced demyelinating leukoencephalitis favor a biphasic mode of demyelination. PLoS ONE 2014, 9, e95917. [Google Scholar] [CrossRef]
- Fernández, O.; Fernández, V.; Guerrero, V. Enfermedades desmielinizantes del sistema nervioso central. Medicine 2015, 11, 4601–4609. [Google Scholar] [CrossRef]
- Pueyo, V.; Ara, J.R.; Martín, J. La retina como marcador biológico de daño neuronal [The retina as a biologicalmarker of neuronal damage]. Arch. Soc. Española Oftalmol. 2010, 85, 163–164. [Google Scholar] [CrossRef]
- Shaharabani, R.; Ram-On, M.; Talmon, Y.; Beck, R. Pathological transitions in myelin membranes driven by environmental and multiple sclerosis conditions. Proc. Natl. Acad. Sci. USA 2018, 115, 11156–11161. [Google Scholar] [CrossRef] [PubMed]
- Söderström, M. Optic neuritis and multiple sclerosis. Acta Ophthalmol. Scand. 2001, 79, 223–227. [Google Scholar] [CrossRef]
- Barton, J.; Garber, J.; Klistorner, A.; Barnett, M. The electrophysiological assessment of visual function in Multiple Sclerosis. Clin. Neurophysiol. Pract. 2019, 4, 90–96. [Google Scholar] [CrossRef]
- Hardmeier, M.; Leocani, L.; Fuhr, P. A new role for evoked potentials in MS? Repurposing evoked potentials as biomarkers for clinical trials in MS. Mult. Scler. J. 2017, 23, 1309–1319. [Google Scholar] [CrossRef]
- Ramanathan, S.; Prelog, K.; Barnes, E.; Tantsis, E.; Reddel, S.; Henderson, A.; Vucic, S.; Gorman, M.; Benson, L.; Alper, G.; et al. The utility of multimodal evoked potentials in multiple sclerosis prognostication. Clin. Neurosci. 2013, 20, 1576–1581. [Google Scholar] [CrossRef]
- Cambron, M.; D’Haeseleer, M.; Laureys, G.; Clinckers, R.; Debruyne, J.; De Keyser, J. White-matter astrocytes, axonal energy metabolism, and axonal degeneration in multiple sclerosis. J. Cereb. Blood Flow Metab. 2012, 32, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Di Maggio, G.; Santangelo, R.; Guerrieri, S.; Bianco, M.; Ferrari, L.; Medaglini, S.; Rodegher, M.; Colombo, B.; Moiola, L.; Chieffo, R.; et al. Optical coherence tomography and visual evoked potentials: Which is more sensitive in multiple sclerosis? Mult. Scler. 2014, 20, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Kiiski, S.; Riada, S.N.; Lalor, C.; Goncalves, R.; Nolan, H.; Whelan, R.; Bramham, J. Delayed P100-Like latencies in multiple sclerosis: A preliminary investigation using visual evoked spread spectrum analysis. PLoS ONE 2016, 11, e0146084. [Google Scholar] [CrossRef]
- Kraft, H. Evoked potentials in multiple sclerosis. Phys. Med. Rehabil. Clin. N. Am. 2013, 24, 717–720. [Google Scholar] [CrossRef]
- Klistorner, A.; Graham, S.; Fraser, C.; Garrick, R.; Nguyen, T.; Paine, M.; Billson, A. Electrophysiological evidence for heterogeneity of lesions in optic neuritis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4549–4556. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Klistorner, A.; Thie, J.; Graham, S.L. Latency delay of visual evoked potential is a real measurement of demyelination in a rat model of optic neuritis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6911–6918. [Google Scholar] [CrossRef]
- You, Y.; Klistorner, A.; Thie, J.; Gupta, V.K.; Graham, S.L. Axonal loss in a rat model of optic neuritis is closely correlated with visual evoked potential amplitudes using electroencephalogram-based scaling. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3662. [Google Scholar] [CrossRef]
- Chiappa, K.H. Pattern shift visual, brainstem auditory, and short-latency somatosensory evoked potentials in multiple sclerosis. Neurology 1980, 30, 110–123. [Google Scholar] [CrossRef]
- Chiappa, K.H. Chapter 2. Pattern-Shift Visual Evoked Potential: Methodology. In Evoked Potential in Clinical Medicine, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1997; pp. 30–94. [Google Scholar]
- Chiappa, K.H. Chapter 3. Pattern-Shift Visual Evoked Potential: Interpretation. In Evoked Potential in Clinical Medicine, 3rd ed.; Chiappa, K., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1997; pp. 95–130. [Google Scholar]
- Cantore, A. Optic neuritis. Penn Med. 1996, 99, 96–98. [Google Scholar]
- Fernández, O.; Arroyo-González, R.; Rodríguez-Antigüedad, A.; García-Merino, A.; Comabella, M.; Villar, M.; Meca-Lallana, E. Biomarkers in multiple sclerosis. Rev. Neurol. 2013, 56, 375–390. [Google Scholar]
- Ochikubo, F.; Nagata, T.; Yoshikawa, Y.; Matsubara, Y.; Kai, C.; Yamanouchi, Y. Electroencephalogram and evoked potentials in the primate model of viral encephalitis. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1993, 88, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.; Whelan, N.; Pinard, C.; Alcala, F.; Wolfe, K. Optic neuritis caused by canine distemper virus in a Jack Russell terrier. Can. Vet. J. 2011, 52, 398–402. [Google Scholar] [PubMed]
- Gutiérrez, M.; Feijóo, G.; Delucchi, L.J. Neurophysiological evaluation of canine congenital hydrocephalus in three dogs. Vet. Rec. Case Rep. 2020, 8, e000949. [Google Scholar] [CrossRef]
- Pellegrino, F.; Sica, R. Canine electroencephalographic recording technique: Findings in normal and epileptic dogs. Clin. Neurophysiol. 2004, 115, 477–487. [Google Scholar] [CrossRef]
- Pellegrino, F. Neuropatología y síndromes clínicos del virus del moquillo canino: Estado actual del conocimiento. NeuroVet 2015, 31, 2–59. [Google Scholar]
- Kimotsuki, T.; Yasuda, M.; Tamahara, S.; Matsuki, N.; Ono, K. Topographic analysis of flash visual evoked potentials in dogs. J. Vet. Med. Sci. 2005, 67, 869–875. [Google Scholar] [CrossRef]
- Strain, G.; Jackson, M.; Tedford, L. Visual evoked potentials in the clinically normal dog. J. Vet. Intern. Med. 1990, 4, 222–225. [Google Scholar] [CrossRef]
- Tovar-Sahuquillo, M.; Torres-Soriano, D. Protocolo Combinado de PEV y ERG en perros Beagle para evaluar la integridad funcional de las vías visuales. Arch. Med. Vet. 2014, 46, 289–297. [Google Scholar]
- Lee, E.; Seok, H.; Park, K.; Seo, D. Evoked potential: Basic requirements and guidelines for writing reports. Ann. Clin. Neuriphysiol. 2018, 20, 18–25. [Google Scholar] [CrossRef]
- Torres, D.; Tovar, M. Clinical guideline for assessing flash visual evoked potentials in laboratory dogs and normal data for beagle dogs. Scand. J. Lab. Anim. Sci. 2016, 42, 1–8. [Google Scholar]
- Kimotsuki, T.; Yasuda, M.; Tamahara, S.; Tomihari, M.; Matsuki, N.; Ono, K. Age associated changes on flash visual evoked potentials in dogs. J. Vet. Med. Sci. 2006, 68, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Fox, M. Neuronal development and ontogeny of evoked potentials in auditory and visual cortex of the dog. Electroencephalogr. Clin. Neurophysiol. 1968, 24, 213–226. [Google Scholar] [CrossRef]
- Leocani, L.; Guerrieri, S.; Comi, G. Visual evoked potentials as a biomarker in multiple sclerosis and associated optic neuritis. J. Neuro-Ophthalmol. 2018, 38, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Comi, G.; Leocani, L.; Medaglini, S.; Locatelli, T.; Martinelli, V.; Santuccio, G.; Rossi, P. Measuring evoked responses in multiple sclerosis. Mult. Scler. J. 1999, 5, 263–267. [Google Scholar] [CrossRef]
- Alshowaeir, D.; Yiannikas, C.; Garrick, R.; Parratt, J.; Barnett, M.; Graham, S.; Klistorner, A. Latency of multifocal visual evoked potentials in nonoptic neuritis eyes of multiple sclerosis patients associated with optic radiation lesions. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3758–3764. [Google Scholar] [CrossRef] [PubMed]
- Schobesberger, M.; Zurbriggen, A.; Doherr, M.G.; Weissenbóck, H.; Vandevelde, M.; Lassman, H.; Griot, C. Demyelination precedes oligodendrocyte loss in canine distemper virus-induced encephalitis. Acta Neuropathol. 2002, 103, 11–19. [Google Scholar] [CrossRef]
- Mike, B.; Carithers, W. Chorioretinitis and detached retina as post-distemper lesions in the canine. Iowa State Univ. Vet. 1975, 37, 1. [Google Scholar]
| Distemper | Control | |||
|---|---|---|---|---|
| Left Eyes | Right Eyes | Left Eyes | Right Eyes | |
| N1 | 56.5 ± 21.0 a | 54.9 ± 21.9 a | 27.6 ± 3.1 b | 27.4 ± 3.1 b |
| P1 | 79.5 ± 25.5 a | 76.1 ± 26.0 a | 45.3 ± 4.4 b | 45.0 ± 3.6 b |
| N2 | 107.0 ± 26.2 a | 101.0 ± 29.3 a | 67.4 ± 7.0 b | 70.0 ± 11.2 b |
| P2 | 129.3 ± 39.7 a | 123.3 ± 35.1 a | 96.5 ± 11.0 b | 95.8 ± 14.9 b |
| N3 | 159.9 ± 31.7 a | 171.7 ± 33.7 a | 127.0 ± 6.7 b | 125.4 ± 20.8 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, M.; Delucchi, L.; Bielli, A.; Verdes, J.M. Prolonged Visual Evoked Potential Latencies in Dogs Naturally Infected with Canine Distemper Virus. Viruses 2024, 16, 1721. https://doi.org/10.3390/v16111721
Gutiérrez M, Delucchi L, Bielli A, Verdes JM. Prolonged Visual Evoked Potential Latencies in Dogs Naturally Infected with Canine Distemper Virus. Viruses. 2024; 16(11):1721. https://doi.org/10.3390/v16111721
Chicago/Turabian StyleGutiérrez, Mary, Luis Delucchi, Alejandro Bielli, and José Manuel Verdes. 2024. "Prolonged Visual Evoked Potential Latencies in Dogs Naturally Infected with Canine Distemper Virus" Viruses 16, no. 11: 1721. https://doi.org/10.3390/v16111721
APA StyleGutiérrez, M., Delucchi, L., Bielli, A., & Verdes, J. M. (2024). Prolonged Visual Evoked Potential Latencies in Dogs Naturally Infected with Canine Distemper Virus. Viruses, 16(11), 1721. https://doi.org/10.3390/v16111721





