Comparison between the Viral Illness Caused by SARS-CoV-2, Influenza Virus, Respiratory Syncytial Virus and Other Respiratory Viruses in Pediatrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Definition of Exposure
2.3. Definition of the Outcomes of Interest
2.4. Data Source and Collection
2.5. Statistical Analysis
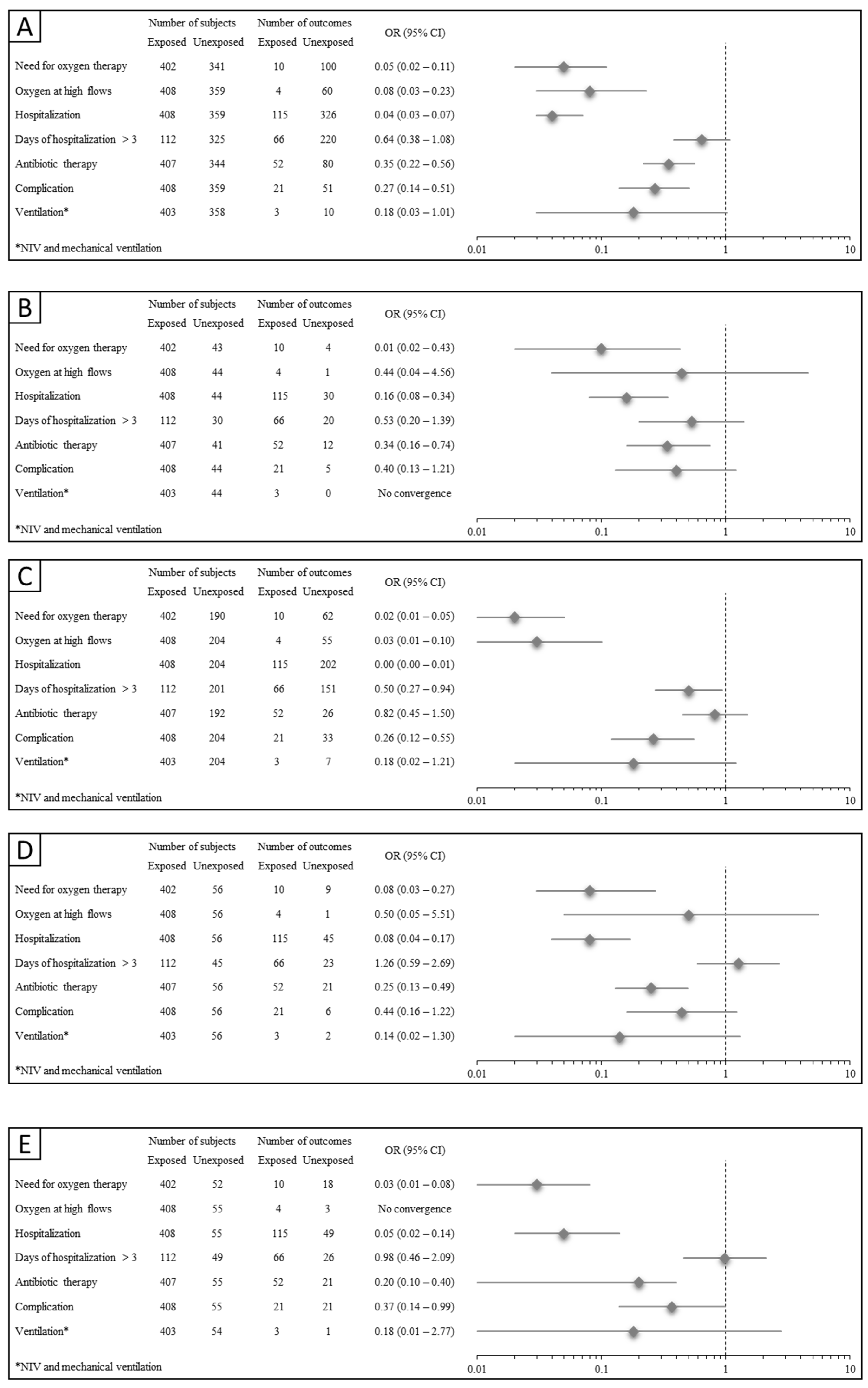
3. Results
3.1. Study Population
3.2. Patient Signs and Symptoms
3.3. Clinical Outcomes
3.4. Illness Courses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maitreyi, R.; Broor, S.; Kabra, S.; Ghosh, M.; Seth, P.; Dar, L.; Prasad, A. Rapid detection of respiratory viruses by centrifugation enhanced cultures from children with acute lower respiratory tract infections. J. Clin. Virol. 2000, 16, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-M.; Shim, J.W.; Kim, D.S.; Jung, H.L.; Park, M.S.; Shim, J.Y. Prevalence of respiratory viral infection in children hospitalized for acute lower respiratory tract diseases, and association of rhinovirus and influenza virus with asthma exacerbations. Korean J. Pediatr. 2014, 57, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.; Chenever, E.; Durbin, J.E. Respiratory Virus Infection in Infants and Children. Pediatr. Dev. Pathol. 2007, 10, 172–180. [Google Scholar] [CrossRef] [PubMed]
- File, T.M. Viral respiratory tract infections: Increasing importance and a new pathogen. Curr. Opin. Infect. Dis. 2003, 16, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Pattemore, P.K.; Jennings, L.C. Epidemiology of Respiratory Infections. In Pediatric Respiratory Medicine, 2nd ed.; Mosby: St. Louis, MO, USA, 2008; pp. 435–452. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; A Madhi, S.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- EuroMOMO. 2023. Available online: https://euromomo.eu (accessed on 1 June 2023).
- Glass, R.; Glass, L.; Beyeler, W.; Min, H. Targeted social distancing designs for pandemic influenza. Emerg. Infect. Dis. 2006, 12, 1671–1681. [Google Scholar] [CrossRef]
- eCDC. Data on 14-Day Notification Rate of New COVID-19 Cases and Deaths. Available online: https://www.ecdc.europa.eu/en/publications-data/data-national-14-day-notification-rate-covid-19 (accessed on 17 November 2023).
- Fricke, L.M.; Glöckner, S.; Dreie, M.; Lange, B. Impact of non-pharmaceutical interventions targeted at COVID-19 pandemic. J. Infect. 2021, 82, 1–35. [Google Scholar] [CrossRef]
- Rotulo, G.A.; Percivale, B.; Molteni, M.; Naim, A.; Brisca, G.; Piccotti, E.; Castagnola, E. The impact of COVID-19 lockdown on infectious diseases epidemiology: The experience of a tertiary Italian Pediatric Emergency Department. Am. J. Emerg. Med. 2021, 43, 115–117. [Google Scholar] [CrossRef]
- Chiapinotto, S.; Sarria, E.E.; Mocelin, H.T.; Lima, J.A.; Mattiello, R.; Fischer, G.B. Impact of non-pharmacological initiatives for COVID-19 on hospital admissions due to pediatric acute respiratory illnesses. Paediatr. Respir. Rev. 2021, 39, 3–8. [Google Scholar] [CrossRef]
- de Boer, G.; Braunstahl, G.-J.; Hendriks, R.; Tramper-Stranders, G. Asthma exacerbation prevalence during the COVID-19 lockdown in a moderate-severe asthma cohort. BMJ Open Respir. Res. 2021, 8, e000758. [Google Scholar] [CrossRef]
- De Conto, F.; Conversano, F.; Medici, M.C.; Ferraglia, F.; Pinardi, F.; Arcangeletti, M.C.; Chezzi, C.; Calderaro, A. Epidemiology of human respiratory viruses in children with acute respiratory tract infection in a 3-year hospital-based survey in Northern Italy. Diagn. Microbiol. Infect. Dis. 2019, 94, 260–267. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, P. Coronavirus Disease 2019 (COVID-19) in Neonates and Children From China: A Review. Front. Pediatr. 2020, 8, 287. [Google Scholar] [CrossRef]
- She, J.; Liu, L.; Liu, W. COVID-19 epidemic: Disease characteristics in children. J. Med. Virol. 2020, 92, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. COVID-19 in Children, Pregnancy and Neonates: A Review of Epidemiologic and Clinical Features. Pediatr. Infect. Dis. J. 2020, 39, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Wu, J.; Hong, L.; Luo, Y.; Song, Q.; Chen, D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: An observational cohort study. Lancet Infect. Dis. 2020, 20, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Rong, L.; Nian, W.; He, Y. Review article: Gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020, 51, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Q.; Chen, J.; Xiang, R.; Song, H.; Shu, S.; Chen, L.; Liang, L.; Zhou, J.; You, L.; et al. Detection of COVID-19 in Children in Early January 2020 in Wuhan, China. N. Engl. J. Med. 2020, 382, 1370–1371. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Daleno, C.; Prunotto, G.; Scala, A.; Tagliabue, C.; Borzani, I.; Fossali, E.; Pelucchi, C.; Principi, N. Impact of viral infections in children with community-acquired pneumonia: Results of a study of 17 respiratory viruses. Influenza Other Respir. Viruses 2013, 7, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.S.; Jackson, M.A.; Tamma, P.D.; Zaoutis, T.E.; Maldonado, Y.A.; O’leary, S.T.; Banerjee, R.; Barnett, E.D.; Campbell, J.D.; Caserta, M.T.; et al. Policy Statement: Antibiotic Stewardship in Pediatrics. J. Pediatr. Infect. Dis. Soc. 2021, 10, 641–649. [Google Scholar] [CrossRef]
- Kronman, M.P.; Gerber, J.S.; Grundmeier, R.W.; Zhou, C.; Robinson, J.D.; Heritage, J.; Stout, J.; Burges, D.; Hedrick, B.; Warren, L.; et al. Reducing Antibiotic Prescribing in Primary Care for Respiratory Illness. Available online: http://publications.aap.org/pediatrics/article-pdf/146/3/e20200038/1080752/peds_20200038.pdf?casa_token=aAIfB-s8cX8AAAAA:l2iXExC_YbjMIaK7DPfxpjJmCaRVwv1dDiqJmPfhFbqs2_tFHMN2TfZ7V (accessed on 16 January 2022).
- EpiCentro (ISS). Le EpiCentro (ISS): Le Vaccinazioni in Italia. Available online: https://www.epicentro.iss.it/vaccini/dati_ita (accessed on 2 January 2024).
- Committee on Infectious Diseases; O’leary, S.T.; Campbell, J.D.; Ardura, M.I.; Banerjee, R.; Bryant, K.A.; Caserta, M.T.; Frenck, R.W.; Gerber, J.S.; John, C.C.; et al. Recommendations for Prevention and Control of Influenza in Children, 2023–2024. Pediatrics 2023, 152, e2023063773. [Google Scholar] [CrossRef]
- Tejada, S.; Martinez-Reviejo, R.; Karakoc, H.N.; Peña-López, Y.; Manuel, O.; Rello, J. Ribavirin for Treatment of Subjects with Respiratory Syncytial Virus-Related Infection: A Systematic Review and Meta-Analysis. Adv. Ther. 2022, 39, 4037–4051. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Cots, M.B.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef]
- Drysdale, S.B.; Cathie, K.; Flamein, F.; Knuf, M.; Collins, A.M.; Hill, H.C.; Kaiser, F.; Cohen, R.; Pinquier, D.; Felter, C.T.; et al. Nirsevimab for Prevention of Hospitalizations Due to RSV in Infants. N. Engl. J. Med. 2023, 389, 2425–2435. [Google Scholar] [CrossRef]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccines Immunother. 2022, 18, 2002083. [Google Scholar] [CrossRef]
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic strategies for COVID-19: Progress and lessons learned. Nat. Rev. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, P.; Liang, Y.; Du, B.; Li, L.; Yu, Z.; Wang, H.; Wang, Q.; Zhang, X.; Zhang, W. A systematic review of current status and challenges of vaccinating children against SARS-CoV-2. J. Infect. Public Health 2022, 15, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- AAP. COVID-19 Interim Guidance—Management Strategies in Children and Adolescents with Mild to Moderate COVID-19. Available online: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/outpatient-covid-19-management-strategies-in-children-and-adolescents/ (accessed on 2 January 2024).
- Camporesi, A.; Morello, R.; Ferro, V.; Pierantoni, L.; Rocca, A.; Lanari, M.; Trobia, G.L.; Sciacca, T.; Bellinvia, A.G.; De Ferrari, A.; et al. Epidemiology, Microbiology and Severity of Bronchiolitis in the First Post-Lockdown Cold Season in Three Different Geographical Areas in Italy: A Prospective, Observational Study. Children 2022, 9, 491. [Google Scholar] [CrossRef]
- Hatter, L.; Eathorne, A.; Hills, T.; Bruce, P.; Beasley, R. Respiratory syncytial virus: Paying the immunity debt with interest. Lancet Child Adolesc. Health 2021, 5, e44–e45. [Google Scholar] [CrossRef]
- Curatola, A.; Lazzareschi, I.; Bersani, G.; Covino, M.; Gatto, A.; Chiaretti, A. Impact of COVID-19 outbreak in acute bronchiolitis: Lesson from a tertiary Italian Emergency Department. Pediatr. Pulmonol. 2021, 56, 2484–2488. [Google Scholar] [CrossRef] [PubMed]
- Kuitunen, I.; Artama, M.M.; Mäkelä, L.; Backman, K.; Heiskanen-Kosma, T.; Renko, M. Effect of Social Distancing Due to the COVID-19 Pandemic on the Incidence of Viral Respiratory Tract Infections in Children in Finland during Early 2020. Pediatr. Infect. Dis. J. 2020, 39, e423–e427. [Google Scholar] [CrossRef] [PubMed]
- Di Chiara, C.; Boracchini, R.; Sturniolo, G.; Barbieri, A.; Costenaro, P.; Cozzani, S.; De Pieri, M.; Liberati, C.; Zin, A.; Padoan, A.; et al. Clinical features of COVID-19 in Italian outpatient children and adolescents during Parental, Delta, and Omicron waves: A prospective, observational, cohort study. Front. Pediatr. 2023, 11, 1193857. [Google Scholar] [CrossRef]
- Hedberg, P.; Abdel-Halim, L.; Valik, J.K.; Alfvén, T.; Nauclér, P. Outcomes of Pediatric SARS-CoV-2 Omicron Infection vs. Influenza and Respiratory Syncytial Virus Infections. JAMA Pediatr. 2023, e235734. [Google Scholar] [CrossRef] [PubMed]
| Overall | SARS-CoV-2 | RSV | Adenovirus | Rhinovirus | Influenza A and B | Metapneumovirus | Coronavirus | |
|---|---|---|---|---|---|---|---|---|
| n = 767 | n = 408 | n = 204 | n = 56 | n = 32 | n = 44 | n = 17 | n = 6 | |
| Socio-demographic characteristics | ||||||||
| Age—mo. Median (p25–p75) | 15.77 (3.44–69.80) | 46.46 (7.93–120.98) | 3.25 (1.52–9.33) | 22.31 (12.11–39.54) | 5.77 (1.23–76.70) | 18.59 (4.70–31.05) | 10.03 (6.07–24.69) | 4.93 (1.90–17.41) |
| Gender | ||||||||
| Female | 345 (44.98) | 182 (44.61) | 95 (46.57) | 27 (48.21) | 12 (37.50) | 21 (47.73) | 6 (35.29) | 2 (3.33) |
| Male | 422 (55.02) | 226 (55.39) | 109 (53.43) | 29 (51.79) | 20 (62.50) | 23 (52.27) | 11 (64.71) | 4 (66.67) |
| Ethnicity | ||||||||
| Caucasian | 597 (77.84) | 316 (77.45) | 151 (74.02) | 47 (83.93) | 28 (87.50) | 39 (88.64) | 13 (76.47) | 3 (50) |
| Other | 170 (22.16) | 92 (22.55) | 53 (25.98) | 9 (16.07) | 4 (12.50) | 5 (11.36) | 4 (23.53) | 3 (50) |
| Pediatric visit 3 days previous | 151 (19.69) | 15 (3.68) | 75 (36.76) | 20 (35.71) | 11 (34.38) | 17 (38.64) | 8 (47.06) | 5 (83.33) |
| Sent by pediatrician | 99 (12.91) | 26 (6.37) | 387 (18.63) | 13 (23.21) | 4 (12.50) | 11 (25) | 6 (35.29) | 1 (16.67) |
| Comorbidities | ||||||||
| No comorbidities | 546 (71.19) | 293 (71.81) | 145 (71.08) | 38 (67.86) | 21 (65.63) | 32 (72.73) | 12 (70.59) | 5 (83.33) |
| At least one comorbidity | 221 (28.8) | 115 (28.19) | 59 (28.9) | 18 (32.14) | 11 (34.38) | 12 (27.27) | 5 (29.41) | 11 (16.67) |
| Most frequent comorbidities | ||||||||
| Prematurity | 78 (10.17) | 32 (7.84) | 34 (16.67) | 3 (5.36) | 5 (15.63) | 4 (9.09) | 0 (0) | 0 (0) |
| Chronic neurologic disease | 22 (2.87) | 9 (2.21) | 5 (2.45) | 4 (7.14) | 3 (9.38) | 0 (0) | 0 (0) | 1 (16.67) |
| Onco-hematological disease | 17 (2.22) | 11 (2.70) | 2 (0.98) | 1 (1.79) | 1 (3.13) | 1 (2.27) | 1 (5.88) | 0 (0) |
| Overall | SARS-CoV-2 | RSV | Adenovirus | Rhinovirus | Influenza A and B | Metapneumovirus | Coronavirus | |
|---|---|---|---|---|---|---|---|---|
| n = 767 | n = 408 | n = 204 | n = 56 | n = 32 | n = 44 | n = 17 | n = 6 | |
| Symptoms | ||||||||
| Fever > 37.5 °C | 472 (61.54) | 236 (57.84) | 106 (51.96) | 50 (89.29) | 23 (71.88) | 39 (88.64) | 14 (82.35) | 4 (66.67) |
| Rhinitis | 347 (45.24) | 122 (29.90) | 147 (72.09) | 23 (41.07) | 20 (62.50) | 20 (45.45) | 12 (70.59) | 3 (50) |
| Cough | 385 (50.20) | 111 (27.21) | 176 (86.27) | 28 (50) | 20 (62.50) | 31 (70.45) | 16 (94.12) | 3 (50) |
| Dyspnea | 164 (21.38) | 17 (4.17) | 103 (50.49) | 13 (23.21) | 11 (34.38) | 7 (15.91) | 8 (47.06) | 5 (83.33) |
| Earache | 16 (2.09) | 10 (2.45) | 0 (0) | 3 (5.36) | 1 (3.13) | 1 (2.27) | 1 (5.88) | 0 (0) |
| Conjunctivitis | 16 (2.09) | 9 (2.21) | 2 (0.98) | 2 (3.57) | 1 (3.13) | 2 (4.55) | 0 (0) | 0 (0) |
| Weakness | 19 (2.48) | 11 (2.70) | 0 (0) | 2 (3.57) | 1 (3.13) | 5 (11.36) | 0 (0) | 0 (0) |
| Mental confusion, drowsiness | 13 (1.69) | 2 (0.49) | 3 (1.47) | 1 (1.79) | 6 (18.75) | 1 (2.27) | 0 (0) | 0 (0) |
| Abdominal pain | 40 (5.22) | 29 (7.11) | 3 (1.47) | 6 (10.71) | 1 (3.13) | 1 (2.27) | 0 (0) | 0 (0) |
| Nausea/vomiting | 99 (12.91) | 50 (12.25) | 23 (11.27) | 7 (12.50) | 5 (15.63) | 7 (15.91) | 6 (35.29) | 1 (16.67) |
| Diarrhea | 72 (9.39) | 49 (12.01) | 9 (4.41) | 6 (10.71) | 4 (12.50) | 3 (6.82) | 1 (5.88) | 0 (0) |
| Poor feeding | 233 (30.38) | 32 (7.84) | 133 (65.20) | 27 (48.21) | 12 (37.50) | 20 (45.45) | 7 (41.18) | 2 (33.33) |
| Lymphadenopathy | 33 (4.30) | 6 (1.47) | 2 (0.98) | 11 (19.64) | 2 (6.25) | 11 (25) | 1 (5.88) | 0 (0) |
| Skin rash | 28 (3.65) | 13 (3.49) | 5 (2.45) | 4 (7.14) | 1 (3.13) | 5 (11.36) | 0 (0) | 0 (0) |
| Lung crackles | 9 (1.17) | 3 (0.74) | 3 (1.47) | 2 (3.57) | 0 (0) | 1 (2.27) | 0 (0) | 0 (0) |
| Other | 97 (12.65) | 44 (10.78) | 20 (9.80) | 13 (23.21) | 8 (25) | 8 (18.18) | 2 (11.76) | 2 (33.33) |
| Overall | SARS-CoV-2 | RSV | Adenovirus | Rhinovirus | Influenza A and B | Metapneumovirus | Coronavirus | |
|---|---|---|---|---|---|---|---|---|
| n = 767 | n = 408 | n = 204 | n = 56 | n = 32 | n = 44 | n = 17 | n = 6 | |
| Outcome | ||||||||
| Need for O2 therapy | 110 (14.80) | 10 (2.49) | 69 (36.32) | 9 (16.07) | 10 (32.26) | 4 (9.30) | 5 (33.33) | 3 (50) |
| O2 high flow | 64 (8.34) | 4 (0.98) | 55 (26.96) | 1 (1.79) | 2 (6.25) | 1 (2.27) | 0 (0) | 1 (16.67) |
| Admission | 441 (57.50) | 115 (28.19) | 202 (99.02) | 45 (80.36) | 28 (87.50) | 30 (68.18) | 15 (88.24) | 6 (100) |
| Days of hospitalization > 3 | 286 (65.45) | 66 (58.93) | 151 (75.12) | 23 (51.11) | 15 (53.57) | 20 (66.67) | 10 (66.67) | 1 (16.67) |
| Antibiotic therapy | 132 (17.58) | 52 (12.78) | 26 (13.54) | 21 (37.50) | 12 (37.50) | 12 (29.27) | 6 (35.29) | 3 (50) |
| Complication | 72 (9.39) | 21 (5.15) | 33 (16.18) | 6 (10.71) | 4 (12.50) | 5 (11.36) | 3 (17.65) | 0 (0) |
| Mechanical ventilation | 13 (1.71) | 3 (0.74) | 7 (3.43) | 2 (3.57) | 1 (3.23) | 0 (0) | 0 (0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brigadoi, G.; Demarin, G.C.; Boracchini, R.; Pierantoni, L.; Rossin, S.; Barbieri, E.; Tirelli, F.; Cantarutti, A.; Tempo, G.; Giaquinto, C.; et al. Comparison between the Viral Illness Caused by SARS-CoV-2, Influenza Virus, Respiratory Syncytial Virus and Other Respiratory Viruses in Pediatrics. Viruses 2024, 16, 199. https://doi.org/10.3390/v16020199
Brigadoi G, Demarin GC, Boracchini R, Pierantoni L, Rossin S, Barbieri E, Tirelli F, Cantarutti A, Tempo G, Giaquinto C, et al. Comparison between the Viral Illness Caused by SARS-CoV-2, Influenza Virus, Respiratory Syncytial Virus and Other Respiratory Viruses in Pediatrics. Viruses. 2024; 16(2):199. https://doi.org/10.3390/v16020199
Chicago/Turabian StyleBrigadoi, Giulia, Giulia Camilla Demarin, Riccardo Boracchini, Luca Pierantoni, Sara Rossin, Elisa Barbieri, Francesca Tirelli, Anna Cantarutti, Gaia Tempo, Carlo Giaquinto, and et al. 2024. "Comparison between the Viral Illness Caused by SARS-CoV-2, Influenza Virus, Respiratory Syncytial Virus and Other Respiratory Viruses in Pediatrics" Viruses 16, no. 2: 199. https://doi.org/10.3390/v16020199
APA StyleBrigadoi, G., Demarin, G. C., Boracchini, R., Pierantoni, L., Rossin, S., Barbieri, E., Tirelli, F., Cantarutti, A., Tempo, G., Giaquinto, C., Lanari, M., Da Dalt, L., & Donà, D. (2024). Comparison between the Viral Illness Caused by SARS-CoV-2, Influenza Virus, Respiratory Syncytial Virus and Other Respiratory Viruses in Pediatrics. Viruses, 16(2), 199. https://doi.org/10.3390/v16020199









