Current Advances in Japanese Encephalitis Virus Drug Development
Abstract
1. Introduction
2. Global Distribution of JEV
3. Molecular Biology of JEV
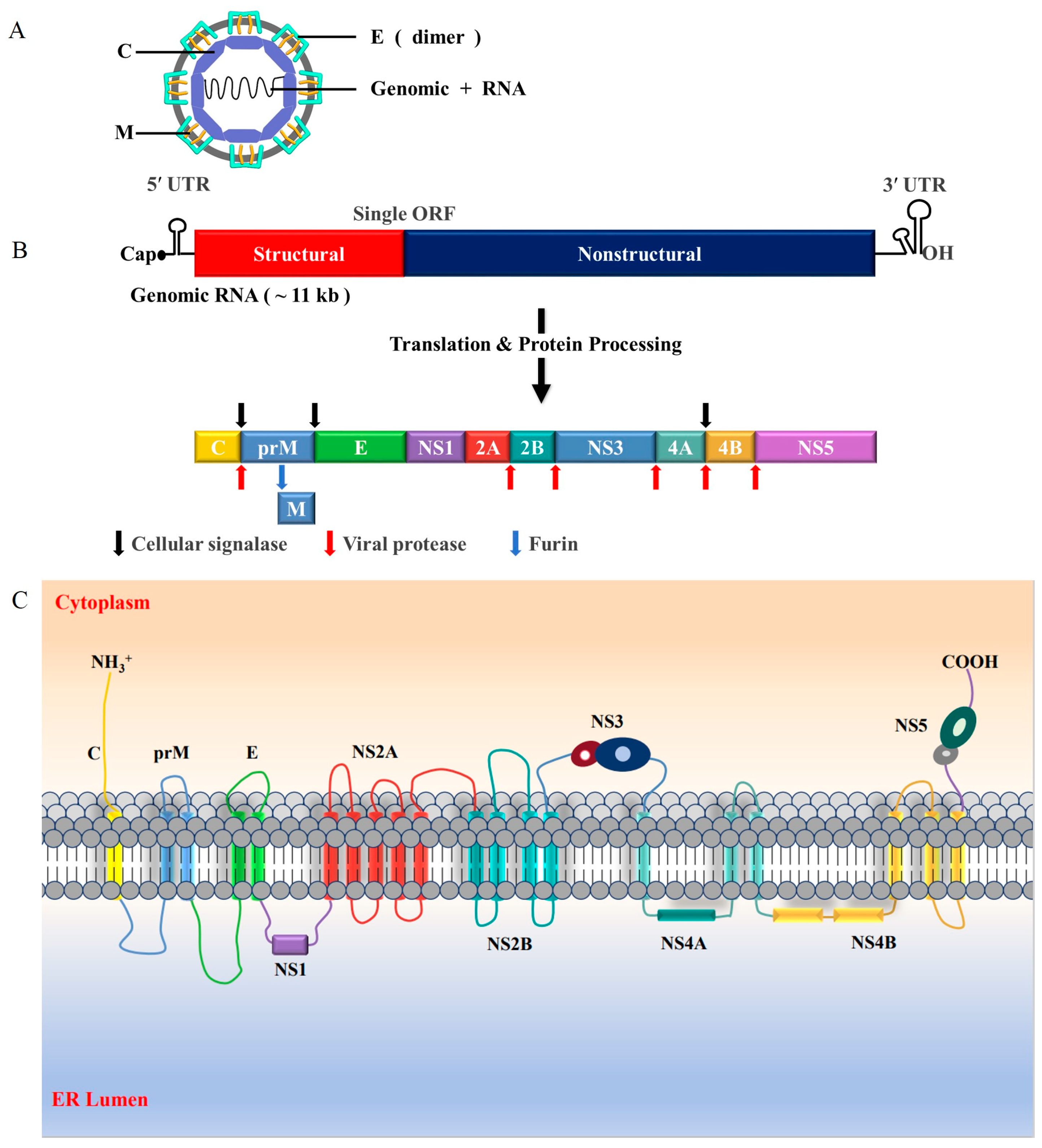
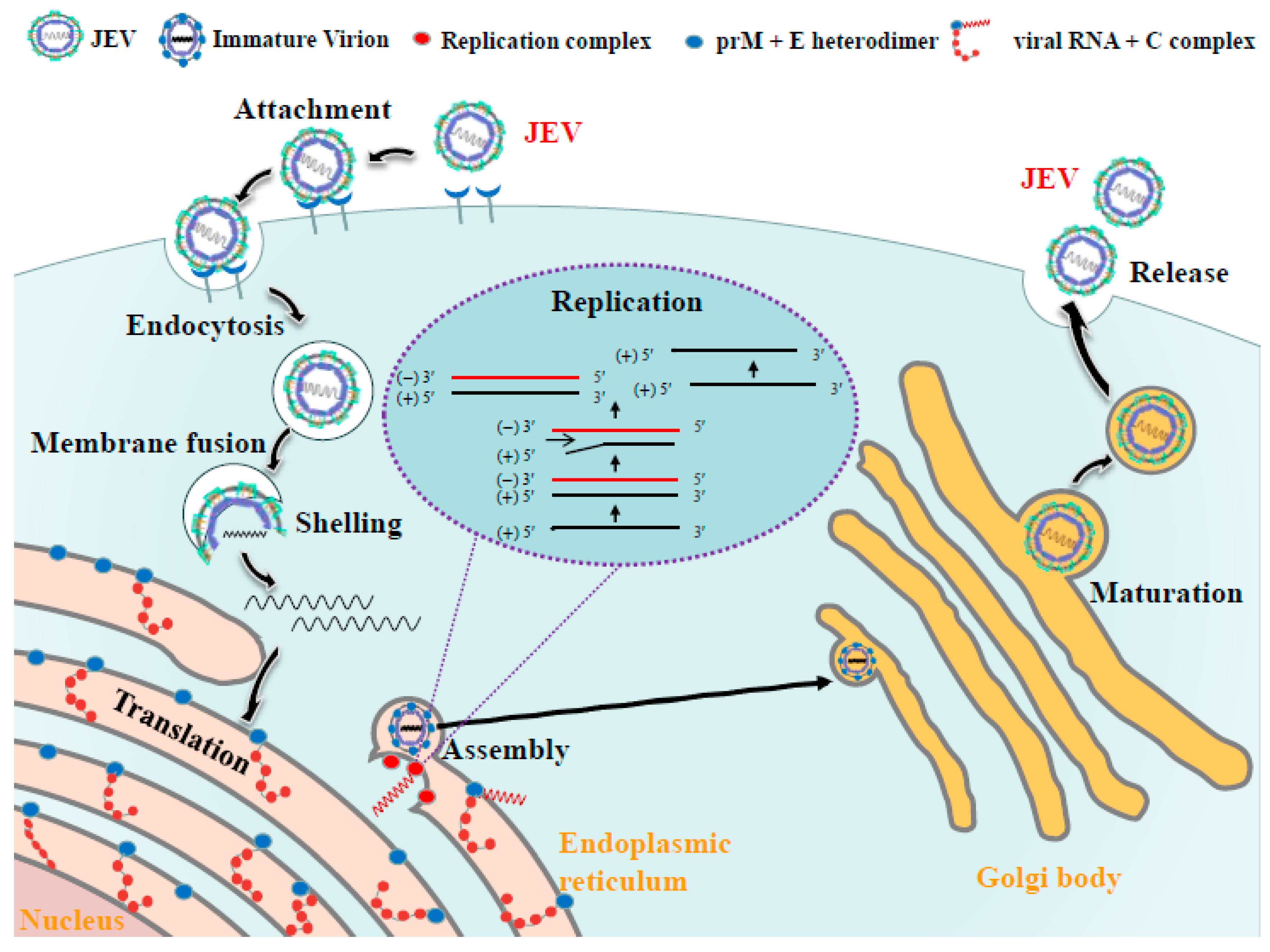
4. Life Cycle of JEV
5. Target Product Profile (TPP)
6. Drug Development
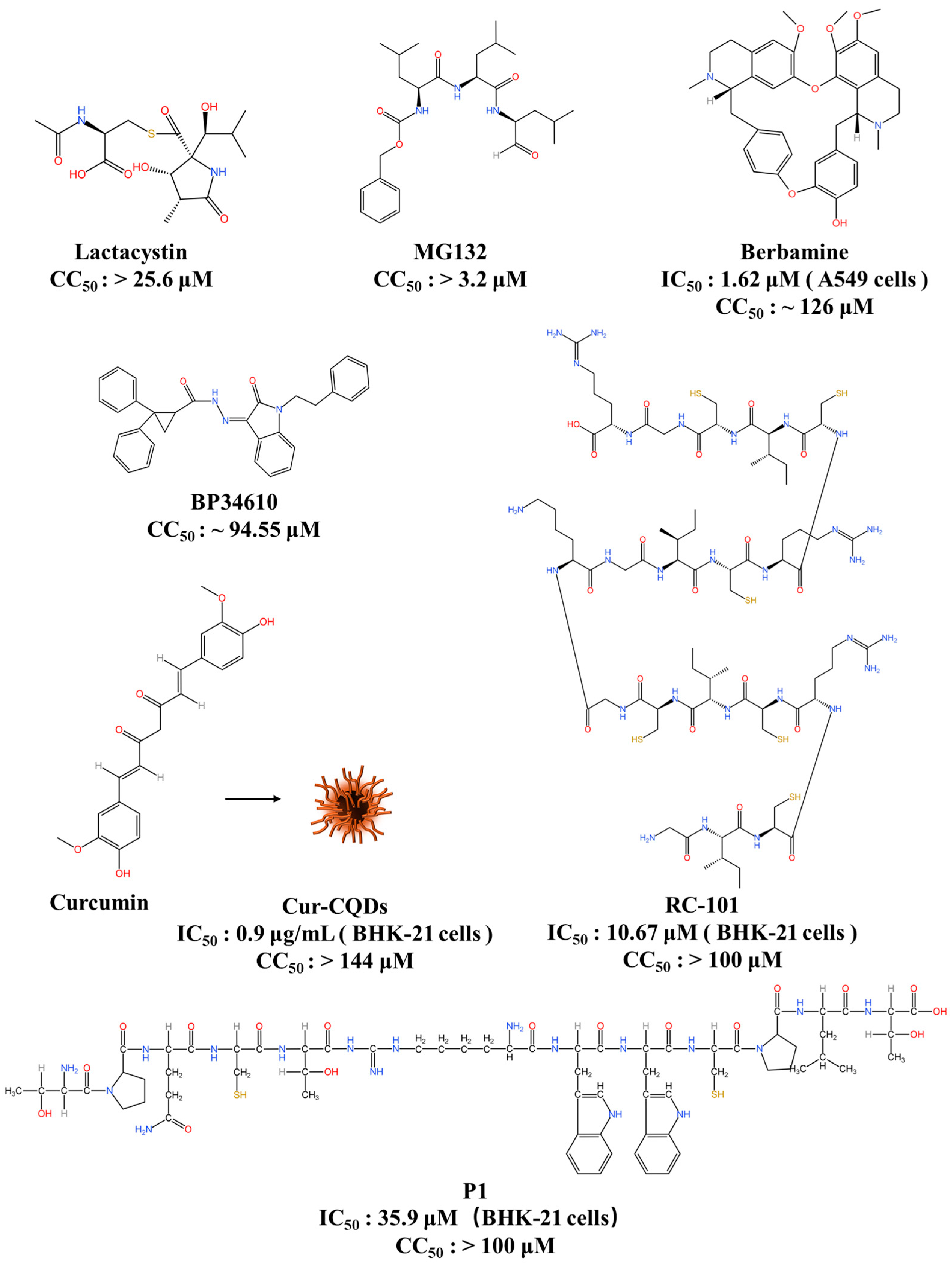
6.1. Entry Inhibitors
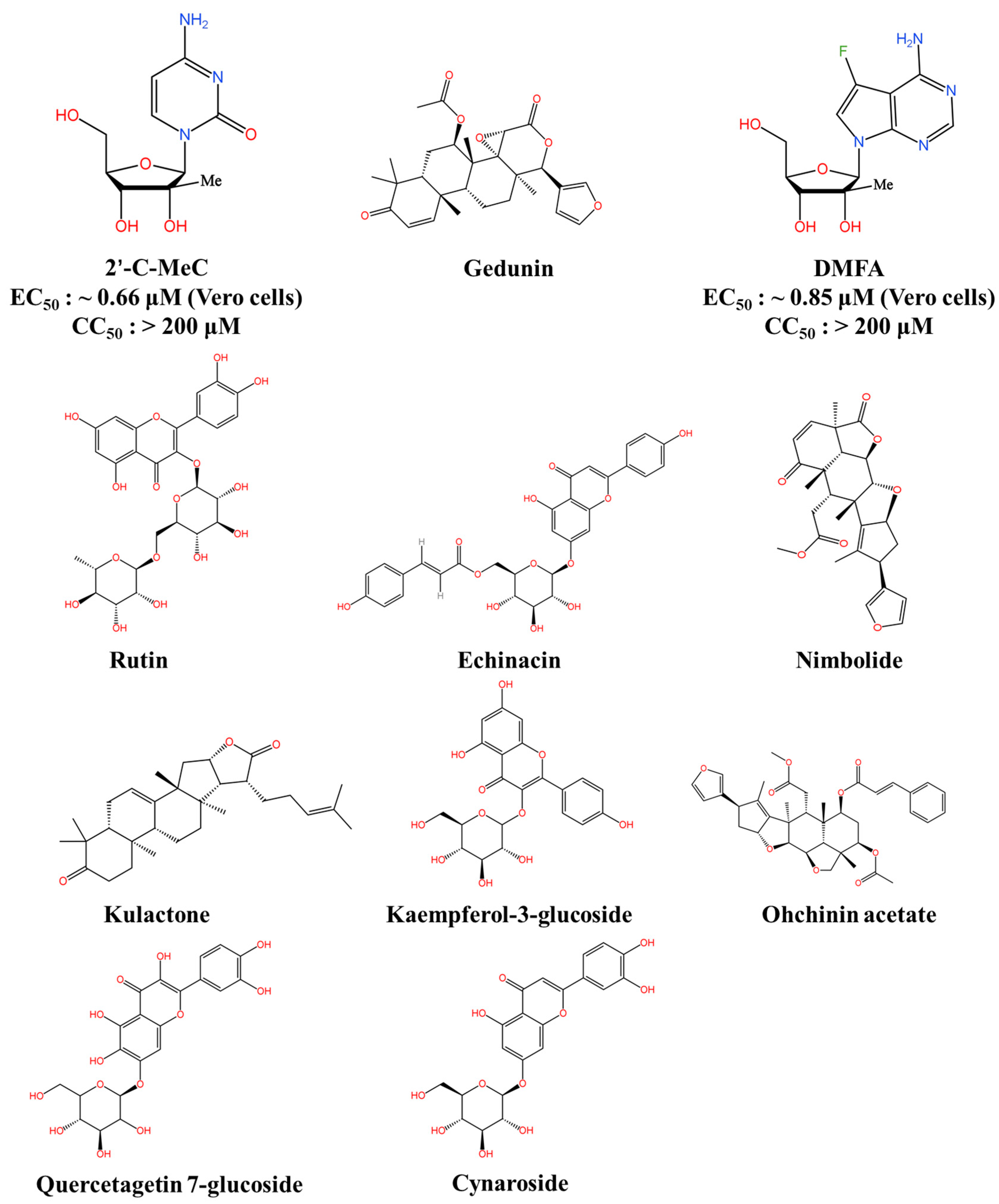
6.2. RdRp Inhibitors
6.3. Protease Inhibitors
6.4. Bioactive Natural Products and Their Derivatives
6.5. Host-Directed Antiviral Agents
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Solomon, T. Flavivirus encephalitis. N. Engl. J. Med. 2004, 351, 370–378. [Google Scholar] [CrossRef]
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef]
- Misra, U.K.; Kalita, J. Overview: Japanese encephalitis. Prog. Neurobiol. 2010, 91, 108–120. [Google Scholar] [CrossRef]
- Ghosh, D.; Basu, A.; Brooker, S. Japanese Encephalitis—A Pathological and Clinical Perspective. PLoS Negl. Trop. Dis. 2009, 3, e437. [Google Scholar] [CrossRef] [PubMed]
- Maximova, O.A.; Pletnev, A.G. Flaviviruses and the Central Nervous System: Revisiting Neuropathological Concepts. Annu. Rev. Virol. 2018, 5, 255–272. [Google Scholar] [CrossRef]
- Solomon, T.; Dung, N.M.; Kneen, R.; Gainsborough, M.; Vaughn, D.W.; Khanh, V.T. Japanese encephalitis. J. Neurol. Neurosurg. Psychiatry 2000, 68, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.S.; Nisalak, A. Detection of Japanese encephalitis virus immunoglobulin M antibodies in serum by antibody capture radioimmunoassay. J. Clin. Microbiol. 1982, 15, 353–361. [Google Scholar] [CrossRef]
- Batchelor, P.; Petersen, K. Japanese encephalitis: A review of clinical guidelines and vaccine availability in Asia. Trop. Dis. Travel Med. Vaccines 2015, 1, 11. [Google Scholar] [CrossRef]
- Hills, S.L.; Walter, E.B.; Atmar, R.L.; Fischer, M. Japanese Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunization Practices. MMWR. Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2019, 68, 1–33. [Google Scholar] [CrossRef]
- Endy, T.P.; Nisalak, A. Japanese Encephalitis Virus: Ecology and Epidemiology. Jpn. Enceph. West Nile Viruses 2002, 267, 11–48. [Google Scholar]
- Mackenzie, J.S.; Williams, D.T.; van den Hurk, A.F.; Smith, D.W.; Currie, B.J. Japanese Encephalitis Virus: The Emergence of Genotype IV in Australia and Its Potential Endemicity. Viruses 2022, 14, 2480. [Google Scholar] [CrossRef] [PubMed]
- van den Hurk, A.F.; Ritchie, S.A.; Mackenzie, J.S. Ecology and geographical expansion of Japanese encephalitis virus. Annu. Rev. Entomol. 2009, 54, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, H.; Li, X.; Fu, S.; Cao, L.; Shao, N.; Zhang, W.; Wang, Q.; Lu, Z.; Lei, W.; et al. Changing Geographic Distribution of Japanese Encephalitis Virus Genotypes, 1935-2017. Vector Borne Zoonotic Dis. 2019, 19, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.S.; Jeswani, V.; Pal, N.; Bohra, B.; Vishwakarma, V.; Bapat, A.A.; Patnaik, Y.P.; Khanna, N.; Shukla, R. Japanese Encephalitis Virus: An Update on the Potential Antivirals and Vaccines. Vaccines 2023, 11, 742. [Google Scholar] [CrossRef]
- Solomon, T.; Ni, H.; Beasley, D.W.; Ekkelenkamp, M.; Cardosa, M.J.; Barrett, A.D. Origin and evolution of Japanese encephalitis virus in southeast Asia. J. Virol. 2003, 77, 3091–3098. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Gubler, D.J.; Petersen, L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004, 10, S98–S109. [Google Scholar] [CrossRef]
- Chen, W.R.; Tesh, R.B.; Rico-Hesse, R. Genetic variation of Japanese encephalitis virus in nature. J. Gen. Virol. 1990, 71 Pt 12, 2915–2922. [Google Scholar] [CrossRef]
- Tiwari, S.; Singh, R.K.; Tiwari, R.; Dhole, T.N. Japanese encephalitis: A review of the Indian perspective. Braz. J. Infect. Dis. Off. Publ. Braz. Soc. Infect. Dis. 2012, 16, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.B.; Vrati, S.; Kalia, M. Pathobiology of Japanese encephalitis virus infection. Mol. Asp. Med. 2021, 81, 100994. [Google Scholar] [CrossRef]
- Simon-Loriere, E.; Faye, O.; Prot, M.; Casademont, I.; Fall, G.; Fernandez-Garcia, M.D.; Diagne, M.M.; Kipela, J.M.; Fall, I.S.; Holmes, E.C.; et al. Autochthonous Japanese Encephalitis with Yellow Fever Coinfection in Africa. N. Engl. J. Med. 2017, 376, 1483–1485. [Google Scholar] [CrossRef]
- Le Flohic, G.; Porphyre, V.; Barbazan, P.; Gonzalez, J.P. Review of climate, landscape, and viral genetics as drivers of the Japanese encephalitis virus ecology. PLoS Negl. Trop. Dis. 2013, 7, e2208. [Google Scholar] [CrossRef]
- Pan, X.L.; Liu, H.; Wang, H.Y.; Fu, S.H.; Liu, H.Z.; Zhang, H.L.; Li, M.H.; Gao, X.Y.; Wang, J.L.; Sun, X.H.; et al. Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia. J. Virol. 2011, 85, 9847–9853. [Google Scholar] [CrossRef]
- Li, Y.X.; Li, M.H.; Fu, S.H.; Chen, W.X.; Liu, Q.Y.; Zhang, H.L.; Da, W.; Hu, S.L.; Mu, S.D.; Bai, J.; et al. Japanese encephalitis, Tibet, China. Emerg. Infect. Dis. 2011, 17, 934–936. [Google Scholar] [CrossRef]
- Takhampunya, R.; Kim, H.C.; Tippayachai, B.; Kengluecha, A.; Klein, T.A.; Lee, W.J.; Grieco, J.; Evans, B.P. Emergence of Japanese encephalitis virus genotype V in the Republic of Korea. Virol. J. 2011, 8, 449. [Google Scholar] [CrossRef]
- Mulvey, P.; Duong, V. The Ecology and Evolution of Japanese Encephalitis Virus. Pathogens 2021, 10, 1534. [Google Scholar] [CrossRef]
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Niu, Y.; Liang, G. The 5’ and 3’ Untranslated Regions of the Japanese Encephalitis Virus (JEV): Molecular Genetics and Higher Order Structures. Front. Microbiol. 2021, 12, 730045. [Google Scholar] [CrossRef]
- Poonsiri, T.; Wright, G.S.A. Crystal Structure of the Japanese Encephalitis Virus Capsid Protein. Viruses 2019, 11, 623. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.K.; Carletti, T.; Marcello, A. Cellular Targets for the Treatment of Flavivirus Infections. Front. Cell. Infect. Microbiol. 2018, 8, 398. [Google Scholar] [CrossRef]
- Ivanyi-Nagy, R.; Lavergne, J.P.; Gabus, C.; Ficheux, D.; Darlix, J.L. RNA chaperoning and intrinsic disorder in the core proteins of Flaviviridae. Nucleic Acids Res. 2008, 36, 712–725. [Google Scholar] [CrossRef]
- Luca, V.C.; AbiMansour, J.; Nelson, C.A.; Fremont, D.H. Crystal structure of the Japanese encephalitis virus envelope protein. J. Virol. 2012, 86, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. Degrees of maturity: The complex structure and biology of flaviviruses. Curr. Opin. Virol. 2012, 2, 168–175. [Google Scholar] [CrossRef]
- Li, L.; Lok, S.M.; Yu, I.M.; Zhang, Y.; Kuhn, R.J.; Chen, J.; Rossmann, M.G. The flavivirus precursor membrane-envelope protein complex: Structure and maturation. Science 2008, 319, 1830–1834. [Google Scholar] [CrossRef]
- Joe, S.; Salam, A.A.A.; Neogi, U.; N, N.B.; Mudgal, P.P. Antiviral drug research for Japanese encephalitis: An updated review. Pharmacol. Rep. 2022, 74, 273–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, S.H.; Zhu, L.; Nian, Q.G.; Yuan, S.; Gao, Q.; Hu, Z.; Ye, Q.; Li, X.F.; Xie, D.Y.; et al. Near-atomic structure of Japanese encephalitis virus reveals critical determinants of virulence and stability. Nat. Commun. 2017, 8, 14. [Google Scholar] [CrossRef]
- Leng, S.L.; Huang, R.; Feng, Y.N.; Peng, L.J.; Yang, J.; Li, Y.H. The pre membrane and envelope protein is the crucial virulence determinant of Japanese encephalitis virus. Microb. Pathog. 2020, 148, 104492. [Google Scholar] [CrossRef]
- Jia, X.; Guo, J.; Yuan, W.; Sun, L.; Liu, Y.; Zhou, M.; Xiao, G.; Lu, W.; Garzino-Demo, A.; Wang, W. Mechanism through Which Retrocyclin Targets Flavivirus Multiplication. J. Virol. 2021, 95, e0056021. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Alcon-LePoder, S.; Sivard, P.; Drouet, M.T.; Talarmin, A.; Rice, C.; Flamand, M. Secretion of flaviviral non-structural protein NS1: From diagnosis to pathogenesis. Novartis Found. Symp. 2006, 277, 233–247. [Google Scholar] [CrossRef]
- Lindenbach, B.D.; Rice, C.M. Genetic interaction of flavivirus nonstructural proteins NS1 and NS4A as a determinant of replicase function. J. Virol. 1999, 73, 4611–4621. [Google Scholar] [CrossRef]
- Youn, S.; Li, T.; McCune, B.T.; Edeling, M.A.; Fremont, D.H.; Cristea, I.M.; Diamond, M.S. Evidence for a genetic and physical interaction between nonstructural proteins NS1 and NS4B that modulates replication of West Nile virus. J. Virol. 2012, 86, 7360–7371. [Google Scholar] [CrossRef]
- Akey, D.L.; Brown, W.C.; Dutta, S.; Konwerski, J.; Jose, J.; Jurkiw, T.J.; DelProposto, J.; Ogata, C.M.; Skiniotis, G.; Kuhn, R.J.; et al. Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science 2014, 343, 881–885. [Google Scholar] [CrossRef]
- Khromykh, A.A.; Sedlak, P.L.; Westaway, E.G. cis- and trans-acting elements in flavivirus RNA replication. J. Virol. 2000, 74, 3253–3263. [Google Scholar] [CrossRef]
- Liao, C.L.; Lin, Y.L.; Shen, S.C.; Shen, J.Y.; Su, H.L.; Huang, Y.L.; Ma, S.H.; Sun, Y.C.; Chen, K.P.; Chen, L.K. Antiapoptotic but not antiviral function of human bcl-2 assists establishment of Japanese encephalitis virus persistence in cultured cells. J. Virol. 1998, 72, 9844–9854. [Google Scholar] [CrossRef]
- Halstead, S.B.; Thomas, S.J. New Japanese encephalitis vaccines: Alternatives to production in mouse brain. Expert Rev. Vaccines 2011, 10, 355–364. [Google Scholar] [CrossRef]
- Leung, J.Y.; Pijlman, G.P.; Kondratieva, N.; Hyde, J.; Mackenzie, J.M.; Khromykh, A.A. Role of nonstructural protein NS2A in flavivirus assembly. J. Virol. 2008, 82, 4731–4741. [Google Scholar] [CrossRef] [PubMed]
- Bhimaneni, S.; Kumar, A. Abscisic acid and aloe-emodin against NS2B-NS3A protease of Japanese encephalitis virus. Environ. Sci. Pollut. Res. 2022, 29, 8759–8766. [Google Scholar] [CrossRef]
- Bera, A.K.; Kuhn, R.J.; Smith, J.L. Functional characterization of cis and trans activity of the Flavivirus NS2B-NS3 protease. J. Biol. Chem. 2007, 282, 12883–12892. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Li, X.F.; Ye, H.Q.; Deng, C.L.; Ye, Q.; Shan, C.; Shang, B.D.; Xu, L.L.; Li, S.H.; Cao, S.B.; et al. Recovery of a chemically synthesized Japanese encephalitis virus reveals two critical adaptive mutations in NS2B and NS4A. J. Gen. Virol. 2014, 95, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Cheng, C.W.; Yang, T.C.; Li, S.W.; Cheng, M.H.; Wan, L.; Lin, Y.J.; Lai, C.H.; Lin, W.Y.; Kao, M.C. Interferon antagonist function of Japanese encephalitis virus NS4A and its interaction with DEAD-box RNA helicase DDX42. Virus Res. 2008, 137, 49–55. [Google Scholar] [CrossRef]
- Miller, S.; Kastner, S.; Krijnse-Locker, J.; Bühler, S.; Bartenschlager, R. The non-structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K-regulated manner. J. Biol. Chem. 2007, 282, 8873–8882. [Google Scholar] [CrossRef]
- Stern, O.; Hung, Y.F.; Valdau, O.; Yaffe, Y.; Harris, E.; Hoffmann, S.; Willbold, D.; Sklan, E.H. An N-terminal amphipathic helix in dengue virus nonstructural protein 4A mediates oligomerization and is essential for replication. J. Virol. 2013, 87, 4080–4085. [Google Scholar] [CrossRef] [PubMed]
- Cahour, A.; Falgout, B.; Lai, C.J. Cleavage of the dengue virus polyprotein at the NS3/NS4A and NS4B/NS5 junctions is mediated by viral protease NS2B-NS3, whereas NS4A/NS4B may be processed by a cellular protease. J. Virol. 1992, 66, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.; Shi, P.Y. Recent advances in flavivirus antiviral drug discovery and vaccine development. Recent Pat. Anti-Infect. Drug Discov. 2006, 1, 45–55. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, A.; Yadav, P.; Dubey, S.K.; Azhar, E.I.; Maitra, S.S.; Dwivedi, V.D. Molecular pathogenesis of Japanese encephalitis and possible therapeutic strategies. Arch. Virol. 2022, 167, 1739–1762. [Google Scholar] [CrossRef]
- Knyazhanskaya, E.; Morais, M.C.; Choi, K.H. Flavivirus enzymes and their inhibitors. Enzymes 2021, 49, 265–303. [Google Scholar] [CrossRef] [PubMed]
- Utama, A.; Shimizu, H.; Morikawa, S.; Hasebe, F.; Morita, K.; Igarashi, A.; Hatsu, M.; Takamizawa, K.; Miyamura, T. Identification and characterization of the RNA helicase activity of Japanese encephalitis virus NS3 protein. FEBS Lett. 2000, 465, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Phoo, W.W.; Luo, D. Functional interplay among the flavivirus NS3 protease, helicase, and cofactors. Virol. Sin. 2014, 29, 74–85. [Google Scholar] [CrossRef][Green Version]
- Lin, R.J.; Chang, B.L.; Yu, H.P.; Liao, C.L.; Lin, Y.L. Blocking of interferon-induced Jak-Stat signaling by Japanese encephalitis virus NS5 through a protein tyrosine phosphatase-mediated mechanism. J. Virol. 2006, 80, 5908–5918. [Google Scholar] [CrossRef]
- Zhao, Y.; Soh, T.S.; Lim, S.P.; Chung, K.Y.; Swaminathan, K.; Vasudevan, S.G.; Shi, P.Y.; Lescar, J.; Luo, D. Molecular basis for specific viral RNA recognition and 2′-O-ribose methylation by the dengue virus nonstructural protein 5 (NS5). Proc. Natl. Acad. Sci. USA 2015, 112, 14834–14839. [Google Scholar] [CrossRef]
- Li, C.; Di, D.; Huang, H.; Wang, X.; Xia, Q.; Ma, X.; Liu, K.; Li, B.; Shao, D.; Qiu, Y.; et al. NS5-V372A and NS5-H386Y variations are responsible for differences in interferon α/β induction and co-contribute to the replication advantage of Japanese encephalitis virus genotype I over genotype III in ducklings. PLoS Pathog. 2020, 16, e1008773. [Google Scholar] [CrossRef]
- Kao, Y.T.; Chang, B.L.; Liang, J.J.; Tsai, H.J.; Lee, Y.L.; Lin, R.J.; Lin, Y.L. Japanese encephalitis virus nonstructural protein NS5 interacts with mitochondrial trifunctional protein and impairs fatty acid β-oxidation. PLoS Pathog. 2015, 11, e1004750. [Google Scholar] [CrossRef]
- Lubick, K.J.; Robertson, S.J.; McNally, K.L.; Freedman, B.A.; Rasmussen, A.L.; Taylor, R.T.; Walts, A.D.; Tsuruda, S.; Sakai, M.; Ishizuka, M.; et al. Flavivirus Antagonism of Type I Interferon Signaling Reveals Prolidase as a Regulator of IFNAR1 Surface Expression. Cell Host Microbe 2015, 18, 61–74. [Google Scholar] [CrossRef]
- Best, S.M. The Many Faces of the Flavivirus NS5 Protein in Antagonism of Type I Interferon Signaling. J. Virol. 2017, 91, e01970-16. [Google Scholar] [CrossRef]
- Latanova, A.; Starodubova, E. Flaviviridae Nonstructural Proteins: The Role in Molecular Mechanisms of Triggering Inflammation. Viruses 2022, 14, 1808. [Google Scholar] [CrossRef]
- Upadhyay, A.K.; Cyr, M.; Longenecker, K.; Tripathi, R.; Sun, C.; Kempf, D.J. Crystal structure of full-length Zika virus NS5 protein reveals a conformation similar to Japanese encephalitis virus NS5. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2017, 73, 116–122. [Google Scholar] [CrossRef]
- Turtle, L.; Solomon, T. Japanese encephalitis—The prospects for new treatments. Nat. Rev. Neurol. 2018, 14, 298–313. [Google Scholar] [CrossRef]
- Lee, C.J.; Lin, H.R.; Liao, C.L.; Lin, Y.L. Cholesterol effectively blocks entry of flavivirus. J. Virol. 2008, 82, 6470–6480. [Google Scholar] [CrossRef]
- Bressanelli, S.; Stiasny, K.; Allison, S.L.; Stura, E.A.; Duquerroy, S.; Lescar, J.; Heinz, F.X.; Rey, F.A. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004, 23, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Stiasny, K.; Kössl, C.; Lepault, J.; Rey, F.A.; Heinz, F.X. Characterization of a structural intermediate of flavivirus membrane fusion. PLoS Pathog. 2007, 3, e20. [Google Scholar] [CrossRef]
- Villordo, S.M.; Gamarnik, A.V. Genome cyclization as strategy for flavivirus RNA replication. Virus Res. 2009, 139, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.M.; Zhang, W.; Holdaway, H.A.; Li, L.; Kostyuchenko, V.A.; Chipman, P.R.; Kuhn, R.J.; Rossmann, M.G.; Chen, J. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science 2008, 319, 1834–1837. [Google Scholar] [CrossRef] [PubMed]
- Nain, M.; Mukherjee, S.; Karmakar, S.P.; Paton, A.W.; Paton, J.C.; Abdin, M.Z.; Basu, A.; Kalia, M.; Vrati, S. GRP78 Is an Important Host Factor for Japanese Encephalitis Virus Entry and Replication in Mammalian Cells. J. Virol. 2017, 91, e02274-16. [Google Scholar] [CrossRef]
- Chen, S.T.; Liu, R.S.; Wu, M.F.; Lin, Y.L.; Chen, S.Y.; Tan, D.T.; Chou, T.Y.; Tsai, I.S.; Li, L.; Hsieh, S.L. CLEC5A regulates Japanese encephalitis virus-induced neuroinflammation and lethality. PLoS Pathog. 2012, 8, e1002655. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.J.; Chen, W.J.; Hsu, W.L.; Chiou, S.S. Bovine lactoferrin inhibits Japanese encephalitis virus by binding to heparan sulfate and receptor for low density lipoprotein. Virology 2008, 379, 143–151. [Google Scholar] [CrossRef]
- Liang, Z.; Pan, J.; Xie, S.; Yang, X.; Cao, R. Interaction between hTIM-1 and Envelope Protein Is Important for JEV Infection. Viruses 2023, 15, 1589. [Google Scholar] [CrossRef]
- Wang, P.; Hu, K.; Luo, S.; Zhang, M.; Deng, X.; Li, C.; Jin, W.; Hu, B.; He, S.; Li, M.; et al. DC-SIGN as an attachment factor mediates Japanese encephalitis virus infection of human dendritic cells via interaction with a single high-mannose residue of viral E glycoprotein. Virology 2016, 488, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Yu, C.Y.; Liao, C.L.; Lin, Y.L. Vimentin binding is critical for infection by the virulent strain of Japanese encephalitis virus. Cell. Microbiol. 2011, 13, 1358–1370. [Google Scholar] [CrossRef]
- Thongtan, T.; Wikan, N.; Wintachai, P.; Rattanarungsan, C.; Srisomsap, C.; Cheepsunthorn, P.; Smith, D.R. Characterization of putative Japanese encephalitis virus receptor molecules on microglial cells. J. Med. Virol. 2012, 84, 615–623. [Google Scholar] [CrossRef]
- Altmeyer, R. Virus attachment and entry offer numerous targets for antiviral therapy. Curr. Pharm. Des. 2004, 10, 3701–3712. [Google Scholar] [CrossRef]
- Castel, G.; Chtéoui, M.; Heyd, B.; Tordo, N. Phage display of combinatorial peptide libraries: Application to antiviral research. Molecules 2011, 16, 3499–3518. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Hameed, M.; Wang, X.; Zhang, J.; Guo, S.; Anwar, M.N.; Pang, L.; Liu, K.; Li, B.; Shao, D.; et al. Antiviral activity of phage display-selected peptides against Japanese encephalitis virus infection in vitro and in vivo. Antivir. Res. 2020, 174, 104673. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, H.; Ye, Z.; Xu, Q.; Fu, Q.; Sun, W.; Qi, W.; Yue, J. Berbamine inhibits Japanese encephalitis virus (JEV) infection by compromising TPRMLs-mediated endolysosomal trafficking of low-density lipoprotein receptor (LDLR). Emerg. Microbes Infect. 2021, 10, 1257–1271. [Google Scholar] [CrossRef]
- Go, G.W.; Mani, A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J. Biol. Med. 2012, 85, 19–28. [Google Scholar] [PubMed]
- Mokhber-Dezfuli, N.; Saeidnia, S.; Gohari, A.R.; Kurepaz-Mahmoodabadi, M. Phytochemistry and pharmacology of berberis species. Pharmacogn. Rev. 2014, 8, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, T.; Meng, Z.; Gan, Y.; Xu, X.; Lou, G.; Li, H.; Gan, X.; Zhou, H.; Tang, J.; et al. CaMKII γ, a critical regulator of CML stem/progenitor cells, is a target of the natural product berbamine. Blood 2012, 120, 4829–4839. [Google Scholar] [CrossRef]
- Yang, C.C.; Hu, H.S.; Lin, H.M.; Wu, P.S.; Wu, R.H.; Tian, J.N.; Wu, S.H.; Tsou, L.K.; Song, J.S.; Chen, H.W.; et al. A novel flavivirus entry inhibitor, BP34610, discovered through high-throughput screening with dengue reporter viruses. Antivir. Res. 2019, 172, 104636. [Google Scholar] [CrossRef]
- Arnett, E.; Lehrer, R.I.; Pratikhya, P.; Lu, W.; Seveau, S. Defensins enable macrophages to inhibit the intracellular proliferation of Listeria monocytogenes. Cell. Microbiol. 2011, 13, 635–651. [Google Scholar] [CrossRef]
- Leonova, L.; Kokryakov, V.N.; Aleshina, G.; Hong, T.; Nguyen, T.; Zhao, C.; Waring, A.J.; Lehrer, R.I. Circular minidefensins and posttranslational generation of molecular diversity. J. Leukoc. Biol. 2001, 70, 461–464. [Google Scholar] [CrossRef]
- Tran, D.; Tran, P.A.; Tang, Y.Q.; Yuan, J.; Cole, T.; Selsted, M.E. Homodimeric theta-defensins from rhesus macaque leukocytes: Isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J. Biol. Chem. 2002, 277, 3079–3084. [Google Scholar] [CrossRef] [PubMed]
- Prantner, D.; Shirey, K.A.; Lai, W.; Lu, W.; Cole, A.M.; Vogel, S.N.; Garzino-Demo, A. The θ-defensin retrocyclin 101 inhibits TLR4- and TLR2-dependent signaling and protects mice against influenza infection. J. Leukoc. Biol. 2017, 102, 1103–1113. [Google Scholar] [CrossRef]
- Glickman, M.H.; Ciechanover, A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol. Rev. 2002, 82, 373–428. [Google Scholar] [CrossRef]
- Pickart, C.M. Ubiquitin enters the new millennium. Mol. Cell 2001, 8, 499–504. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Zu, X.; Liu, Y.; Chen, L.; Zhu, X.; Zhang, L.; Zhou, Z.; Xiao, G.; Wang, W. The ubiquitin-proteasome system is essential for the productive entry of Japanese encephalitis virus. Virology 2016, 498, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Lin, C.J.; Anand, A.; Lin, H.J.; Lin, H.Y.; Mao, J.Y.; Wang, P.H.; Tseng, Y.J.; Tzou, W.S.; Huang, C.C.; et al. Development of antiviral carbon quantum dots that target the Japanese encephalitis virus envelope protein. J. Biol. Chem. 2022, 298, 101957. [Google Scholar] [CrossRef]
- Lin, C.J.; Chang, L.; Chu, H.W.; Lin, H.J.; Chang, P.C.; Wang, R.Y.L.; Unnikrishnan, B.; Mao, J.Y.; Chen, S.Y.; Huang, C.C. High Amplification of the Antiviral Activity of Curcumin through Transformation into Carbon Quantum Dots. Small 2019, 15, e1902641. [Google Scholar] [CrossRef]
- Rawlinson, S.M.; Pryor, M.J.; Wright, P.J.; Jans, D.A. Dengue virus RNA polymerase NS5: A potential therapeutic target? Curr. Drug Targets 2006, 7, 1623–1638. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.P.; Noble, C.G.; Shi, P.Y. The dengue virus NS5 protein as a target for drug discovery. Antivir. Res. 2015, 119, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, M.; Raffi, F.; Allavena, C. Tenofovir DF/emtricitabine/rilpivirine as HIV post-exposure prophylaxis: Results from a multicentre prospective study-authors’ response. J. Antimicrob. Chemother. 2019, 74, 3403–3404. [Google Scholar] [CrossRef]
- Bhatia, H.K.; Singh, H.; Grewal, N.; Natt, N.K. Sofosbuvir: A novel treatment option for chronic hepatitis C infection. J. Pharmacol. Pharmacother. 2014, 5, 278–284. [Google Scholar] [CrossRef]
- Bullard-Feibelman, K.M.; Govero, J.; Zhu, Z.; Salazar, V.; Veselinovic, M.; Diamond, M.S.; Geiss, B.J. The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antivir. Res. 2017, 137, 134–140. [Google Scholar] [CrossRef]
- Meerbach, A.; Klöcking, R.; Meier, C.; Lomp, A.; Helbig, B.; Wutzler, P. Inhibitory effect of cycloSaligenyl-nucleoside monophosphates (cycloSal-NMP) of acyclic nucleoside analogues on HSV-1 and EBV. Antivir. Res. 2000, 45, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Gong, P. A structural view of the RNA-dependent RNA polymerases from the Flavivirus genus. Virus Res. 2017, 234, 34–43. [Google Scholar] [CrossRef] [PubMed]
- El Sahili, A.; Soh, T.S.; Schiltz, J.; Gharbi-Ayachi, A.; Seh, C.C.; Shi, P.Y.; Lim, S.P.; Lescar, J. NS5 from Dengue Virus Serotype 2 Can Adopt a Conformation Analogous to That of Its Zika Virus and Japanese Encephalitis Virus Homologues. J. Virol. 2019, 94, e01294-19. [Google Scholar] [CrossRef]
- Zandi, K.; Bassit, L.; Amblard, F.; Cox, B.D.; Hassandarvish, P.; Moghaddam, E.; Yueh, A.; Libanio Rodrigues, G.O.; Passos, I.; Costa, V.V.; et al. Nucleoside Analogs with Selective Antiviral Activity against Dengue Fever and Japanese Encephalitis Viruses. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Yadav, P.; El-Kafrawy, S.A. Discovery of Small Molecules from Echinacea angustifolia Targeting RNA-Dependent RNA Polymerase of Japanese Encephalitis Virus. Life 2022, 12, 952. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, V.D.; Singh, A.; El-Kafraway, S.A.; Alandijany, T.A.; Faizo, A.A.; Bajrai, L.H.; Kamal, M.A.; Azhar, E.I. Mechanistic insights into the Japanese encephalitis virus RNA dependent RNA polymerase protein inhibition by bioflavonoids from Azadirachta indica. Sci. Rep. 2021, 11, 18125. [Google Scholar] [CrossRef] [PubMed]
- Wahaab, A.; Mustafa, B.E.; Hameed, M.; Stevenson, N.J. Potential Role of Flavivirus NS2B-NS3 Proteases in Viral Pathogenesis and Anti-flavivirus Drug Discovery Employing Animal Cells and Models: A Review. Viruses 2021, 14, 44. [Google Scholar] [CrossRef]
- de Leuw, P.; Stephan, C. Protease inhibitor therapy for hepatitis C virus-infection. Expert Opin. Pharmacother. 2018, 19, 577–587. [Google Scholar] [CrossRef]
- Yuan, S.; Chan, J.F.; den-Haan, H.; Chik, K.K.; Zhang, A.J.; Chan, C.C.; Poon, V.K.; Yip, C.C.; Mak, W.W.; Zhu, Z.; et al. Structure-based discovery of clinically approved drugs as Zika virus NS2B-NS3 protease inhibitors that potently inhibit Zika virus infection in vitro and in vivo. Antivir. Res. 2017, 145, 33–43. [Google Scholar] [CrossRef]
- Pinkham, A.M.; Yu, Z.; Cowan, J.A. Broad-spectrum catalytic metallopeptide inactivators of Zika and West Nile virus NS2B/NS3 proteases. Chem. Commun. 2018, 54, 12357–12360. [Google Scholar] [CrossRef]
- Huang, S.H.; Lien, J.C.; Chen, C.J.; Liu, Y.C.; Wang, C.Y.; Ping, C.F.; Lin, Y.F.; Huang, A.C.; Lin, C.W. Antiviral Activity of a Novel Compound CW-33 against Japanese Encephalitis Virus through Inhibiting Intracellular Calcium Overload. Int. J. Mol. Sci. 2016, 17, 1386. [Google Scholar] [CrossRef]
- Chen, K.C.; Lin, Y.F.; Huang, A.C.; Gao, J.Y.; Lin, C.W.; Lien, J.C. Molecular interaction of the antiviral compound CW-33 and its analogues with the NS2B-NS3 protease of the Japanese encephalitis virus. Int. J. Mol. Med. 2019, 43, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.; Kumar, A. Screening of phytoconstituents of Andrographis paniculata against various targets of Japanese encephalitis virus: An in-silico and in-vitro target-based approach. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100043. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chen, L.J.; Huang, T.; Ying, J.Q.; Li, J. The pharmacology, toxicology and therapeutic potential of anthraquinone derivative emodin. Chin. J. Nat. Med. 2020, 18, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Wu, C.F.; Hsiao, N.W.; Chang, C.Y.; Li, S.W.; Wan, L.; Lin, Y.J.; Lin, W.Y. Aloe-emodin is an interferon-inducing agent with antiviral activity against Japanese encephalitis virus and enterovirus 71. Int. J. Antimicrob. Agents 2008, 32, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Sydiskis, R.J.; Owen, D.G.; Lohr, J.L.; Rosler, K.H.; Blomster, R.N. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob. Agents Chemother. 1991, 35, 2463–2466. [Google Scholar] [CrossRef] [PubMed]
- Barnard, D.L.; Huffman, J.H.; Morris, J.L.; Wood, S.G.; Hughes, B.G.; Sidwell, R.W. Evaluation of the antiviral activity of anthraquinones, anthrones and anthraquinone derivatives against human cytomegalovirus. Antivir. Res. 1992, 17, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.I.; Sheikh, W.M.; Rather, M.A.; Venkatesalu, V. Medicinal plants: Treasure for antiviral drug discovery. Phytotherapy Res. 2021, 35, 3447–3483. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Wang, Y.W.; Yang, J.; Tong, Z.J.; Wu, J.Z.; Wang, Y.B.; Wang, Q.X.; Li, Q.Q.; Yu, Y.C.; Leng, X.J.; et al. Natural products as potential lead compounds to develop new antiviral drugs over the past decade. Eur. J. Med. Chem. 2023, 260, 115726. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jia, X.; Liu, Y.; Wang, S.; Cao, J.; Zhang, B.; Xiao, G.; Wang, W. Screening of Natural Extracts for Inhibitors against Japanese Encephalitis Virus Infection. Antimicrob. Agents Chemother. 2020, 64, e02373-19. [Google Scholar] [CrossRef]
- Liu, M.; Wang, W.G.; Sun, H.D.; Pu, J.X. Diterpenoids from Isodon species: An update. Nat. Prod. Rep. 2017, 34, 1090–1140. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Fu, Q.; Dai, J.M.; Yan, B.C.; Wang, D.; Puno, P.T.; Yue, J. High-content screening of diterpenoids from Isodon species as autophagy modulators and the functional study of their antiviral activities. Cell Biol. Toxicol. 2021, 37, 695–713. [Google Scholar] [CrossRef]
- Johari, J.; Kianmehr, A.; Mustafa, M.R.; Abubakar, S.; Zandi, K. Antiviral activity of baicalein and quercetin against the Japanese encephalitis virus. Int. J. Mol. Sci. 2012, 13, 16785–16795. [Google Scholar] [CrossRef]
- Peake, P.W.; Pussell, B.A.; Martyn, P.; Timmermans, V.; Charlesworth, J.A. The inhibitory effect of rosmarinic acid on complement involves the C5 convertase. Int. J. Immunopharmacol. 1991, 13, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Swarup, V.; Ghosh, J.; Ghosh, S.; Saxena, A.; Basu, A. Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrob. Agents Chemother. 2007, 51, 3367–3370. [Google Scholar] [CrossRef]
- Sharma, R.; Bhattu, M.; Tripathi, A.; Verma, M.; Acevedo, R.; Kumar, P.; Rajput, V.D.; Singh, J. Potential medicinal plants to combat viral infections: A way forward to environmental biotechnology. Environ. Res. 2023, 227, 115725. [Google Scholar] [CrossRef]
- Gould, E.A.; de Lamballerie, X.; Zanotto, P.M.; Holmes, E.C. Origins, evolution, and vector/host coadaptations within the genus Flavivirus. Adv. Virus Res. 2003, 59, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.F.; Lee, C.J.; Liao, C.L.; Dwek, R.A.; Zitzmann, N.; Lin, Y.L. Antiviral effects of an iminosugar derivative on flavivirus infections. J. Virol. 2002, 76, 3596–3604. [Google Scholar] [CrossRef]
- Thirumal Kumar, D.; Iyer, S.; Christy, J.P.; Siva, R.; Tayubi, I.A.; George Priya Doss, C.; Zayed, H. A comparative computational approach toward pharmacological chaperones (NN-DNJ and ambroxol) on N370S and L444P mutations causing Gaucher’s disease. Adv. Protein Chem. Struct. Biol. 2019, 114, 315–339. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Guo, J.; Wang, P.; Zhang, L.; Xiao, G.; Wang, W. Screening of FDA-Approved Drugs for Inhibitors of Japanese Encephalitis Virus Infection. J. Virol. 2017, 91, e01055-17. [Google Scholar] [CrossRef]
- Mizuno, K.; Haga, H.; Takahashi, M.; Watanabe, Y.; Fukuchi, S. Clinical evaluation of the efficacy and safety of manidipine in hypertensive patients with renal disorders. Blood Press. Suppl. 1992, 3, 119–123. [Google Scholar]
- Ravi, V.; Parida, S.; Desai, A.; Chandramuki, A.; Gourie-Devi, M.; Grau, G.E. Correlation of tumor necrosis factor levels in the serum and cerebrospinal fluid with clinical outcome in Japanese encephalitis patients. J. Med. Virol. 1997, 51, 132–136. [Google Scholar] [CrossRef]
- Genovese, T.; Mazzon, E.; Crisafulli, C.; Di Paola, R.; Muià, C.; Bramanti, P.; Cuzzocrea, S. Immunomodulatory effects of etanercept in an experimental model of spinal cord injury. J. Pharmacol. Exp. Ther. 2006, 316, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Jiang, R.; Cui, M.; Zhu, B.; Sun, L.; Wang, Y.; Zohaib, A.; Dong, Q.; Ruan, X.; Song, Y.; et al. Etanercept reduces neuroinflammation and lethality in mouse model of Japanese encephalitis. J. Infect. Dis. 2014, 210, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.H.; Ilowite, N.T.; Lovell, D.J.; Wallace, C.A.; Rabinovich, C.E.; Reiff, A.; Higgins, G.; Gottlieb, B.; Singer, N.G.; Chon, Y.; et al. Long-term safety and effectiveness of etanercept in children with selected categories of juvenile idiopathic arthritis. Arthritis Rheum. 2009, 60, 2794–2804. [Google Scholar] [CrossRef]
- Elliott, M.J.; Maini, R.N.; Feldmann, M.; Long-Fox, A.; Charles, P.; Bijl, H.; Woody, J.N. Repeated therapy with monoclonal antibody to tumour necrosis factor alpha (cA2) in patients with rheumatoid arthritis. Lancet 1994, 344, 1125–1127. [Google Scholar] [CrossRef]
- Zou, J.X.; Braun, J.; Sieper, J. Immunological basis for the use of TNFalpha-blocking agents in ankylosing spondylitis and immunological changes during treatment. Clin. Exp. Rheumatol. 2002, 20, S34–S37. [Google Scholar] [PubMed]
- Yong, V.W.; Wells, J.; Giuliani, F.; Casha, S.; Power, C.; Metz, L.M. The promise of minocycline in neurology. Lancet Neurol. 2004, 3, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Carney, S.; Butcher, R.A.; Dawborn, J.K.; Pattison, G. Minocycline excretion and distribution in relation to renal function in man. Clin. Exp. Pharmacol. Physiol. 1974, 1, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I. Glycylcyclines: Third-generation tetracycline antibiotics. Curr. Opin. Pharmacol. 2001, 1, 464–469. [Google Scholar] [CrossRef]
- Mishra, M.K.; Basu, A. Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J. Neurochem. 2008, 105, 1582–1595. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.L.; Sasaki, D.T.; Murray, B.W.; O’Leary, E.C.; Sakata, S.T.; Xu, W.; Leisten, J.C.; Motiwala, A.; Pierce, S.; Satoh, Y.; et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 13681–13686. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, H.; He, W.; Zhu, B.; Zhou, D.; Chen, Z.; Ashraf, U.; Wei, Y.; Liu, Z.; Fu, Z.F.; et al. Quantitative phosphoproteomic analysis identifies the critical role of JNK1 in neuroinflammation induced by Japanese encephalitis virus. Sci. Signal. 2016, 9, ra98. [Google Scholar] [CrossRef]
- Yang, X.J.; Seto, E. Lysine acetylation: Codified crosstalk with other posttranslational modifications. Mol. Cell 2008, 31, 449–461. [Google Scholar] [CrossRef]
- Liu, H.M.; Jiang, F.; Loo, Y.M.; Hsu, S.; Hsiang, T.Y.; Marcotrigiano, J.; Gale, M., Jr. Regulation of Retinoic Acid Inducible Gene-I (RIG-I) Activation by the Histone Deacetylase 6. EBioMedicine 2016, 9, 195–206. [Google Scholar] [CrossRef]
- Lu, C.Y.; Chang, Y.C.; Hua, C.H.; Chuang, C.; Huang, S.H.; Kung, S.H.; Hour, M.J.; Lin, C.W. Tubacin, an HDAC6 Selective Inhibitor, Reduces the Replication of the Japanese Encephalitis Virus via the Decrease of Viral RNA Synthesis. Int. J. Mol. Sci. 2017, 18, 954. [Google Scholar] [CrossRef]
- Han, Y.W.; Choi, J.Y.; Uyangaa, E.; Kim, S.B.; Kim, J.H.; Kim, B.S.; Kim, K.; Eo, S.K. Distinct dictation of Japanese encephalitis virus-induced neuroinflammation and lethality via triggering TLR3 and TLR4 signal pathways. PLoS Pathog. 2014, 10, e1004319. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Lee, K.C.; Wu, P.S.; Huo, T.I.; Huang, Y.H. Eritoran Attenuates Hepatic Inflammation and Fibrosis in Mice with Chronic Liver Injury. Cells 2021, 10, 1562. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Chakraborty, P. Molecular Mechanism and Role of Japanese Encephalitis Virus Infection in Central Nervous System-Mediated Diseases. Viruses 2022, 14, 2686. [Google Scholar] [CrossRef] [PubMed]
- Erlanger, T.E.; Weiss, S.; Keiser, J.; Utzinger, J.; Wiedenmayer, K. Past, present, and future of Japanese encephalitis. Emerg. Infect. Dis. 2009, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tiroumourougane, S.V.; Raghava, P.; Srinivasana, S.; Badrinath. Management parameters affecting the outcome of Japanese encephalitis. J. Trop. Pediatr. 2003, 49, 153–156. [Google Scholar] [CrossRef]
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011, 89, 766–774. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, S.; Lurong, Q.; Qi, Z. Recent Advances in Antivirals for Japanese Encephalitis Virus. Viruses 2023, 15, 1033. [Google Scholar] [CrossRef]
- Calvert, A.E.; Bennett, S.L.; Dixon, K.L.; Blair, C.D.; Roehrig, J.T. A Monoclonal Antibody Specific for Japanese Encephalitis Virus with High Neutralizing Capability for Inclusion as a Positive Control in Diagnostic Neutralization Tests. Am. J. Trop. Med. Hyg. 2019, 101, 233–236. [Google Scholar] [CrossRef]
- Kant, K.; Lal, U.R.; Kumar, A.; Ghosh, M. A merged molecular docking, ADME-T and dynamics approaches towards the genus of Arisaema as herpes simplex virus type 1 and type 2 inhibitors. Comput. Biol. Chem. 2019, 78, 217–226. [Google Scholar] [CrossRef]
- Majerová, T.; Konvalinka, J. Viral proteases as therapeutic targets. Mol. Asp. Med. 2022, 88, 101159. [Google Scholar] [CrossRef] [PubMed]
- Brecher, M.; Zhang, J.; Li, H. The flavivirus protease as a target for drug discovery. Virol. Sin. 2013, 28, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Li, H.; Kong, D.; Cao, S.; Peng, G.; Zhou, R.; Chen, H.; Song, Y. Structure-based discovery of two antiviral inhibitors targeting the NS3 helicase of Japanese encephalitis virus. Sci. Rep. 2016, 6, 34550. [Google Scholar] [CrossRef]
- Kim, Y.G.; Yoo, J.S.; Kim, J.H.; Kim, C.M.; Oh, J.W. Biochemical characterization of a recombinant Japanese encephalitis virus RNA-dependent RNA polymerase. BMC Mol. Biol. 2007, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Bian, P.; Zhang, J.; Xiao, H.; Zhang, L.; Ye, W.; Dong, Y.; Zhou, Y.; Jia, Z.; Lei, Y. Structure-based discovery of antiviral inhibitors targeting the E dimer interface of Japanese encephalitis virus. Biochem. Biophys. Res. Commun. 2019, 515, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Hombach, J.; Barrett, A.D.; Cardosa, M.J.; Deubel, V.; Guzman, M.; Kurane, I.; Roehrig, J.T.; Sabchareon, A.; Kieny, M.P. Review on flavivirus vaccine development. Proceedings of a meeting jointly organised by the World Health Organization and the Thai Ministry of Public Health, 26-27 April 2004, Bangkok, Thailand. Vaccine 2005, 23, 2689–2695. [Google Scholar] [CrossRef] [PubMed]

| Optimum/Ideal | Minimum/Acceptable | |
|---|---|---|
| Target population | Population presenting clinical symptoms of JEV infection in endemic areas | Population that recently visited an endemic area and presented a clinical manifestation of JEV infection |
| Efficacy | No circulating JEV in the bloodstream, as assessed by specific quantitative PCR assays | Eliminates or reduces the risks of the neurological impairment of patients |
| Administration and dosage | Single oral dose | The duration of treatment is extended throughout pregnancy; oral dose |
| Pharmacokinetic profile | Permeable to the blood–brain barrier | Repair of damaged blood–brain barrier |
| Safety and tolerability | Safe to be taken during pregnancy (category A a) | Safe to be taken during pregnancy (category B b) |
| Adverse reactions | No observed adverse reaction | Reversible and mild adverse reactions |
| Drug interactions | No drug interactions | No interaction with common drugs against depression, diabetes, and high blood pressure during pregnancy |
| Contraindications | No contraindications | / |
| Precautions and warnings | No precautions or warnings | No teratogenicity and genotoxicity |
| Storage and handling | Stable for 1 year at room temperature | Require refrigeration (−20 °C) for stability |
| Cost of goods | Less than USD 5 per treatment course | Less than USD 100 per treatment course |
| Drug Target | Compound/ Drug Name | Mechanism of Action | In Vitro Activity: IC50 or EC50 and CC50 or LD50 (Utilised Cell Line) | In Vivo Efficacy: % Survival (Explored Animal Model) | References |
|---|---|---|---|---|---|
| Entry Inhibitors | P1 | Interacts with the JEV E protein | IC50: 35.9 μM (BHK-21 cells) CC50: >100 μM | 70% (C57/BL6) | [83] |
| Berbamine | Decreases LDLR levels at the cell surface | IC50: 1.62 μM (A549 cells) CC50: ~126 μM | 75% (BALB/c) | [84] | |
| BP34610 | A broad-spectrum flavivirus inhibitor that may target the flavivirus E protein | ND * CC50: ~94.55 μM | ND | [88] | |
| RC-101 | Targets the DE loop of the E protein | IC50: 10.67 μM (BHK-21 cells) CC50: >100 μM | ND | [38] | |
| MG132 | Interferes with JEV intracellular trafficking | ND * CC50: >3.2 μM | ND | [95] | |
| Lactacystin | Interferes with JEV intracellular trafficking | ND * CC50: >25.6 μM | ND | [95] | |
| Cur-CQDs | Binds to the JEV E protein | IC50: 0.9 μg/mL (BHK-21 cells) CC50: >144 μM | ND | [98] | |
| RdRp Inhibitors | 2′-C-MeC | Inhibits JEV replication | EC50: ~0.66 μM (Vero cells) CC50: >200 μM | ND | [108] |
| DMFA | Inhibits JEV replication | EC50: ~0.85 μM (Vero cells) CC50: >200 μM | ND | [108] | |
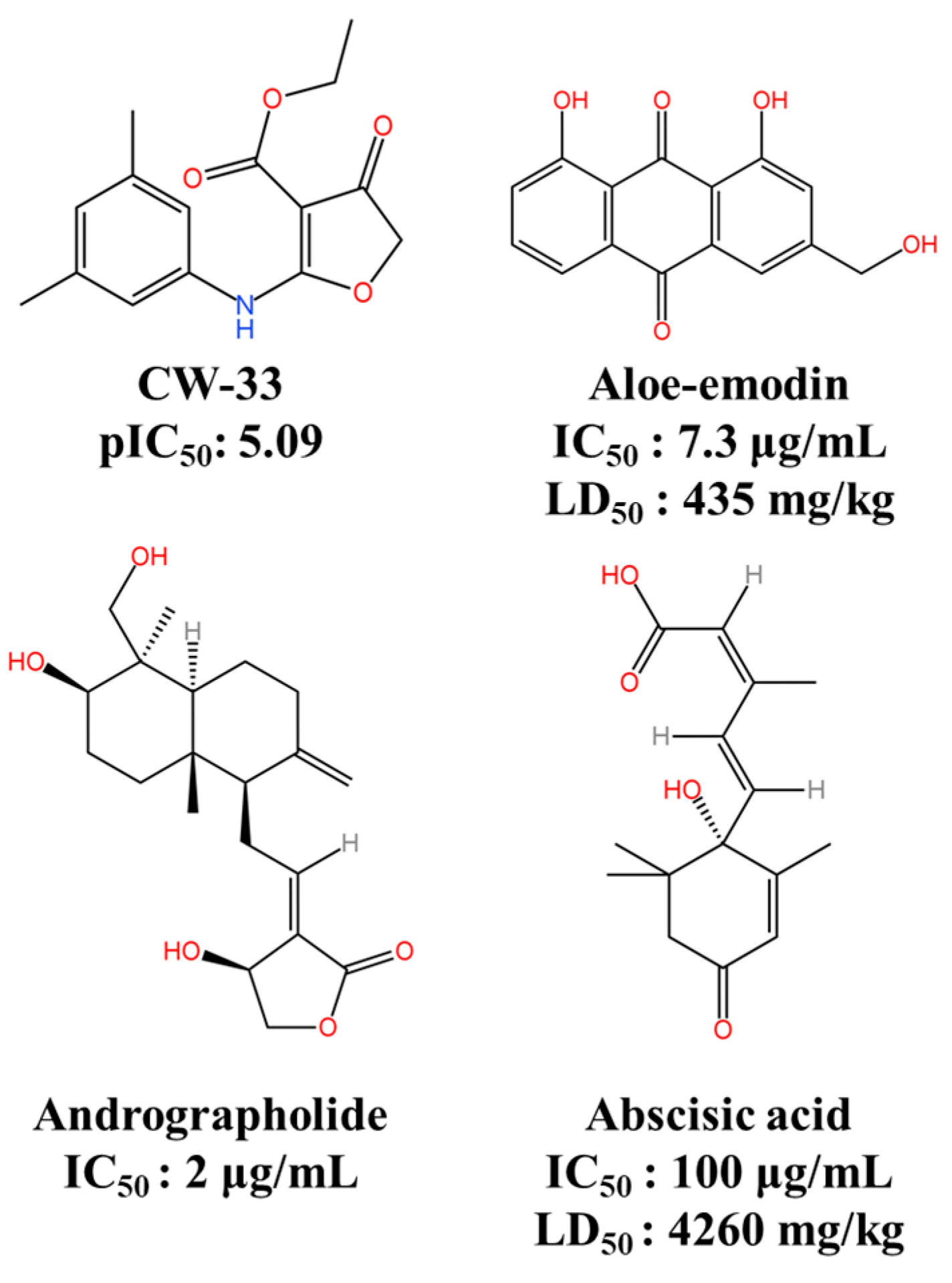
| Protease Inhibitors | CW-33 | Targets JEV NS2B-NS3 | pIC50: 5.09 | ND | [116] |
| Andrographolide | Inhibits JEV NS3 protease | IC50: 2 μg/mL | ND | [117] | |
| Abscisic acid | Binds with JEV NS2B-NS3 protease | IC50: 100 μg/mL LD50: 4260 mg/kg | ND | [48] | |
| Aloe-emodin | Binds with JEV NS2B-NS3 protease | IC50: 7.3 μg/mL LD50: 435 mg/kg | ND | [48] | |
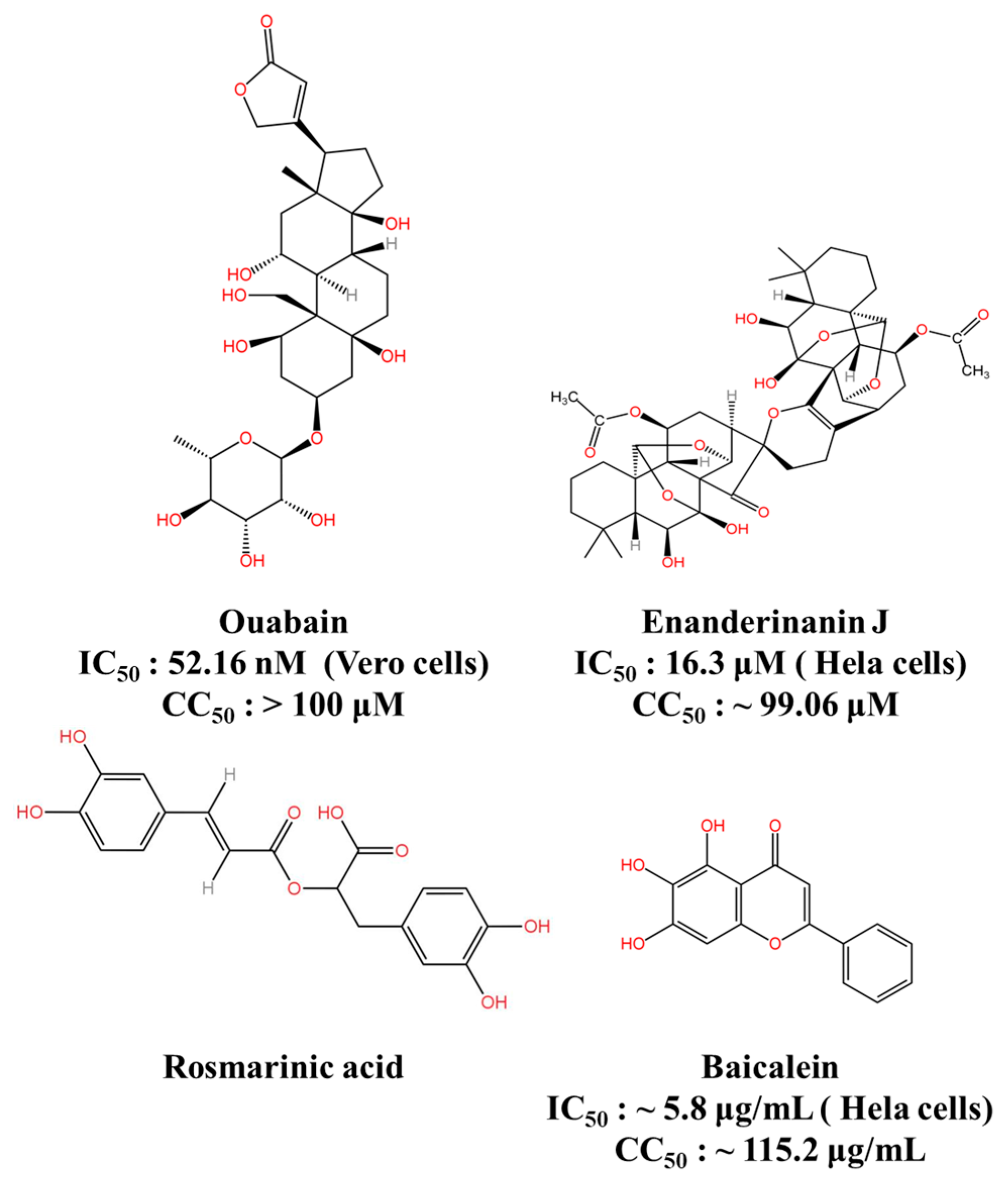
| Bioactive Natural Products and Their Derivatives | Ouabain | Targeting the Na+/K+-ATPase | IC50: 52.16 nM (Vero cells) CC50: >100 μM | 67% (BALB/c) | [124] |
| Enanderinanin J | Inhibits autophagosome–lysosome fusion | IC50: 16.3 μM (Hela cells) CC50: ~99.06 μM | ND | [126] | |
| Baicalein | Virucidal activity; inhibits adsorption activity | IC50: ~5.8 μg/mL (Hela cells) CC50: ~115.2 μg/mL | ND | [127] | |
| Rosmarinic acid | Anti-inflammatory effect | ND * | 80% (BALB/c) | [129] | |
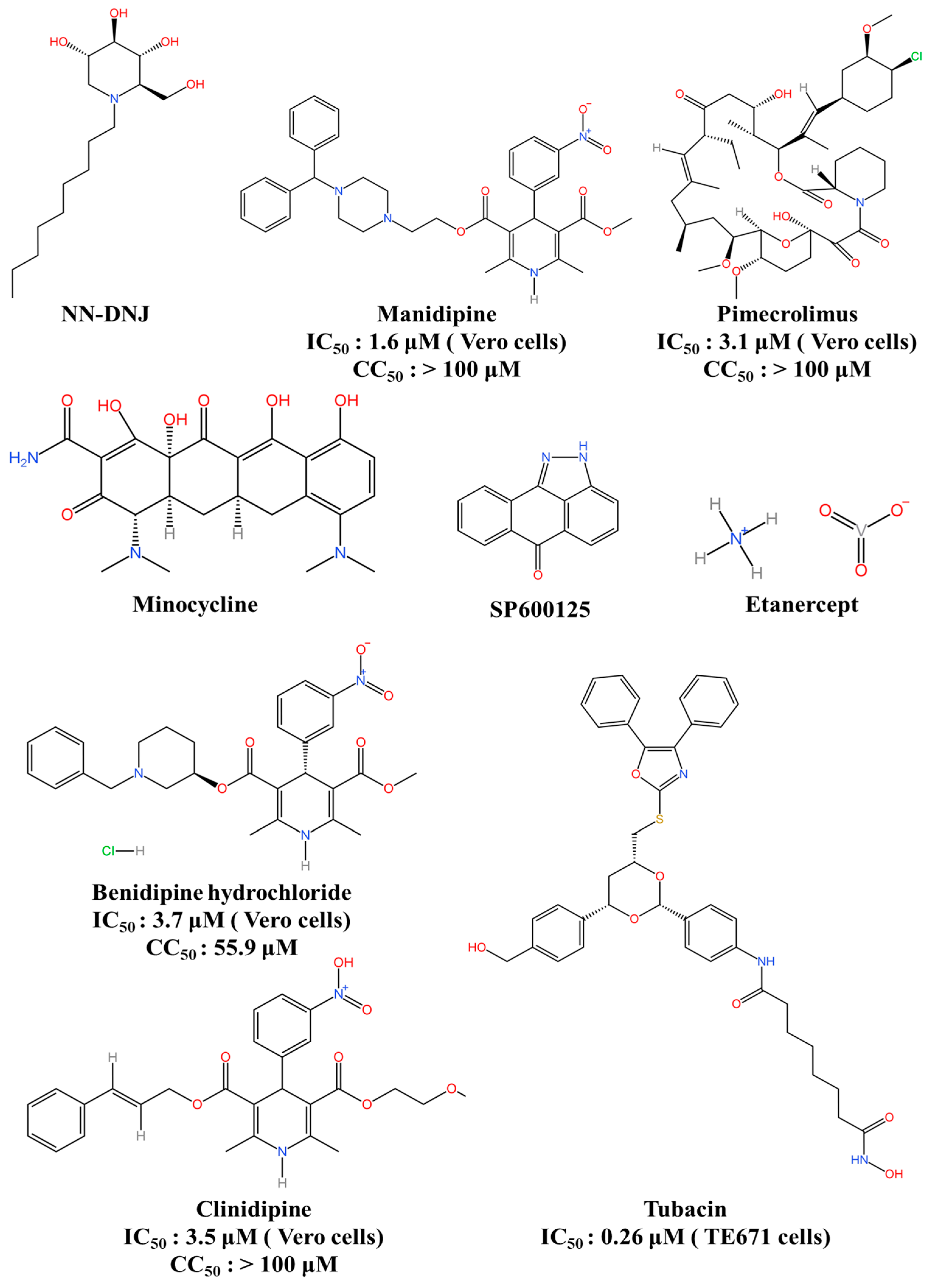
| Host-Directed Antiviral Agent | NN-DNJ | Inhibits α-glucosidases enzymes | ND * | 54% (ICR) | [132] |
| Manidipine | Targets NS4B and calcium channel | IC50: 1.6 μM (Vero cells) CC50: >100 μM | 80% (BALB/c) | [134] | |
| Cilnidipine | Inhibits calcium channel | IC50: 3.5 μM (Vero cells) CC50: >100 μM | ND | [134] | |
| Benidipine hydrochloride | Inhibits L-, N-, and T-type calcium channels | IC50: 3.7 μM (Vero cells) CC50: 55.9 μM | ND | [134] | |
| Pimecrolimus | Inhibits inflammatory cytokine secretion | IC50: 3.1 μM (Vero cells) CC50: >100 μM | ND | [134] | |
| Etanercept | Inhibits the downstream signalling pathways of TNF-α | ND * | 50% (BALB/c) | [138] | |
| Minocycline | Inhibits inflammatory cytokines and active caspase 3 activity | ND * | 70% (BALB/c) | [145] | |
| SP600125 | Inhibits JNK signalling | ND * | 40% (BALB/c) | [147] | |
| Tubacin | Inhibits histone deacetylases | IC50: 0.26 μM (TE671 cells) | ND | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Mi, Y.; Guo, Y.; Bai, Y.; Wang, M.; Wang, W.; Wang, Y. Current Advances in Japanese Encephalitis Virus Drug Development. Viruses 2024, 16, 202. https://doi.org/10.3390/v16020202
Guo J, Mi Y, Guo Y, Bai Y, Wang M, Wang W, Wang Y. Current Advances in Japanese Encephalitis Virus Drug Development. Viruses. 2024; 16(2):202. https://doi.org/10.3390/v16020202
Chicago/Turabian StyleGuo, Jiao, Yunqi Mi, Yan Guo, Yang Bai, Meihua Wang, Wei Wang, and Yang Wang. 2024. "Current Advances in Japanese Encephalitis Virus Drug Development" Viruses 16, no. 2: 202. https://doi.org/10.3390/v16020202
APA StyleGuo, J., Mi, Y., Guo, Y., Bai, Y., Wang, M., Wang, W., & Wang, Y. (2024). Current Advances in Japanese Encephalitis Virus Drug Development. Viruses, 16(2), 202. https://doi.org/10.3390/v16020202






