Using an In Vivo Mouse Model to Determine the Exclusion Criteria of Preexisting Anti-AAV9 Neutralizing Antibody Titer of Pompe Disease Patients in Clinical Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vectors
2.2. Cell Culture
2.3. Animal Studies
2.4. Anti-AAV9 Neutralizing Antibody Assay In Vitro
2.5. Vector Genome Copy Number Detected by Droplet Digital (dd) PCR
2.6. GAA Enzyme Activity Assay
2.7. ELISPOT
2.8. Statistical Analysis
3. Results
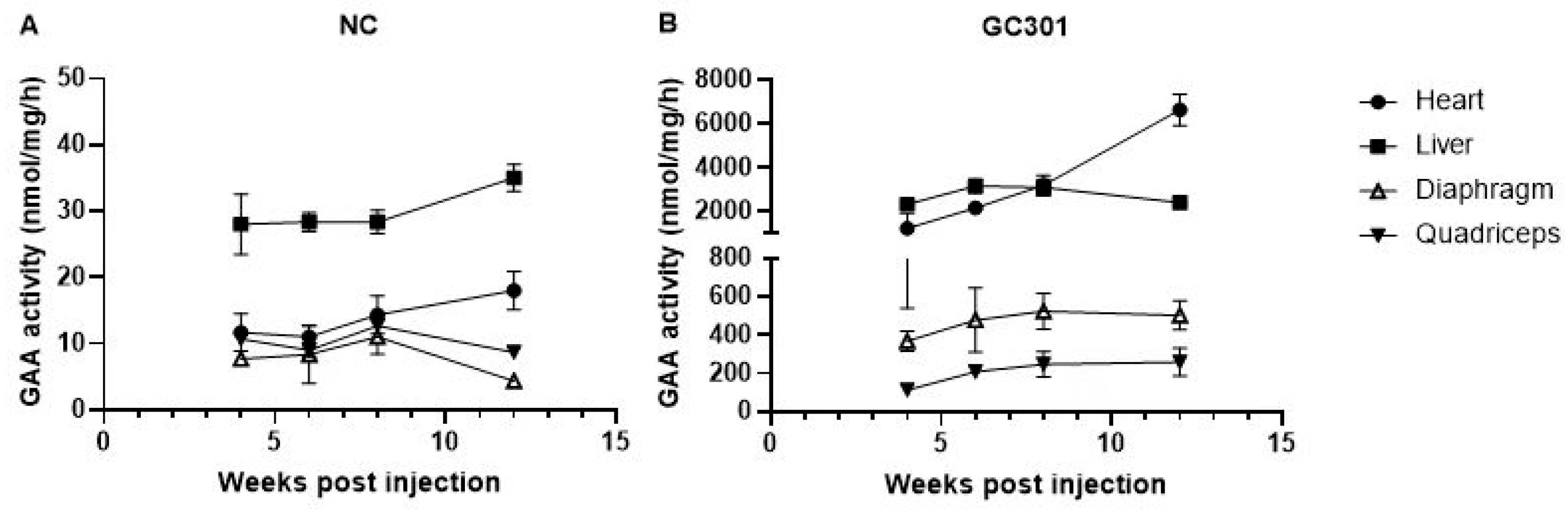
3.1. Effective Transduction of Systemic GC301 Delivery in Multiple Tissues in Balb/C Mice
3.2. In Vitro Neutralizing Assay Validating Ab Titers of a Mice Monoclonal NAb in Human or Mouse Sera Pool and That Delivered In Vivo
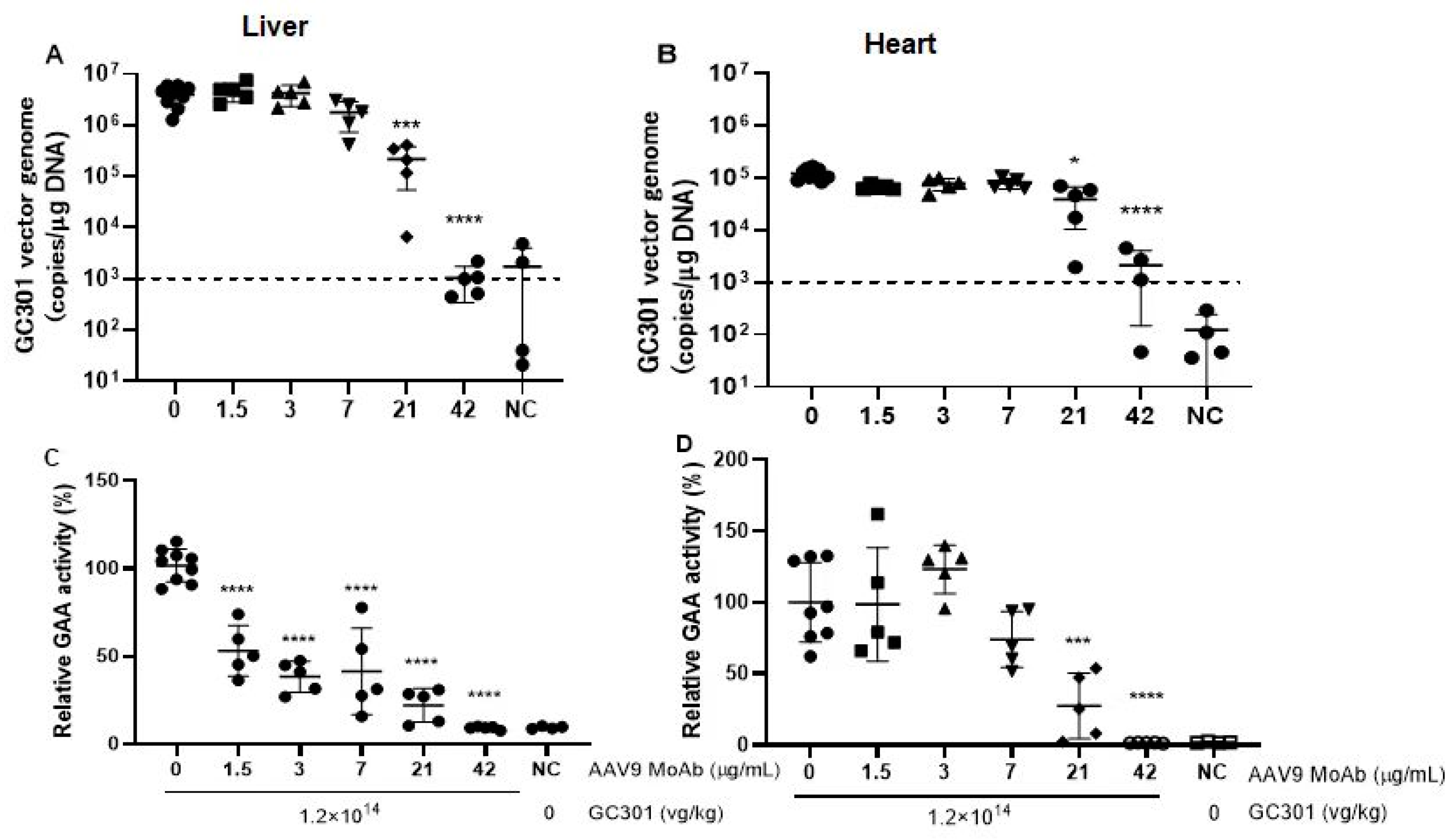
3.3. Accelerated Vector Clearance in Peripheral Blood in Presence of High Level of Preexisting NAb
3.4. Decreased Vector Genome Copies and Transduction of GC301 in Heart and Liver in Presence of Moderate Levels of Preexisting NAb
3.5. Elicited AAV9- and GAA-Specific T Cell Responses
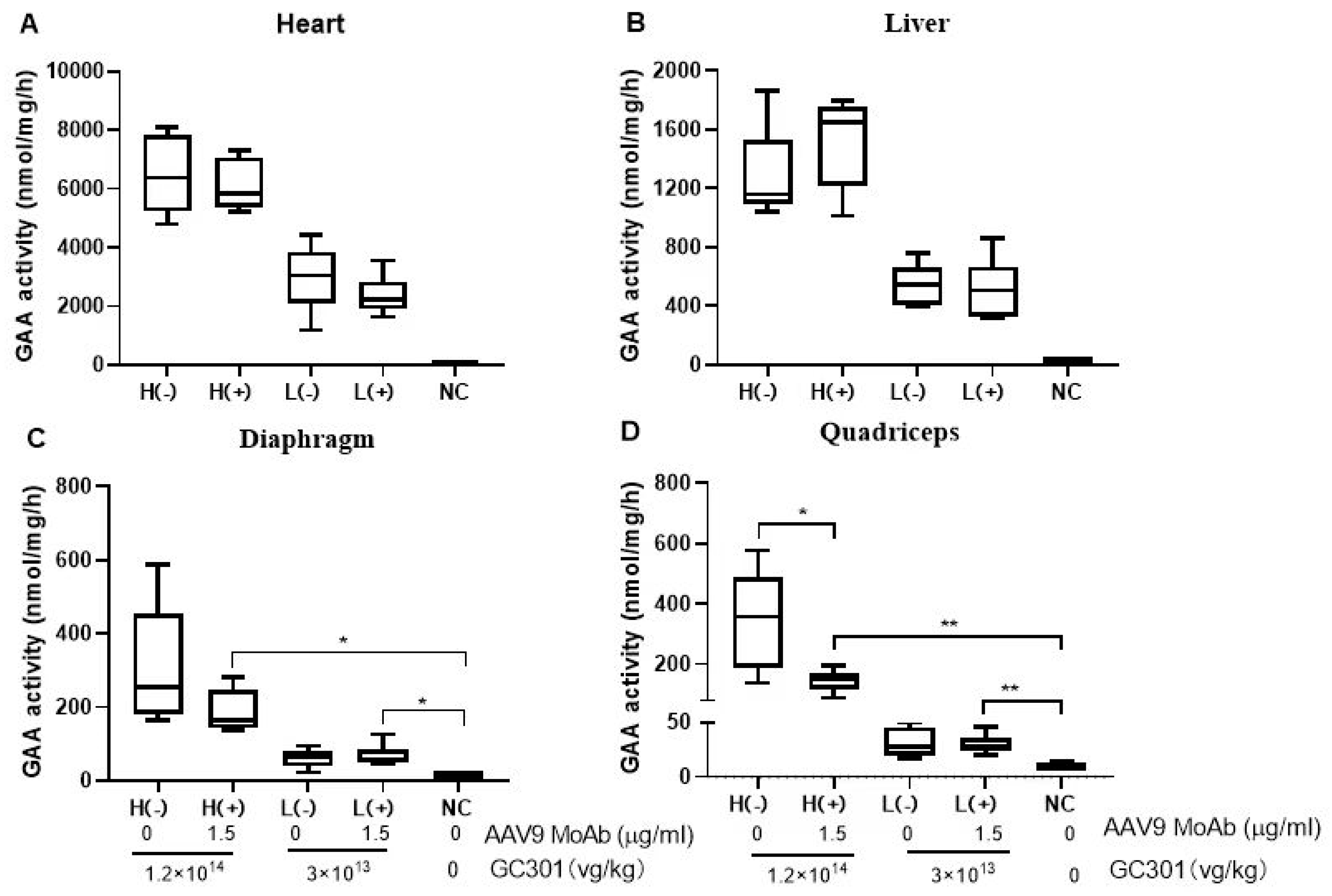
3.6. Efficient Transductions of GC301 in 1.5 μg/mL MoAb-Pretreated Mice at Both High and Low Dosages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Calcedo, R.; Bell, P.; Lin, J.; Grant, R.L.; Siegel, D.L.; Wilson, J.M. Impact of preexisting immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum. Gene Ther. 2011, 22, 1389–1401. [Google Scholar] [CrossRef]
- Wang, L.; Calcedo, R.; Wang, H.; Bell, P.; Grant, R.; Vandenberghe, L.H.; Sanmiguel, J.; Morizono, H.; Batshaw, M.L.; Wilson, J.M. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol. Ther. 2010, 18, 126–134. [Google Scholar] [CrossRef]
- Von Drygalski, A.; Giermasz, A.; Castaman, G.; Key, N.S.; Lattimore, S.; Leebeek, F.W.G.; Miesbach, W.; Recht, M.; Long, A.; Gut, R.; et al. Etranacogene dezaparvovec (AMT-061 phase 2b): Normal/near normal FIX activity and bleed cessation in hemophilia B. Blood Adv. 2019, 3, 3241–3247. [Google Scholar] [CrossRef]
- Aronson, S.J.; Veron, P.; Collaud, F.; Hubert, A.; Delahais, V.; Honnet, G.; de Knegt, R.J.; Junge, N.; Baumann, U.; Di Giorgio, A.; et al. Prevalence and Relevance of Preexisting Anti-Adeno-Associated Virus Immunity in the Context of Gene Therapy for Crigler-Najjar Syndrome. Hum. Gene Ther. 2019, 30, 1297–1305. [Google Scholar] [CrossRef]
- Fitzpatrick, Z.; Leborgne, C.; Barbon, E.; Masat, E.; Ronzitti, G.; van Wittenberghe, L.; Vignaud, A.; Collaud, F.; Charles, S.; Simon Sola, M.; et al. Influence of Preexisting Anti-capsid Neutralizing and Binding Antibodies on AAV Vector Transduction. Mol. Ther. Methods Clin. Dev. 2018, 9, 119–129. [Google Scholar] [CrossRef]
- Zincarelli, C.; Soltys, S.; Rengo, G.; Rabinowitz, J.E. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008, 16, 1073–1080. [Google Scholar] [CrossRef]
- Kohler, L.; Puertollano, R.; Raben, N. Pompe Disease: From Basic Science to Therapy. Neurotherapeutics 2018, 15, 928–942. [Google Scholar] [CrossRef]
- Meena, N.K.; Raben, N. Pompe Disease: New Developments in an Old Lysosomal Storage Disorder. Biomolecules 2020, 10, 1339. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, W.H.; Dong, Z.Y.; Wu, X.; Yang, Y.; Sheng, W. AAV Vector Mediated Gene Therapy in Pompe Model Mice. China Biotechnol. 2022, 42, 24–34. [Google Scholar]
- Yu, Z.; Zhou, S.; Luo, N.; Ho, C.Y.; Chen, M.; Chen, H. TPP Combined with DGUC as an Economic and Universal Process for Large-Scale Purification of AAV Vectors. Mol. Ther. Methods Clin. Dev. 2020, 17, 34–48. [Google Scholar] [CrossRef]
- Reuser, A.J.; Koster, J.F.; Hoogeveen, A.; Galjaard, H. Biochemical, immunological, and cell genetic studies in glycogenosis type II. Am. J. Hum. Genet. 1978, 30, 132–143. [Google Scholar]
- Day, J.W.; Finkel, R.S.; Chiriboga, C.A.; Connolly, A.M.; Crawford, T.O.; Darras, B.T.; Iannaccone, S.T.; Kuntz, N.L.; Peña, L.D.M.; Shieh, P.B.; et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): An open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021, 20, 284–293. [Google Scholar] [CrossRef]
- Vokinger, K.N.; Glaus, C.E.G.; Kesselheim, A.S. Approval and therapeutic value of gene therapies in the US and Europe. Gene Ther. 2023, 30, 756–760. [Google Scholar] [CrossRef]
- Klamroth, R.; Hayes, G.; Andreeva, T.; Gregg, K.; Suzuki, T.; Mitha, I.H.; Hardesty, B.; Shima, M.; Pollock, T.; Slev, P.; et al. Global Seroprevalence of Preexisting Immunity Against AAV5 and Other AAV Serotypes in People with Hemophilia A. Hum. Gene Ther. 2022, 33, 432–441. [Google Scholar] [CrossRef]
- Perocheau, D.P.; Cunningham, S.; Lee, J.; Antinao Diaz, J.; Waddington, S.N.; Gilmour, K.; Eaglestone, S.; Lisowski, L.; Thrasher, A.J.; Alexander, I.E.; et al. Age-Related Seroprevalence of Antibodies Against AAV-LK03 in a UK Population Cohort. Hum. Gene Ther. 2019, 30, 79–87. [Google Scholar] [CrossRef]
- Calcedo, R.; Morizono, H.; Wang, L.; McCarter, R.; He, J.; Jones, D.; Batshaw, M.L.; Wilson, J.M. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 2011, 18, 1586–1588. [Google Scholar] [CrossRef]
- Boutin, S.; Monteilhet, V.; Veron, P.; Leborgne, C.; Benveniste, O.; Montus, M.F.; Masurier, C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010, 21, 704–712. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, W.; Zhang, H.; Wang, Y.; Zhao, J.; Song, A.; Xie, H.; Zhao, C.; Gao, D.; Wang, Y. Neutralizing antibodies against AAV2, AAV5 and AAV8 in healthy and HIV-1-infected subjects in China: Implications for gene therapy using AAV vectors. Gene Ther. 2014, 21, 732–738. [Google Scholar] [CrossRef]
- Bertin, B.; Veron, P.; Leborgne, C.; Deschamps, J.Y.; Moullec, S.; Fromes, Y.; Collaud, F.; Boutin, S.; Latournerie, V.; van Wittenberghe, L.; et al. Capsid-specific removal of circulating antibodies to adeno-associated virus vectors. Sci. Rep. 2020, 10, 864. [Google Scholar] [CrossRef]
- Monteilhet, V.; Saheb, S.; Boutin, S.; Leborgne, C.; Veron, P.; Montus, M.F.; Moullier, P.; Benveniste, O.; Masurier, C. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol. Ther. 2011, 19, 2084–2091. [Google Scholar] [CrossRef]
- Gross, D.A.; Tedesco, N.; Leborgne, C.; Ronzitti, G. Overcoming the Challenges Imposed by Humoral Immunity to AAV Vectors to Achieve Safe and Efficient Gene Transfer in Seropositive Patients. Front. Immunol. 2022, 13, 857276. [Google Scholar] [CrossRef]
- Unnisa, Z.; Yoon, J.K.; Schindler, J.W.; Mason, C.; van Til, N.P. Gene Therapy Developments for Pompe Disease. Biomedicines 2022, 10, 302. [Google Scholar] [CrossRef]
- Baik, A.D.; Calafati, P.; Zhang, X.; Aaron, N.A.; Mehra, A.; Moller-Tank, S.; Miloscio, L.; Praggastis, M.; Giovannone, N.; Pan, C.; et al. Cell type-selective targeted delivery of a recombinant lysosomal enzyme for enzyme therapies. Mol. Ther. 2021, 29, 3512–3524. [Google Scholar] [CrossRef]
- Hordeaux, J.; Ramezani, A.; Tuske, S.; Mehta, N.; Song, C.; Lynch, A.; Lupino, K.; Chichester, J.A.; Buza, E.L.; Dyer, C.; et al. Immune transgene-dependent myocarditis in macaques after systemic administration of adeno-associated virus expressing human acid alpha-glucosidase. Front. Immunol. 2023, 14, 1094279. [Google Scholar] [CrossRef]
- Hurlbut, G.D.; Ziegler, R.J.; Nietupski, J.B.; Foley, J.W.; Woodworth, L.A.; Meyers, E.; Bercury, S.D.; Pande, N.N.; Souza, D.W.; Bree, M.P.; et al. Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy. Mol. Ther. 2010, 18, 1983–1994. [Google Scholar] [CrossRef]
- Hamilton, B.A.; Wright, J.F. Challenges Posed by Immune Responses to AAV Vectors: Addressing Root Causes. Front. Immunol. 2021, 12, 675897. [Google Scholar] [CrossRef]
- West, C.; Federspiel, J.D.; Rogers, K.; Khatri, A.; Rao-Dayton, S.; Ocana, M.F.; Lim, S.; D’Antona, A.M.; Casinghino, S.; Somanathan, S. Complement Activation by Adeno-Associated Virus-Neutralizing Antibody Complexes. Hum. Gene Ther. 2023, 34, 554–566. [Google Scholar] [CrossRef]
- Smith, C.J.; Ross, N.; Kamal, A.; Kim, K.Y.; Kropf, E.; Deschatelets, P.; Francois, C.; Quinn, W.J., 3rd; Singh, I.; Majowicz, A.; et al. Preexisting humoral immunity and complement pathway contribute to immunogenicity of adeno-associated virus (AAV) vector in human blood. Front. Immunol. 2022, 13, 999021. [Google Scholar] [CrossRef]
- Murrey, D.A.; Naughton, B.J.; Duncan, F.J.; Meadows, A.S.; Ware, T.A.; Campbell, K.J.; Bremer, W.G.; Walker, C.M.; Goodchild, L.; Bolon, B.; et al. Feasibility and safety of systemic rAAV9-hNAGLU delivery for treating mucopolysaccharidosis IIIB: Toxicology, biodistribution, and immunological assessments in primates. Hum. Gene Ther. Clin. Dev. 2014, 25, 72–84. [Google Scholar] [CrossRef]


| Spiked Anti-AAV9 MoAb (μg/mL) | AAV9 NAb Titer (Reciprocal Dilution, 1:x) | ||
|---|---|---|---|
| Spiked Human Sera | Spiked Mice Sera (Before Infusion) | Geometric Mean of Passively Immunized Mice Sera * (95% CI) | |
| 0 | <1:20 | <1:20 | <1:20 |
| 1.5 | 1:431 | 1:456 | 1:60 (1:52~1:69) |
| 3 | 1:729 | 1:997 | 1:193 (1:158~1:255) |
| 7 | 1:2871 | 1:2976 | 1:290 (1:176~1:479) |
| 21 | 1:8167 | 1:9823 | 1:481 (1:377~1:612) |
| 42 | 1:20,884 | 1:26,671 | 1:851 (1:658~1:1101) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, C.; Dong, Z.; Zhu, X.; Zheng, X.; Liu, Z.; Zhou, J.; Yu, S.; Wu, X.; Dong, X. Using an In Vivo Mouse Model to Determine the Exclusion Criteria of Preexisting Anti-AAV9 Neutralizing Antibody Titer of Pompe Disease Patients in Clinical Trials. Viruses 2024, 16, 400. https://doi.org/10.3390/v16030400
Wang H, Zhang C, Dong Z, Zhu X, Zheng X, Liu Z, Zhou J, Yu S, Wu X, Dong X. Using an In Vivo Mouse Model to Determine the Exclusion Criteria of Preexisting Anti-AAV9 Neutralizing Antibody Titer of Pompe Disease Patients in Clinical Trials. Viruses. 2024; 16(3):400. https://doi.org/10.3390/v16030400
Chicago/Turabian StyleWang, Hanqing, Cengceng Zhang, Zheyue Dong, Xueyang Zhu, Xuchu Zheng, Ziyang Liu, Jianfang Zhou, Shuangqing Yu, Xiaobing Wu, and Xiaoyan Dong. 2024. "Using an In Vivo Mouse Model to Determine the Exclusion Criteria of Preexisting Anti-AAV9 Neutralizing Antibody Titer of Pompe Disease Patients in Clinical Trials" Viruses 16, no. 3: 400. https://doi.org/10.3390/v16030400




