Bactericera cockerelli Picorna-like Virus and Three New Viruses Found Circulating in Populations of Potato/Tomato Psyllids (Bactericera cockerelli)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Psyllid and Plant Sampling and Sample Processing
2.2. RNA Extraction and HTS Analysis
2.3. Nucleic Acid Extraction, RT-PCR Testing, and Sanger Sequencing
2.4. Sequence and Phylogenetic Analysis
3. Results
3.1. Genome Analysis and Confirmation of the Presence of a New Tymo-like Virus in Psyllids
3.2. Genome Analysis and Confirmation of the Presence of New Solemo-like Viruses in Psyllids
3.3. Phylogenetic Analyses and Taxonomy of BcTLV, BcSLV-1, and BcSLV-2
3.4. BcPLV, BcTLV, and BcSLV-1 and -2 Can Be Found in Field-Collected Psyllids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallis, R.L. Ecological Studies on the Potato Psyllid as a Pest of Potatoes; USDA Technical Bulletin; U.S. Department of Agriculture: Washington, DC, USA, 1955; Volume 1107, pp. 1–24. [Google Scholar]
- Butler, C.D.; Trumble, J.T. The potato psyllid, Bactericera cockerelli (Sulc) (Hemiptera: Triozidae): Life history, relationship to plant diseases, and management strategies. Terr. Arthropod Rev. 2012, 5, 87–111. [Google Scholar] [CrossRef]
- Cooper, W.R.; Horton, D.R.; Thinakaran, J.; Karasev, A.V. Dispersal of Bactericera cockerelli (Hemiptera: Triozidae) in relation to phenology of matrimony vine (Lycium spp.; Solanaceae). J. Entomol. Soc. Br. Columbia 2019, 116, 25–39. [Google Scholar]
- Munyaneza, J.E. Zebra chip disease, Candidatus Liberibacter, and potato psyllid: A global threat to the potato industry. Am. J. Potato Res. 2015, 92, 230–235. [Google Scholar] [CrossRef]
- Reyes Corral, C.A.; Cooper, W.R.; Horton, D.R.; Karasev, A.V. Susceptibility of Physalis longifolia (Solanales:Solanaceae) to Bactericera cockerelli (Hemiptera:Triozidae) and ‘Candidatus Liberibacter solanacearum’. J. Econ. Entomol. 2020, 113, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Thinakaran, J.; Horton, D.R.; Cooper, W.R.; Jensen, A.S.; Wohleb, C.H.; Dahan, J.; Mustafa, T.; Karasev, A.V.; Munyaneza, J.E. Association of potato psyllid (Bactericidal cockerelli; Hemiptera: Triozidae) with Lycium spp. (Solanaceae) in potato growing regions of Washington, Idaho, and Oregon. Am. J. Potato Res. 2017, 94, 490–499. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Rashed, A. Biology, ecology, and management of the potato psyllid, Bactericera cockerelli (Hemiptera: Triozidae), and zebra chip disease in potato. Ann. Rev. Entomol. 2024, 69, 139–157. [Google Scholar] [CrossRef]
- Dahan, J.; Cooper, W.R.; Munyaneza, J.; Karasev, A.V. A new picorna-like virus identified in populations of the potato psyllid Bactericera cockerelli. Arch. Virol. 2022, 167, 177–182. [Google Scholar] [CrossRef]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Gottwald, T.R. Current epidemiological understanding of citrus Huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef]
- da Graça, J.V.; Douhan, G.W.; Halbert, S.E.; Keremane, M.L.; Lee, R.F.; Vidalakis, G.; Zhao, H. Huanglongbing: An overview of a complex pathosystem ravaging the world’s citrus. J. Integr. Plant Biol. 2016, 58, 373–387. [Google Scholar] [CrossRef]
- Britt, K.; Gebben, S.; Levy, A.; Al Rwahnih, M.; Batuman, O. The detection and surveillance of Asian citrus psyllid (Diaphorina citri)—Associated viruses in Florida citrus groves. Front. Plant Sci. 2020, 10, 1687. [Google Scholar] [CrossRef]
- Nouri, S.; Salem, N.; Falk, B.W. Complete genome sequence of Diaphorina citri-associated C virus, a novel putative RNA virus of the Asian citrus psyllid, Diaphorina citri. Genome Announc. 2016, 4, e00639-16. [Google Scholar] [CrossRef]
- Nouri, S.; Salem, N.; Nigg, J.C.; Falk, B.W. Diverse array of new viral sequences identified in worldwide populations of the Asian citrus psyllid (Diaphorina citri) using viral metagenomics. J. Virol. 2016, 90, 2434–2445. [Google Scholar] [CrossRef] [PubMed]
- Nouri, S.; Matsumura, E.E.; Kuo, Y.W.; Falk, B.W. Insect-specific viruses: From discovery to potential translational applications. Curr. Opin. Virol. 2018, 33, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Batuman, O.; Levy, A. Identifying the gut virome of Diaphorina citri from Florida groves. Insects 2023, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Britt, K.; Stevens, K.; Gebben, S.; Levy, A.; Al Rwahnih, M.; Batuman, O. Partial genome sequence of a novel Reo-like virus detected in Asian citrus psyllid (Diaphorina citri) populations from Florida citrus groves. Microbiol. Resour. Announc. 2021, 10, e00563-21. [Google Scholar] [CrossRef] [PubMed]
- Stuehler, D.S., Jr.; Hunter, W.B.; Carrillo-Tarazona, Y.; Espitia, H.; Cicero, J.M.; Bell, T.; Mann, H.R.; Clarke, S.-K.V.; Paris, T.M.; Metz, J.L.; et al. Wild lime psyllid Leuronota fagarae Burckhardt (Hemiptera: Psylloidea) picorna-like virus full genome annotation and classification. J. Invertebrate Pathol. 2023, 201, 107995. [Google Scholar] [CrossRef] [PubMed]
- Dreher, T.W.; Edwards, M.C.; Gibbs, A.J.; Haenni, A.-L.; Hammond, R.W.; Jupin, I.; Koenig, R.; Sabanadzovic, S.; Martelli, G.P. Family Tymoviridae. In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Eric, B., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 944–952. [Google Scholar]
- Bejerman, N.; Debat, H. Exploring the tymovirales landscape through metatranscriptomics data. Arch. Virol. 2022, 167, 1785–1803. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.C.; Weiland, J.J. First infectious clone of the propagatively transmitted Oat blue dwarf virus. Arch. Virol. 2010, 155, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lv, X.; Zhai, Y.; Fu, S.; Wang, D.; Rayner, S.; Tang, Q.; Liang, G. Genomic characterization of a novel virus of the family Tymoviridae isolated from mosquitoes. PLoS ONE 2012, 7, e39845. [Google Scholar] [CrossRef]
- De Miranda, J.R.; Cornman, R.S.; Evans, J.D.; Semberg, E.; Haddad, N.; Neumann, P.; Gauthier, L. Genome characterization, prevalence and distribution of a macula-like virus from Apis mellifera and Varroa destructor. Viruses 2015, 7, 3586–3602. [Google Scholar] [CrossRef]
- Li, P.; Lin, Y.; Zhang, H.; Wang, S.; Qiu, D.; Guo, L. Molecular characterization of a novel mycovirus of the family Tymoviridae isolated from the plant pathogenic fungus Fusarium graminearum. Virology 2016, 489, 86–94. [Google Scholar] [CrossRef]
- Charles, J.; Tangudu, C.S.; Hurt, S.L.; Tumescheit, C.; Firth, A.E.; Garcia-Rejon, J.E.; Machain-Williams, C.; Blivich, B.J. Discovery of a novel Tymoviridae-like virus in mosquitoes from Mexico. Arch. Virol. 2019, 164, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Sõmera, M.; Fargette, D.; Hébrard, E.; Sarmiento, C.; ICTV Report Consortium. ICTV Virus Taxonomy Profile: Solemoviridae 2021. J. Gen. Virol. 2021, 102, 001707. [Google Scholar] [CrossRef]
- Pettersson, J.H.; Shi, M.; Bohlin, J.; Eldholm, V.; Brynildsrud, O.B.; Paulsen, K.M.; Andreassen, A.; Holmes, E.C. Characterizing the virome of Ixodes ricinus ticks from northern Europe. Sci. Rep. 2017, 7, 10870. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.D.; Tian, J.H.; Chen, L.J.; Chen, X.; Li, C.X.; Qin, X.C.; Li, J.; Cao, J.P.; Eden, J.S.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef]
- Dahan, J.; Wenninger, E.; Thompson, B.; Eid, S.; Olsen, N.; Karasev, A.V. Relative abundance of psyllid haplotypes in potato fields in Southern Idaho between 2012 and 2015, and occurrence of ‘Candidatus Liberibacter solanacearum’ (Lso) causing Zebra chip disease. Plant Dis. 2017, 101, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Dahan, J.; Wenninger, E.J.; Thompson, B.D.; Eid, S.; Olsen, N.; Karasev, A.V. Prevalence of ‘Candidatus Liberibacter solanacearum’ haplotypes in potato tubers and psyllid vectors in Idaho from 2012 to 2018. Plant Dis. 2019, 103, 2587–2591. [Google Scholar] [CrossRef]
- Dahan, J.; Wenninger, E.J.; Thornton, M.; Corral, C.A.R.; Olsen, N.; Karasev, A.V. Haplotyping the potato psyllid (Hemiptera: Triozidae) and the associated pathogenic bacterium ‘Candidatus Liberibacter solanacearum’ in non-crop alternative hosts in Southern Idaho. Environ. Entomol. 2021, 50, 382–389. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Carroll, A.; Dahan, J.; Karasev, A.V.; Thornton, M.; Miller, J.; Nolte, P.; Olsen, N.; Price, W. Phenology of the potato psyllid, Bactericera cockerelli (Hemiptera: Triozidae), and “Candidatus Liberibacter solanacearum” in Commercial Potato Fields in Idaho. Environ Entomol. 2017, 46, 1179–1188. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Dahan, J.; Thornton, M.; Karasev, A.V. Associations of the potato psyllid and “Candidatus Liberibacter solanacearum” in Idaho with the noncrop host plants bittersweet nightshade and field bindweed. Environ. Entomol. 2019, 48, 747–754. [Google Scholar] [CrossRef]
- Crosslin, J.M.; Lin, H.; Munyaneza, J.E. Detection of “Candidatus Liberibacter solanacearum” in the potato psyllid, Bactericera cockerelli (Sulc), by conventional and real-time PCR. Southwest Entomol. 2011, 36, 125–135. [Google Scholar] [CrossRef]
- Cooper, W.R.; Swisher, K.D.; Garczynski, S.F.; Mustafa, T.; Munyaneza, J.E.; Horton, D.R. Wolbachia infection differs among divergent mitochondrial haplotypes of Bactericera cockerelli (Hemiptera: Triozidae). Ann. Entomol. Soc. Am. 2015, 108, 137–145. [Google Scholar] [CrossRef]
- Swisher, K.D.; Munyaneza, J.E.; Crosslin, J.M. High resolution melting analysis of the Cytochrome Oxidase I gene identifies three haplotypes of the potato psyllid in the United States. Environ. Entomol. 2012, 41, 1019–1028. [Google Scholar] [CrossRef]
- Buchfink, B.; Reuter, K.; Drost, H.G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 2021, 18, 366–368. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation: Version II. Plant Mol. Biol. Rep. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- Dahan, J.; Wolf, Y.I.; Orellana, G.E.; Wenninger, E.J.; Koonin, E.V.; Karasev, A.V. A novel flavi-like virus in alfalfa (Medicago sativa L.) crops along the Snake River valley. Viruses 2022, 14, 1320. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate Maximum-Likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Yu, G.; Smith, D.; Zhu, H.; Guan, Y.; Lam, T.T. ggtree: An R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef]
- Bozarth, C.S.; Weiland, J.J.; Dreher, T.W. Expression of ORF-69 of turnip yellow mosaic virus is necessary for viral spread in plants. Virology 1992, 187, 124–130. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.X.; Xie, D.; Peng, J.R.; Ding, S.W. Viral virulence protein suppresses RNA silencing-mediated defense but upregulates the role of microrna in host gene expression. Plant Cell 2004, 16, 1302–1313. [Google Scholar] [CrossRef]
- Sõmera, M.; Sarmiento, C.; Truve, E. Overview on sobemoviruses and a proposal for the creation of the family Sobemoviridae. Viruses 2015, 7, 3076–3115. [Google Scholar] [CrossRef] [PubMed]
- Csorba, T.; Kontra, L.; Burgyán, J. Viral silencing suppressors: Tools forged to fine-tune host-pathogen coexistence. Virology 2015, 479–480, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Sabanadzovic, S.; Abou Ghanem, N.; Castellano, M.A.; Digiaro, M.; Martelli, G.P. Grapevine fleck virus-like viruses in Vitis. Arch. Virol. 2000, 145, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Sabanadzovic, S.; Abou Ghanem-Sabanadzovic, N.; Saldarelli, P.; Martelli, G.P. Complete nucleotide sequence and genome organization of Grapevine fleck virus. J. Gen. Virol. 2001, 82, 2009–2015. [Google Scholar] [CrossRef]
- Sabanadzovic, S.; Abou Ghanem, N.; Martelli, G.P. Grapevine fleck and similar viruses. In Grapevine Viruses: Molecular Biology, Diagnostics, and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 331–349. [Google Scholar]
- Yang, S.; Shan, T.; Wang, Y.; Yang, J.; Chen, X.; Xiao, Y.; You, Z.; He, Y.; Zhao, M.; Lu, J.; et al. Virome of riverside phytocommunity ecosystem of an ancient canal. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Hodkinson, I.D. The Nearctic Psylloidea (Insecta: Homoptera): An annotated check list. J. Nat. Hist. 1988, 22, 1179–1243. [Google Scholar] [CrossRef]
- Burckhardt, D.; Ouvrard, D. A revised classification of the jumping plant-lice (Hemiptera: Psylloidea). Zootaxa 2012, 3509, 1–34. [Google Scholar] [CrossRef]
- Bonning, B.C. The insect virome: Opportunities and challenges. Curr. Issues Mol. Biol. 2019, 34, 1–12. [Google Scholar] [PubMed]
- Qi, Y.-H.; Ye, Z.-X.; Zhang, C.-X.; Chen, J.-P.; Li, J.-M. Diversity of RNA viruses in agricultural insects. Comput. Struct. Biotechnol. J. 2023, 21, 4312–4321. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Ye, Z.X.; Wang, X.; Yan, X.T.; Zhang, Y.; He, Y.J.; Qi, Y.H.; Zhang, X.D.; Zhuo, J.C.; Lu, G.; et al. Diversity and infectivity of the RNA virome among different cryptic species of an agriculturally important insect vector: Whitefly Bemisia tabaci. NPJ Biofilms Microbiomes 2021, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.I.; Kazlauskas, D.; Iranzo, J.; Lucía-Sanz, A.; Kuhn, J.H.; Krupovic, M.; Dolja, V.V.; Koonin, E.V. Origins and evolution of the global RNA virome. mBio 2018, 9, e02329-18. [Google Scholar] [CrossRef]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M.; Varsani, A.; Wolf, Y.I.; Yutin, N.; Zerbini, F.M.; Kuhn, J.H. Global organization and proposed megataxonomy of the virus world. Microbiol. Mol. Biol. Rev. 2020, 84, e00061-19. [Google Scholar] [CrossRef]
- Adams, M.J.; Candresse, T.; Hammond, J.; Kreuze, J.F.; Martelli, G.P.; Namba, S.; Pearson, M.N.; Ryu, K.H.; Saldarelli, P.; Yoshikawa, N. Family Betaflexiviridae. In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier Academic Press: London, UK, 2012; pp. 920–941. [Google Scholar]
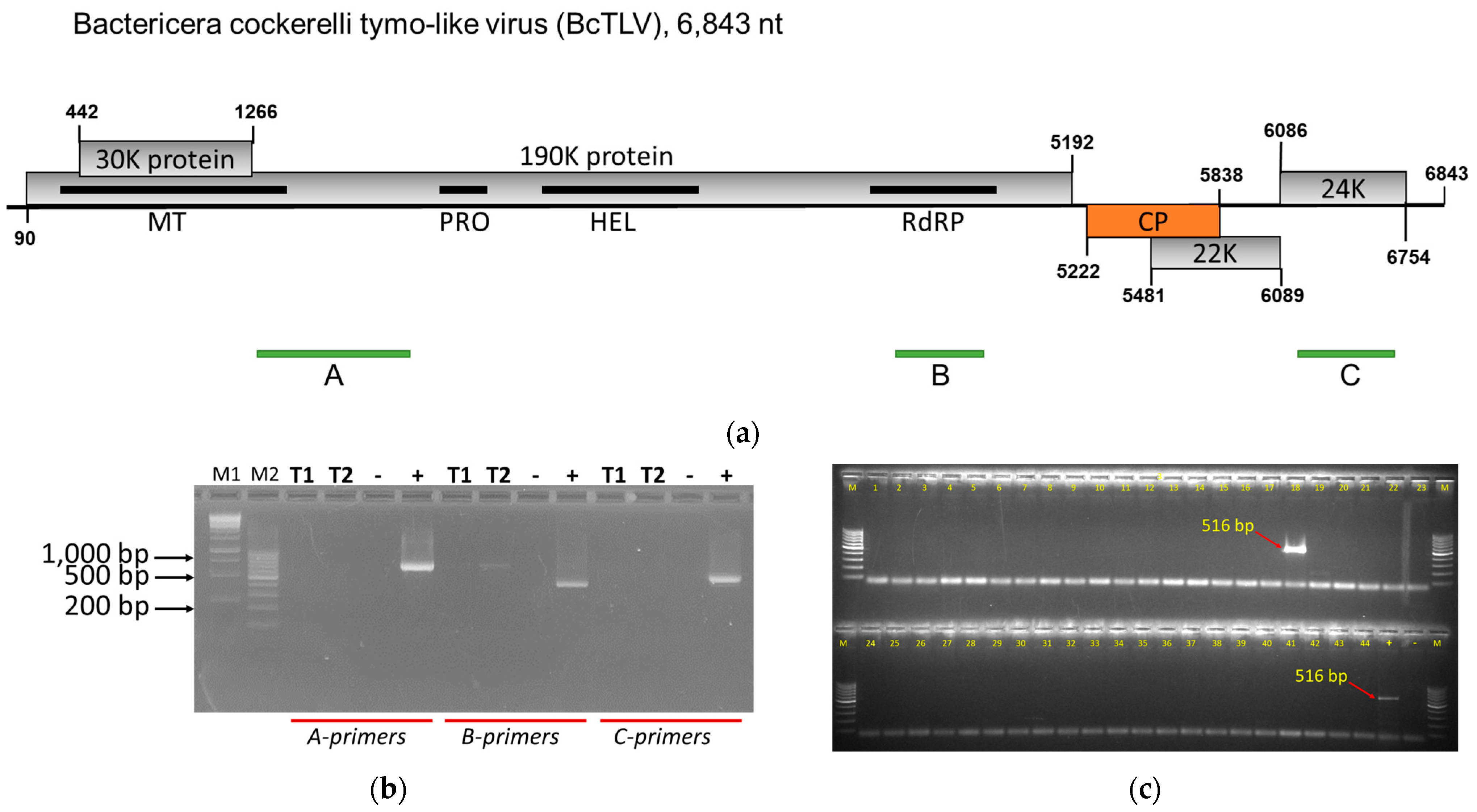

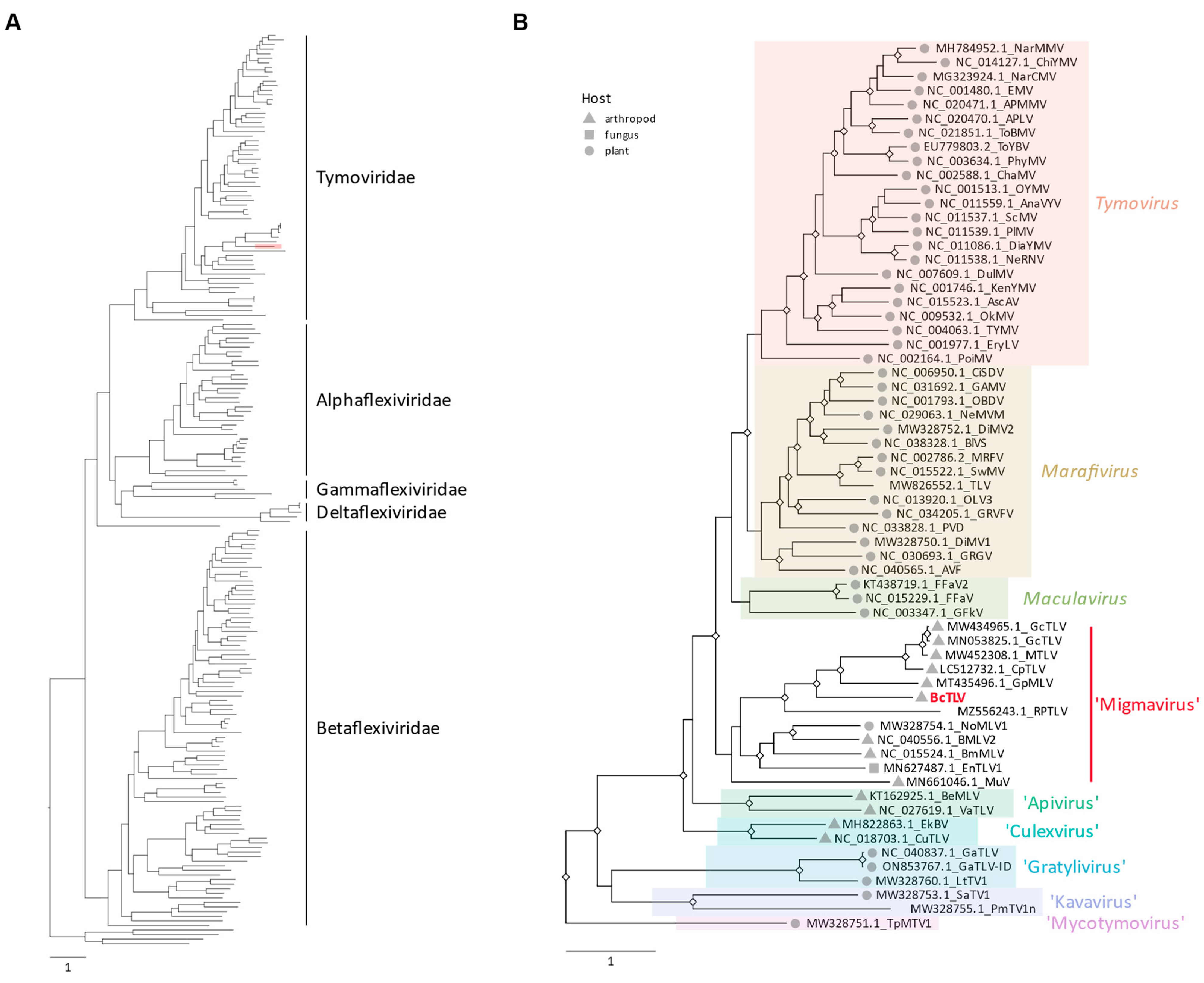

| Psyllid Sample ID a | Origin | Date Collected | Host Plant | Virus Sequences Found b | Largest Contig Size, nt | GenBank Accession Number |
|---|---|---|---|---|---|---|
| PSC | Wapato, WA | Feb-2021 | potato | PVS | 8546 | PP408647 |
| BcPLV | 10,122 | PP408652 | ||||
| PSW | Wapato, WA | Feb 2021 | potato/ tomato | BcTLV | 6843 | PP408654 |
| PVS | 4339 | PP408649 | ||||
| PVY | 3729 | PP408651 | ||||
| BcPLV | 10,150 | PP408653 | ||||
| PSNW | Wapato, WA | Feb 2021 | potato | PVS | 8537 | PP408650 |
| BcSLV-2_1 | 3498 | PP408657 | ||||
| BcSLV-2_2 | 3365 | PP408658 | ||||
| KPF | Kimberly, ID | Aug 2020 | potato | PVS | 8407 | PP408648 |
| BcSLV-1 | 5479 | PP408655 | ||||
| BcSLV-2_1 | 3379 | PP408656 |
| Date of Collection | BcPLV a | BcTLV a | BcSLV-1 a | BcSLV-2 a |
|---|---|---|---|---|
| 7 July | 0/2 | 0/2 | 0/2 | 0/2 |
| 11 July | 1/3 | 0/3 | 2/3 | 1/3 |
| 14 July | 2/14 | 0/14 | 0/14 | 4/14 |
| 21 July | 8/10 | 0/10 | 0/10 | 6/10 |
| 14 August | 0/29 | 0/29 | 0/29 | 5/29 |
| 11 August | 0/23 | 1/23 | 0/23 | 12/23 |
| 15 August | 0/7 | 0/7 | 0/7 | 2/7 |
| 18 August | 9/44 | 1/44 | 5/44 | 29/44 |
| 25 August | 5/25 | 0/25 | 1/25 | 17/25 |
| 28 August | 21/28 | 0/28 | 1/28 | 19/28 |
| 1 September | 9/15 | 0/15 | 0/15 | 7/15 |
| 8 September | 1/6 | 2/6 | 0/6 | 3/6 |
| 14 September | 2/12 | 0/12 | 0/12 | 5/12 |
| Total: | 58/218 | 4/218 | 9/218 | 110/218 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahan, J.; Orellana, G.E.; Wald, K.B.; Wenninger, E.J.; Cooper, W.R.; Karasev, A.V. Bactericera cockerelli Picorna-like Virus and Three New Viruses Found Circulating in Populations of Potato/Tomato Psyllids (Bactericera cockerelli). Viruses 2024, 16, 415. https://doi.org/10.3390/v16030415
Dahan J, Orellana GE, Wald KB, Wenninger EJ, Cooper WR, Karasev AV. Bactericera cockerelli Picorna-like Virus and Three New Viruses Found Circulating in Populations of Potato/Tomato Psyllids (Bactericera cockerelli). Viruses. 2024; 16(3):415. https://doi.org/10.3390/v16030415
Chicago/Turabian StyleDahan, Jennifer, Gardenia E. Orellana, Kaleigh B. Wald, Erik J. Wenninger, W. Rodney Cooper, and Alexander V. Karasev. 2024. "Bactericera cockerelli Picorna-like Virus and Three New Viruses Found Circulating in Populations of Potato/Tomato Psyllids (Bactericera cockerelli)" Viruses 16, no. 3: 415. https://doi.org/10.3390/v16030415
APA StyleDahan, J., Orellana, G. E., Wald, K. B., Wenninger, E. J., Cooper, W. R., & Karasev, A. V. (2024). Bactericera cockerelli Picorna-like Virus and Three New Viruses Found Circulating in Populations of Potato/Tomato Psyllids (Bactericera cockerelli). Viruses, 16(3), 415. https://doi.org/10.3390/v16030415






