The High Capacity of Brazilian Aedes aegypti Populations to Transmit a Locally Circulating Lineage of Chikungunya Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mosquito Collection and Establishment of the Populations
2.2. Virus Strain, Viral Propagation and Titration
2.3. Mice Inoculation with CHIKV
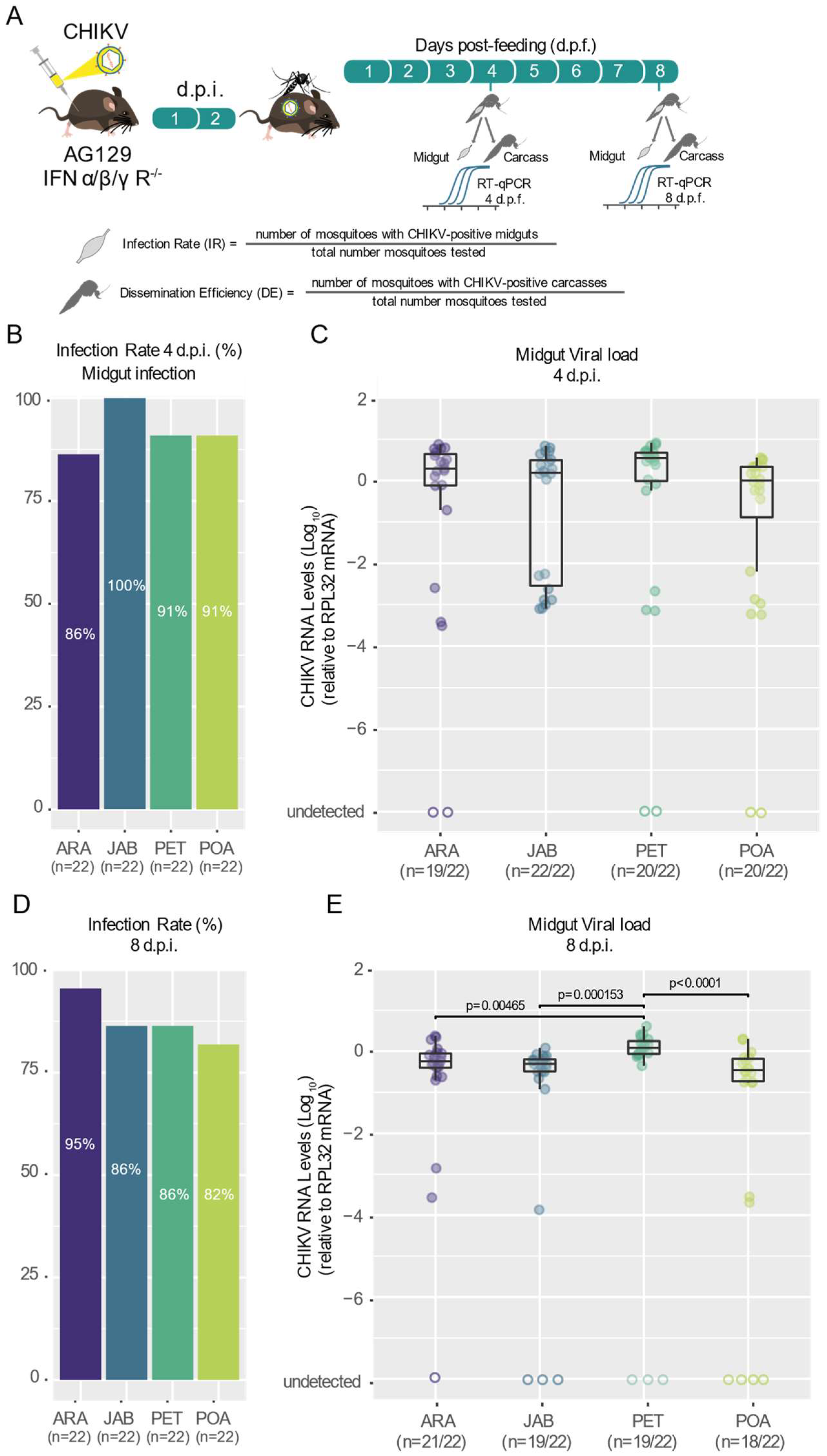
2.4. Mosquito Infection with CHIKV
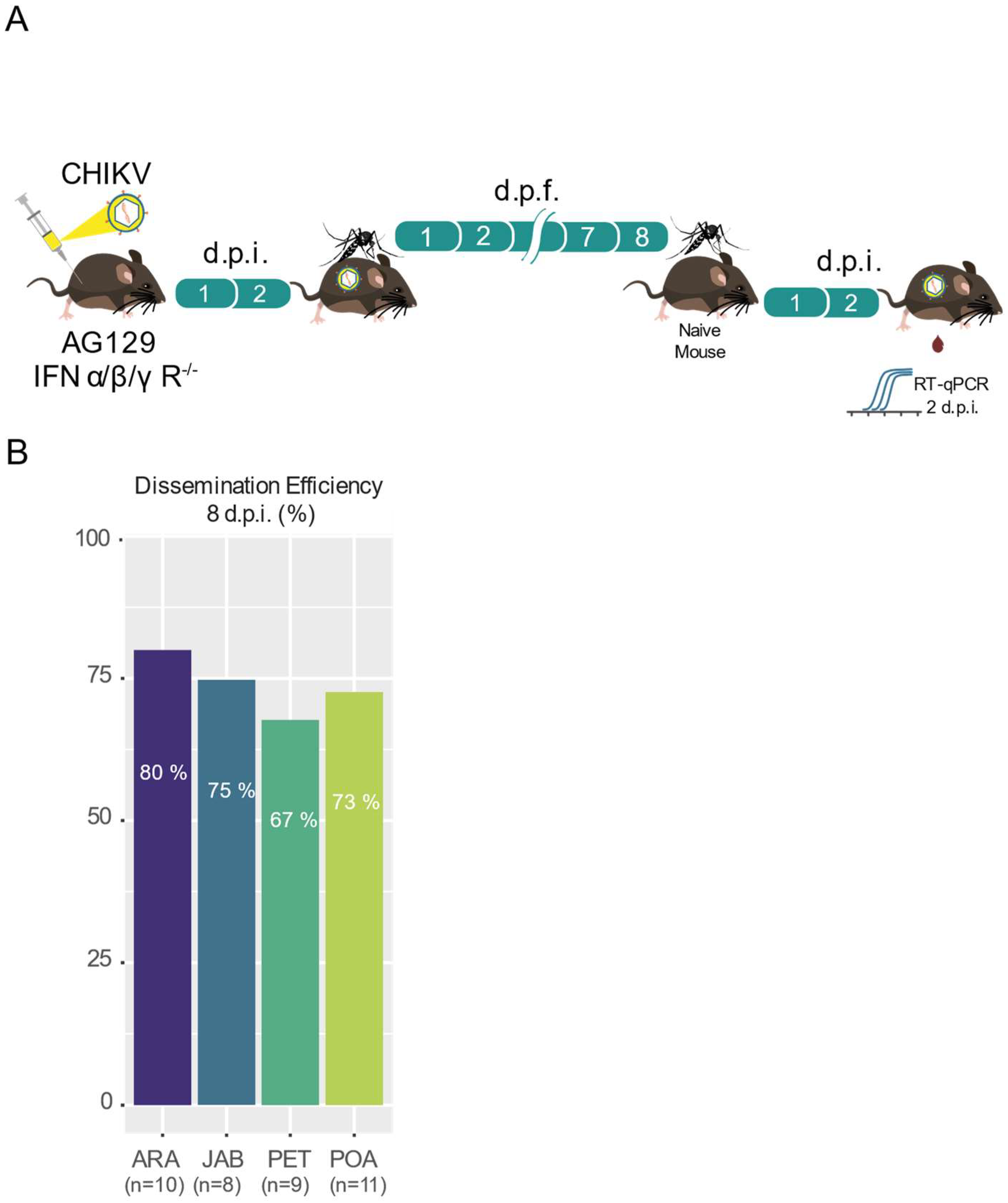
2.5. CHIKV Transmission from Mosquitoes to AG129 Mice
2.6. RNA Extraction and RT-qPCR
2.7. Statistical Analyses
3. Results
3.1. Different Populations of Aedes aegypti across Brazil Exhibit High and Similar CHKIV Infection Rates as Well as Dissemination Efficiency
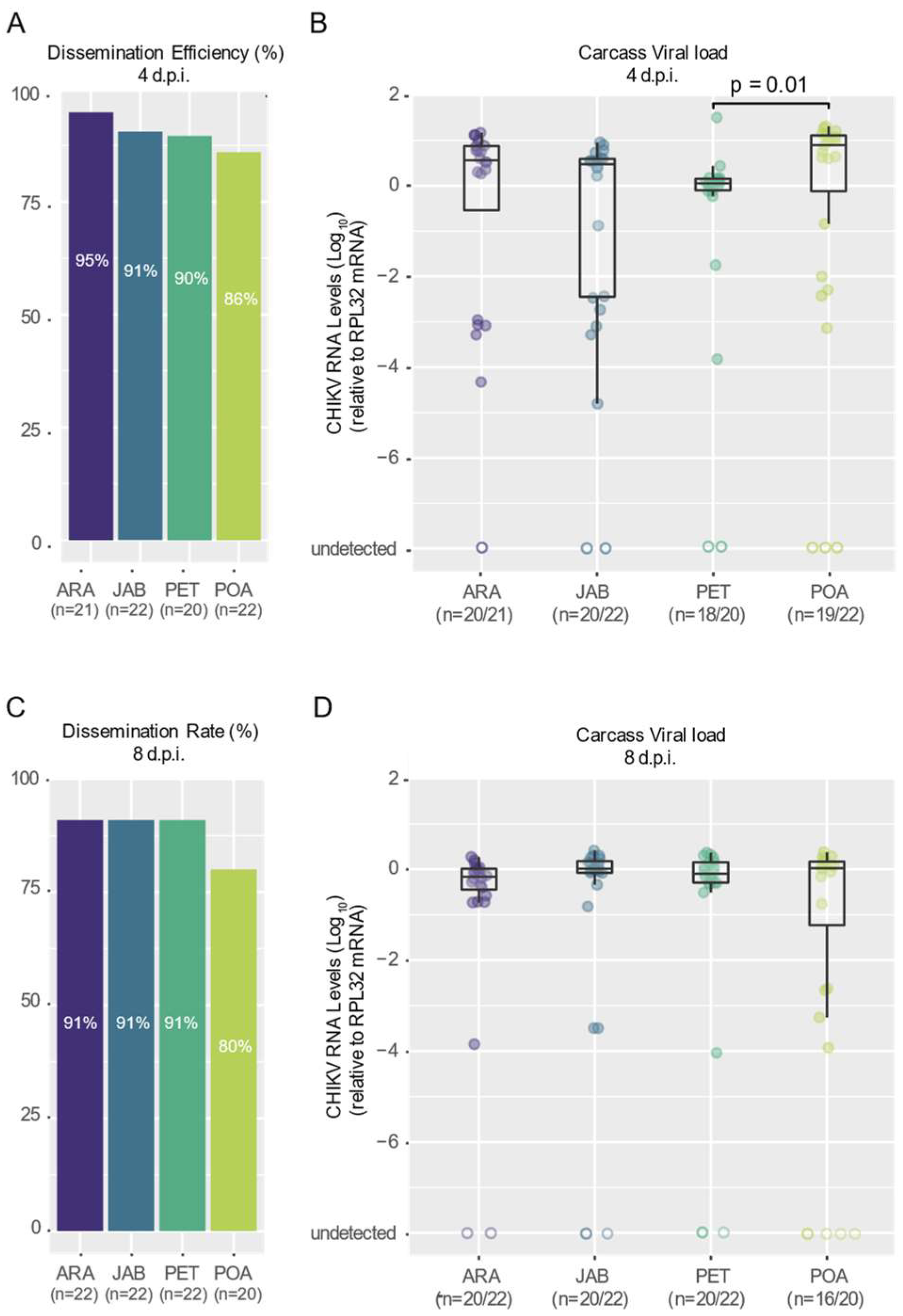
3.2. CHIKV Shows High Dissemination Efficiency in Mosquitoes of Brazilian Aedes aegypti Populations
3.3. High Transmission Efficiency of CHIKV across Brazilian Aedes aegypti Populations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsetsarkin, K.A.; Chen, R.; Sherman, M.B.; Weaver, S.C. Chikungunya Virus: Evolution and Genetic Determinants of Emergence. Curr. Opin. Virol. 2011, 1, 310–317. [Google Scholar] [CrossRef]
- Weaver, S.C.; Forrester, N.L. Chikungunya: Evolutionary History and Recent Epidemic Spread. Antivir. Res. 2015, 120, 32–39. [Google Scholar] [CrossRef]
- Pialoux, G.; Gaüzère, B.A.; Jauréguiberry, S.; Strobel, M. Chikungunya, an Epidemic Arbovirosis. Lancet Infect. Dis. 2007, 7, 319–327. [Google Scholar] [CrossRef]
- Weaver, S.C. Arrival of Chikungunya Virus in the New World: Prospects for Spread and Impact on Public Health. PLoS Neglected Trop. Dis. 2014, 8, e2921. [Google Scholar] [CrossRef]
- Tsetsarkin, K.A.; McGee, C.E.; Volk, S.M.; Vanlandingham, D.L.; Weaver, S.C.; Higgs, S. Epistatic Roles of E2 Glycoprotein Mutations in Adaption of Chikungunya Virus to Aedes albopictus and Ae. aegypti Mosquitoes. PLoS ONE 2009, 4, e6835. [Google Scholar] [CrossRef]
- Tsetsarkin, K.A.; Chen, R.; Leal, G.; Forrester, N.; Higgs, S.; Huang, J.; Weaver, S.C. Chikungunya Virus Emergence Is Constrained in Asia by Lineage-Specific Adaptive Landscapes. Proc. Natl. Acad. Sci. USA 2011, 108, 7872–7877. [Google Scholar] [CrossRef]
- Weaver, S.C.; Lecuit, M. Chikungunya Virus and the Global Spread of a Mosquito-Borne Disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef]
- Simizu, B.; Yamamoto, K.; Hashimoto, K.; Ogata, T. Structural Proteins of Chikungunya Virus. J. Virol. 1984, 51, 254–258. [Google Scholar] [CrossRef]
- Volk, S.M.; Chen, R.; Tsetsarkin, K.A.; Adams, A.P.; Garcia, T.I.; Sall, A.A.; Nasar, F.; Schuh, A.J.; Holmes, E.C.; Higgs, S.; et al. Genome-Scale Phylogenetic Analyses of Chikungunya Virus Reveal Independent Emergences of Recent Epidemics and Various Evolutionary Rates. J. Virol. 2010, 84, 6497–6504. [Google Scholar] [CrossRef]
- Charrel, R.N.; de Lamballerie, X.; Raoult, D. Chikungunya Outbreaks—The Globalization of Vectorborne Diseases. N. Engl. J. Med. 2007, 356, 769–771. [Google Scholar] [CrossRef]
- Rezza, G.; El-Sawaf, G.; Faggioni, G.; Vescio, F.; Al Ameri, R.; De Santis, R.; Helaly, G.; Pomponi, A.; Metwally, D.; Fantini, M.; et al. Co-Circulation of Dengue and Chikungunya Viruses, Al Hudaydah, Yemen, 2012. Emerg. Infect. Dis. 2014, 20, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Leparc-Goffart, I.; Nougairede, A.; Cassadou, S.; Prat, C.; de Lamballerie, X. Chikungunya in the Americas. Lancet 2014, 383, 514. [Google Scholar] [CrossRef] [PubMed]
- Cassadou, S.; Boucau, S.; Petit-Sinturel, M.; Huc, P.; Leparc-Goffart, I.; Ledrans, M. Emergence of Chikungunya Fever on the French Side of Saint Martin Island, October to December 2013. Euro Surveill 2014, 19, 20752. [Google Scholar] [CrossRef] [PubMed]
- Venturi, G.; Di Luca, M.; Fortuna, C.; Remoli, M.E.; Riccardo, F.; Severini, F.; Toma, L.; Del Manso, M.; Benedetti, E.; Caporali, M.G.; et al. Detection of a Chikungunya Outbreak in Central Italy, August to September 2017. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2017, 22, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Burt, F.J.; Chen, W.; Miner, J.J.; Lenschow, D.J.; Merits, A.; Schnettler, E.; Kohl, A.; Rudd, P.A.; Taylor, A.; Herrero, L.J.; et al. Chikungunya Virus: An Update on the Biology and Pathogenesis of This Emerging Pathogen. Lancet Infect. Dis. 2017, 17, e107–e117. [Google Scholar] [CrossRef]
- Silva, M.M.O.; Tauro, L.B.; Kikuti, M.; Anjos, R.O.; Santos, V.C.; Gonçalves, T.S.F.; Paploski, I.A.D.; Moreira, P.S.S.; Nascimento, L.C.J.; Campos, G.S.; et al. Concomitant Transmission of Dengue, Chikungunya, and Zika Viruses in Brazil: Clinical and Epidemiological Findings from Surveillance for Acute Febrile Illness. Clin. Infect. Dis. 2019, 69, 1353–1359. [Google Scholar] [CrossRef]
- Thiboutot, M.M.; Kannan, S.; Kawalekar, O.U.; Shedlock, D.J.; Khan, A.S.; Sarangan, G.; Srikanth, P.; Weiner, D.B.; Muthumani, K. Chikungunya: A Potentially Emerging Epidemic? PLoS Negl. Trop. Dis. 2010, 4, e623. [Google Scholar] [CrossRef]
- Patterson, J.; Sammon, M.; Garg, M. Dengue, Zika and Chikungunya: Emerging Arboviruses in the New World. WestJEM 2016, 17, 671–679. [Google Scholar] [CrossRef]
- Silva, L.A.; Dermody, T.S. Chikungunya Virus: Epidemiology, Replication, Disease Mechanisms, and Prospective Intervention Strategies. J. Clin. Investig. 2017, 127, 737–749. [Google Scholar] [CrossRef]
- Balakrishnan, V.S. WHO Launches Global Initiative for Arboviral Diseases. Lancet Microbe 2022, 3, e407. [Google Scholar] [CrossRef]
- Giovanetti, M.; Vazquez, C.; Lima, M.; Castro, E.; Rojas, A.; de la Fuente, A.G.; Aquino, C.; Cantero, C.; Fleitas, F.; Torales, J.; et al. Rapid Epidemic Expansion of Chikungunya Virus-ECSA Lineage in Paraguay; Infectious Diseases (except HIV/AIDS). 2023. Available online: https://pubmed.ncbi.nlm.nih.gov/37488810/ (accessed on 21 January 2024).
- Fritsch, H.; Giovanetti, M.; Xavier, J.; Adelino, T.E.R.; Fonseca, V.; de Jesus, J.G.; de Jesus, R.; Freitas, C.; Peterka, C.R.L.; Campelo de Albuquerque, C.F.; et al. Retrospective Genomic Surveillance of Chikungunya Transmission in Minas Gerais State, Southeast Brazil. Microbiol. Spectr. 2022, 10, e0128522. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.T.; Faria, N.R.; de Vasconcelos, J.M.; Golding, N.; Kraemer, M.U.G.; de Oliveira, L.F.; Azevedo, R.D.S.D.S.; da Silva, D.E.A.; da Silva, E.V.P.; da Silva, S.P.; et al. Emergence and Potential for Spread of Chikungunya Virus in Brazil. BMC Med. 2015, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Aragão, C.F.; Cruz, A.C.R.; Nunes Neto, J.P.; Monteiro, H.A.D.O.; da Silva, E.V.P.; da Silva, S.P.; Andrade, A.T.d.S.; Tadei, W.P.; Pinheiro, V.C.S. Circulation of Chikungunya Virus in Aedes Aegypti in Maranhão, Northeast Brazil. Acta Tropica 2018, 186, 1–4. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.C.; Fonseca, V.; Xavier, J.; Adelino, T.; Morales Claro, I.; Fabri, A.; Marques Macario, E.; Viniski, A.E.; Campos Souza, C.L.; Gomes da Costa, E.S.; et al. Short Report: Introduction of Chikungunya Virus ECSA Genotype into the Brazilian Midwest and Its Dispersion through the Americas. PLoS Negl. Trop. Dis. 2021, 15, e0009290. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.; Giovanetti, M.; Fonseca, V.; Thézé, J.; Gräf, T.; Fabri, A.; Goes de Jesus, J.; Lima de Mendonça, M.C.; Damasceno Dos Santos Rodrigues, C.; Mares-Guia, M.A.; et al. Circulation of Chikungunya Virus East/Central/South African Lineage in Rio de Janeiro, Brazil. PLoS ONE 2019, 14, e0217871. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.; Fonseca, V.; Bezerra, J.F.; do Monte Alves, M.; Mares-Guia, M.A.; Claro, I.M.; de Jesus, R.; Adelino, T.; Araújo, E.; Cavalcante, K.R.L.J.; et al. Chikungunya Virus ECSA Lineage Reintroduction in the Northeastern most Region of Brazil. Int. J. Infect. Dis 2021, 105, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Fabri, A.A.; Rodrigues, C.D.D.S.; Santos, C.C.D.; Chalhoub, F.L.L.; Sampaio, S.A.; Faria, N.R.D.C.; Torres, M.C.; Fonseca, V.; Brasil, P.; Calvet, G.; et al. Co-Circulation of Two Independent Clades and Persistence of CHIKV-ECSA Genotype during Epidemic Waves in Rio de Janeiro, Southeast Brazil. Pathogens 2020, 9, 984. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rúa, A.; Zouache, K.; Girod, R.; Failloux, A.-B.; Lourenço-de-Oliveira, R. High Level of Vector Competence of Aedes aegypti and Aedes albopictus from Ten American Countries as a Crucial Factor in the Spread of Chikungunya Virus. J. Virol. 2014, 88, 6294–6306. [Google Scholar] [CrossRef]
- Reiskind, M.H.; Pesko, K.; Westbrook, C.J.; Mores, C.N. Susceptibility of Florida Mosquitoes to Infection with Chikungunya Virus. Am. J. Trop. Med. Hyg. 2008, 78, 422–425. [Google Scholar] [CrossRef]
- Richards, S.L.; Anderson, S.L.; Smartt, C.T. Vector Competence of Florida Mosquitoes for Chikungunya Virus. J. Vector Ecol 2010, 35, 439–443. [Google Scholar] [CrossRef]
- Girod, R.; Gaborit, P.; Marrama, L.; Etienne, M.; Ramdini, C.; Rakotoarivony, I.; Dollin, C.; Carinci, R.; Issaly, J.; Dusfour, I.; et al. High Susceptibility to Chikungunya Virus of Aedes aegypti from the French West Indies and French Guiana. Trop. Med. Int. Health 2011, 16, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Dubrulle, M.; Mousson, L.; Moutailler, S.; Vazeille, M.; Failloux, A.-B. Chikungunya Virus and Aedes Mosquitoes: Saliva Is Infectious as Soon as Two Days after Oral Infection. PLoS ONE 2009, 4, e5895. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Beaman, J.R.; Tammariello, R.F. Susceptibility of Selected Strains of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) to Chikungunya Virus. J. Med. Entomol. 1992, 29, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Novelo, M.; Dutra, H.L.; Metz, H.C.; Jones, M.J.; Sigle, L.T.; Frentiu, F.D.; Allen, S.L.; Chenoweth, S.F.; McGraw, E.A. Dengue and Chikungunya Virus Loads in the Mosquito Aedes aegypti Are Determined by Distinct Genetic Architectures. PLoS Pathog. 2023, 19, e1011307. [Google Scholar] [CrossRef] [PubMed]
- Calvez, E.; Miot, E.F.; Keosenhom, S.; Vungkyly, V.; Viengphouthong, S.; Bounmany, P.; Brey, P.T.; Marcombe, S.; Grandadam, M. Low Transmission of Chikungunya Virus by Aedes aegypti from Vientiane Capital, Lao PDR. Pathogens 2022, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Baldon, L.V.R.; de Mendonça, S.F.; Ferreira, F.V.; Rezende, F.O.; Amadou, S.C.G.; Leite, T.H.J.F.; Rocha, M.N.; Marques, J.T.; Moreira, L.A.; Ferreira, A.G.A. AG129 Mice as a Comprehensive Model for the Experimental Assessment of Mosquito Vector Competence for Arboviruses. Pathogens 2022, 11, 879. [Google Scholar] [CrossRef] [PubMed]
- Olmo, R.P.; Todjro, Y.M.H.; Aguiar, E.R.G.R.; de Almeida, J.P.P.; Ferreira, F.V.; Armache, J.N.; de Faria, I.J.S.; Ferreira, A.G.A.; Amadou, S.C.G.; Silva, A.T.S.; et al. Mosquito Vector Competence for Dengue Is Modulated by Insect-Specific Viruses. Nat. Microbiol. 2023, 8, 135–149. [Google Scholar] [CrossRef]
- Olmo, R.P.; Ferreira, A.G.A.; Izidoro-Toledo, T.C.; Aguiar, E.R.G.R.; de Faria, I.J.S.; de Souza, K.P.R.; Osório, K.P.; Kuhn, L.; Hammann, P.; de Andrade, E.G.; et al. Control of Dengue Virus in the Midgut of Aedes aegypti by Ectopic Expression of the DsRNA-Binding Protein Loqs2. Nat. Microbiol. 2018, 3, 1385–1393. [Google Scholar] [CrossRef]
| Population Name | City | State | Species | Stage Collected | Number of Mosquitoes Used to Establish the Laboratory Population | Generation Used in the Experiments | Year of Collection |
|---|---|---|---|---|---|---|---|
| ARA | Araraquara | São Paulo | Aedes aegypti | eggs | 100 females + 100 males | F2 | 2022 |
| JAB | Jaboticatubas | Minas Gerais | Aedes aegypti | eggs | 100 females + 100 males | F2 | 2022 |
| PET | Petrolina | Pernambuco | Aedes aegypti | eggs | 100 females + 100 males | F3 | 2022 |
| POA | Porto Alegre | Rio Grande do Sul | Aedes aegypti | eggs | 100 females + 100 males | F3 | 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Freitas, A.; Rezende, F.; de Mendonça, S.; Baldon, L.; Silva, E.; Ferreira, F.; Almeida, J.; Amadou, S.; Marçal, B.; Comini, S.; et al. The High Capacity of Brazilian Aedes aegypti Populations to Transmit a Locally Circulating Lineage of Chikungunya Virus. Viruses 2024, 16, 575. https://doi.org/10.3390/v16040575
de Freitas A, Rezende F, de Mendonça S, Baldon L, Silva E, Ferreira F, Almeida J, Amadou S, Marçal B, Comini S, et al. The High Capacity of Brazilian Aedes aegypti Populations to Transmit a Locally Circulating Lineage of Chikungunya Virus. Viruses. 2024; 16(4):575. https://doi.org/10.3390/v16040575
Chicago/Turabian Stylede Freitas, Amanda, Fernanda Rezende, Silvana de Mendonça, Lívia Baldon, Emanuel Silva, Flávia Ferreira, João Almeida, Siad Amadou, Bruno Marçal, Sara Comini, and et al. 2024. "The High Capacity of Brazilian Aedes aegypti Populations to Transmit a Locally Circulating Lineage of Chikungunya Virus" Viruses 16, no. 4: 575. https://doi.org/10.3390/v16040575






