Plasma Lipidomic Profiles in cART-Treated Adolescents with Perinatally Acquired HIV Compared to Matched Controls
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Demographics, Disease and Treatment-Related Characteristics
2.3. Lipidomics
2.3.1. Sample Collection, Biomarker Analysis and cART Regimens
2.3.2. Plasma Lipidomic Profiling
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
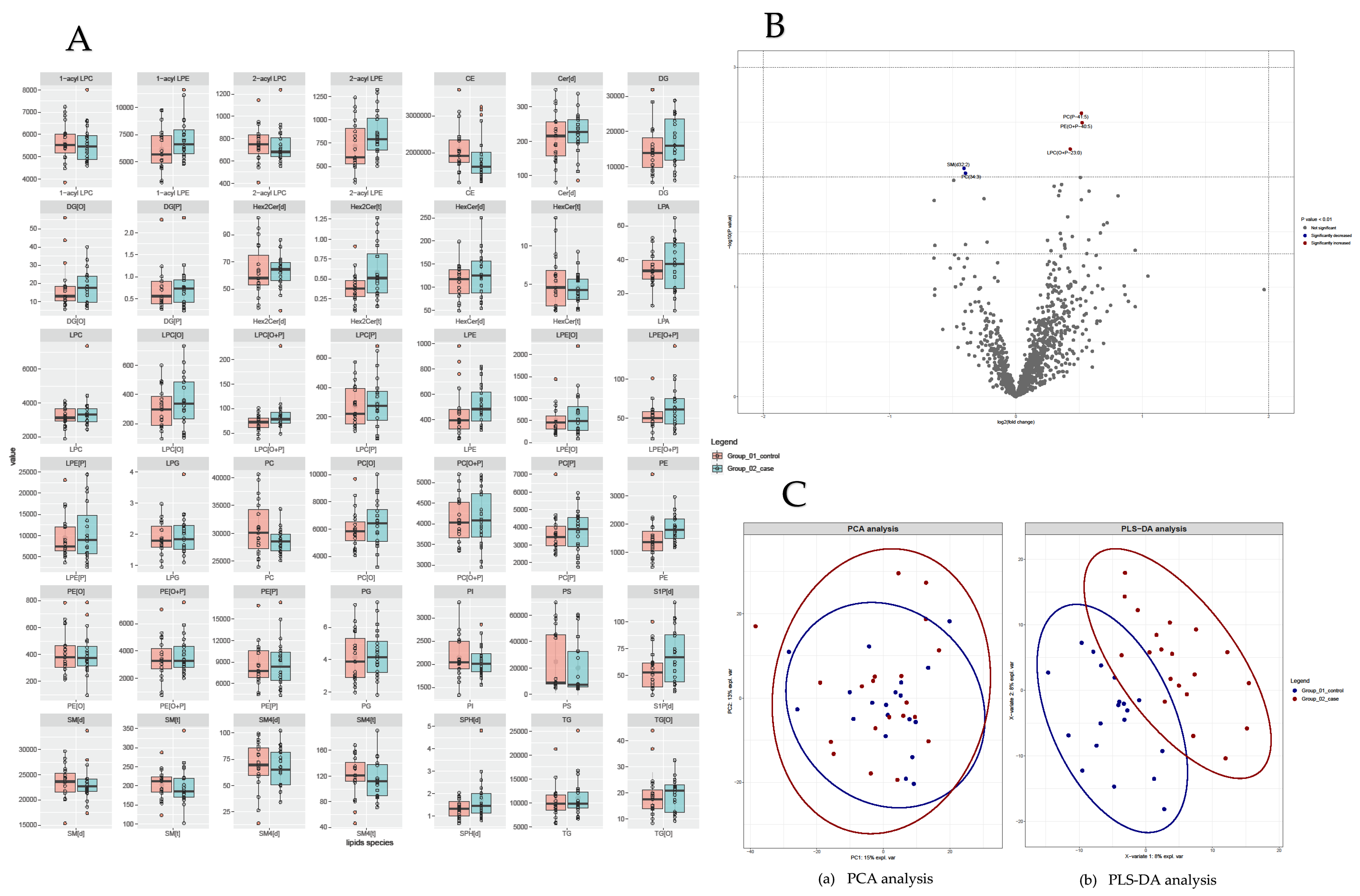
3.2. Lipidome Alterations
3.2.1. Lipidome Composition in PHIV and Control Groups
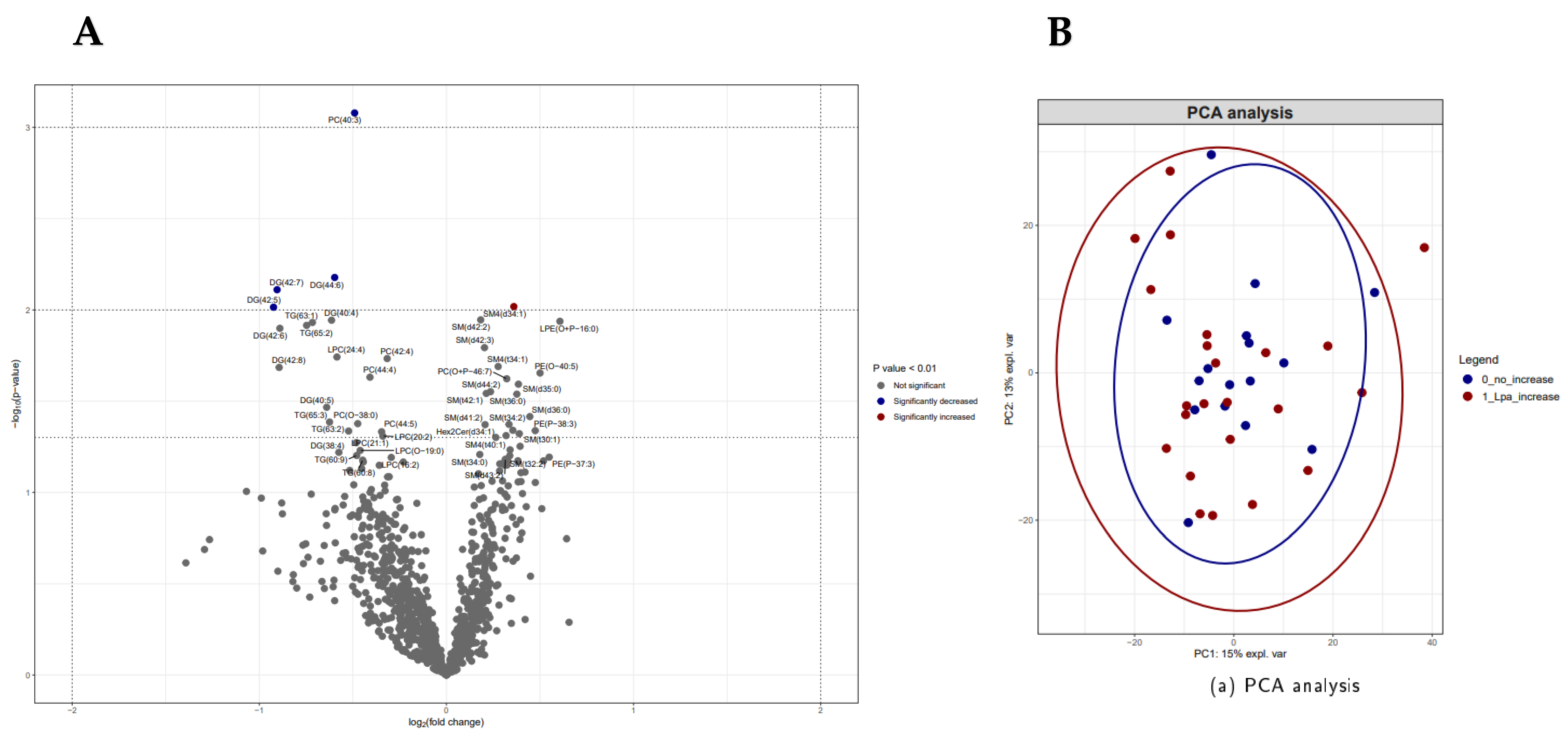
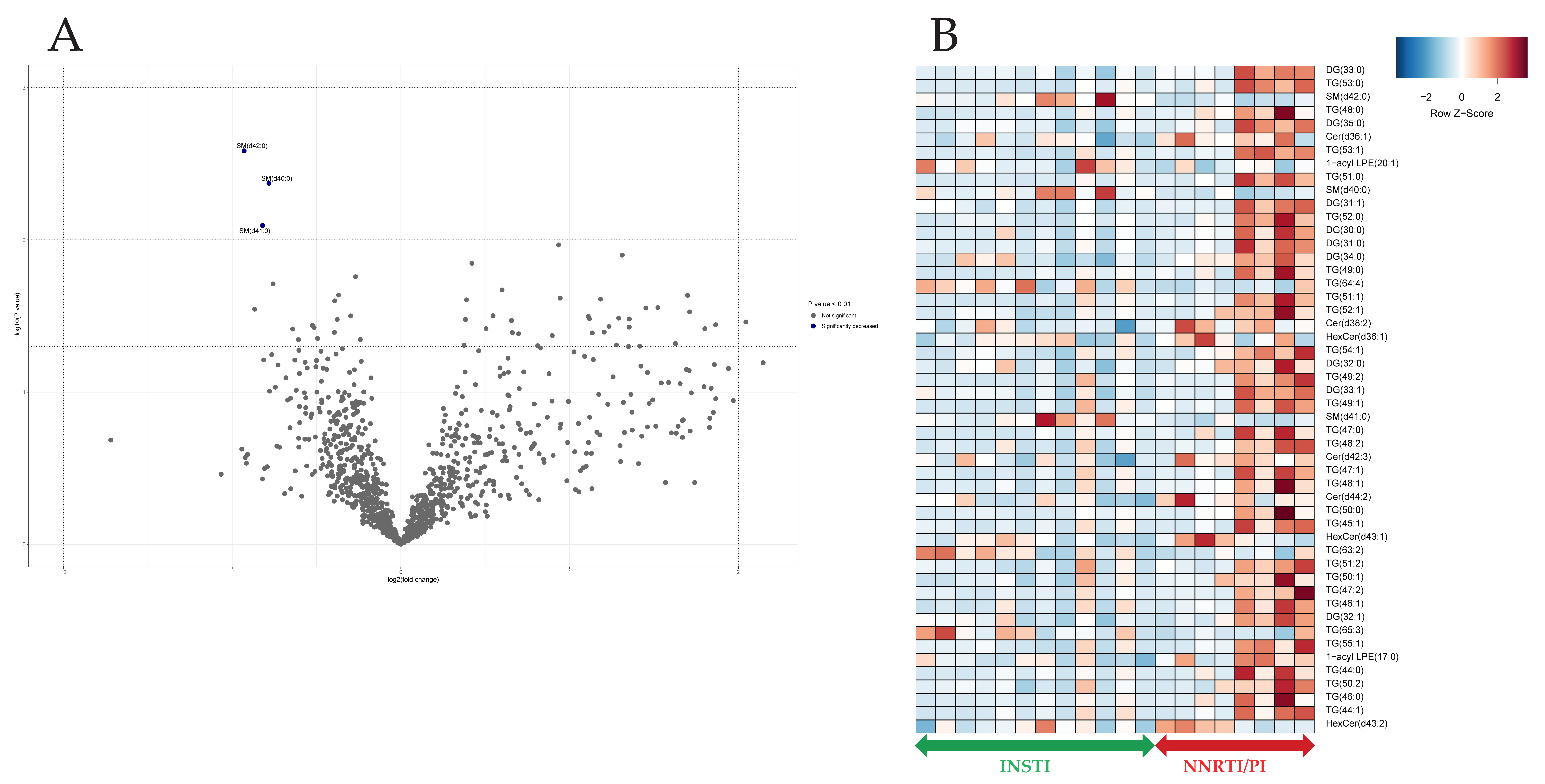
3.2.2. Lp(a) Increase and Various cART Regimes in Relation to Lipidomic Profiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trickey, A.; Sabin, C.A.; Burkholder, G.; Crane, H.; Monforte, A.D.A.; Egger, M.; Sterne, J.A. Life expectancy after 2015 of adults with HIV on long-term antiretroviral therapy in Europe and North America: A collaborative analysis of cohort studies. Lancet HIV 2023, 10, e295–e307. [Google Scholar] [CrossRef] [PubMed]
- Barlow-Mosha, L.; Eckard, A.R.; McComsey, G.A.; Musoke, P.M. Metabolic complications and treatment of perinatally HIV-infected children and adolescents. J. Int. AIDS Soc. 2013, 16, 18600. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.M.; Wu, J.; Jansson, J.; Wilson, D.P. Relative risk of cardiovascular disease among people living with HIV: A systematic review and meta-analysis. HIV Med. 2012, 13, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.V.; Stelzle, D.; Lee, K.K.; Beck, E.J.; Alam, S.; Clifford, S.; Longenecker, C.T.; Strachan, F.; Bagchi, S.; Whiteley, W.; et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV: Systematic Review and Meta-Analysis. Circulation 2018, 138, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Dirajlal-Fargo, S.; Sattar, A.; Kulkarni, M.; Bowman, E.; Funderburg, N.; McComsey, G.A. HIV-positive youth who are perinatally infected have impaired endothelial function. AIDS 2017, 31, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Eckard, A.R.; Raggi, P.; Ruff, J.H.; O’Riordan, M.A.; Rosebush, J.C.; Labbato, D.; Daniels, J.E.; Uribe-Leitz, M.; Longenecker, C.T.; McComsey, G.A. Arterial stiffness in HIV-infected youth and associations with HIV-related variables. Virulence 2017, 8, 1265–1273. [Google Scholar] [CrossRef]
- McComsey, G.A.; O’Riordan, M.; Hazen, S.L.; El-Bejjani, D.; Bhatt, S.; Brennan, M.L.; Storer, N.; Adell, J.; A Nakamoto, D.; Dogra, V. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. AIDS 2007, 21, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Dirajlal-Fargo, S.; McComsey, G.A. Cardiometabolic Complications in Youth with Perinatally Acquired HIV in the Era of Antiretroviral Therapy. Curr. HIV/AIDS Rep. 2021, 18, 424–435. [Google Scholar] [CrossRef] [PubMed]
- van Genderen, J.G.; Van den Hof, M.; de Boer, C.G.; Jansen, H.P.; van Deventer, S.J.; Tsimikas, S.; Witztum, J.L.; Kastelein, J.J.; Pajkrt, D. Longitudinal assessment of lipoprotein (a) levels in perinatally HIV-infected children and adolescents. Viruses 2021, 13, 2067. [Google Scholar] [CrossRef] [PubMed]
- Augustemak de Lima, L.R.; Petroski, E.L.; Moreno, Y.M.; Silva, D.A.; Trindade, E.B.; Carvalho, A.P.; Back, I.D. Dyslipidemia, chronic inflammation, and subclinical atherosclerosis in children and adolescents infected with HIV: The PositHIVe Health Study. PLoS ONE 2018, 13, e0190785. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.; Vaida, F.; Otwombe, K.; Cotton, M.F.; Browne, S.; Innes, S. Longitudinal comparison of insulin resistance and dyslipidemia in children with and without perinatal HIV infection in South Africa. AIDS 2023, 37, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Aurpibul, L.; Namwongprom, S.; Sudjaritruk, T.; Ounjaijean, S. Metabolic syndrome, biochemical markers, and body composition in youth living with perinatal HIV infection on antiretroviral treatment. PLoS ONE 2020, 15, e0230707. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Prasad, A.; Choi, Y.S.; Xing, C.; Clopton, P.; Witztum, J.L.; Tsimikas, S. LPA Gene, Ethnicity, and Cardiovascular Events. Circulation 2017, 135, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Jain, V.; Saeed, A.; Saseen, J.J.; Gulati, M.; Ballantyne, C.M.; Virani, S.S. Lipoprotein (a) and ethnicities. Atherosclerosis 2022, 349, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Lau, F.D.; Giugliano, R.P. Lipoprotein (a) and its significance in cardiovascular disease: A review. JAMA Cardiol. 2022, 7, 760–769. [Google Scholar] [CrossRef]
- Van den Hof, M.; Klein Haneveld, M.J.; Blokhuis, C.; Scherpbier, H.J.; Jansen, H.P.; Kootstra, N.A.; Dallinga-Thie, G.M.; Van Deventer, S.J.; Tsimikas, S.; Pajkrt, D. Elevated lipoprotein (a) in perinatally HIV-infected children compared with healthy ethnicity-matched controls. In InOpen Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2019; Volume 6, p. ofz301. [Google Scholar] [CrossRef]
- Subramanian, S.; Tawakol, A.; Burdo, T.H.; Abbara, S.; Wei, J.; Vijayakumar, J.; Corsini, E.; Abdelbaky, A.; Zanni, M.V.; Hoffmann, U.; et al. Arterial inflammation in patients with HIV. JAMA 2012, 308, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Nou, E.; Lo, J.; Hadigan, C.; Grinspoon, S.K. Pathophysiology and management of cardiovascular disease in patients with HIV. Lancet Diabetes Endocrinol. 2016, 4, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Alvi, R.M.; Neilan, A.M.; Tariq, N.; Awadalla, M.; Afshar, M.; Banerji, D.; Rokicki, A.; Mulligan, C.; Triant, V.A.; Zanni, M.V.; et al. Protease Inhibitors and Cardiovascular Outcomes in Patients with HIV and Heart Failure. J. Am. Coll. Cardiol. 2018, 72, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Diggins, C.E.; Russo, S.C.; Lo, J. Metabolic consequences of antiretroviral therapy. Curr. HIV/AIDS Rep. 2022, 19, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Tsiodras, S.; Mantzoros, C.; Hammer, S.; Samore, M. Effects of Protease Inhibitors on Hyperglycemia, Hyperlipidemia, and Lipodystrophy: A 5-Year Cohort Study. Arch. Intern. Med. 2000, 160, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Koethe, J.R.; Lagathu, C.; Lake, J.E.; Domingo, P.; Calmy, A.; Falutz, J.; Brown, T.T.; Capeau, J. HIV and antiretroviral therapy-related fat alterations. Nat. Rev. Dis. Primers 2020, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Weichseldorfer, M.; Reitz, M.; Latinovic, O.S. Past HIV-1 medications and the current status of combined antiretroviral therapy options for HIV-1 patients. Pharmaceutics 2021, 13, 1798. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Arias, L.; Delgado, R. Update and latest advances in antiretroviral therapy. Trends Pharmacol. Sci. 2022, 43, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevención con Dieta Mediterránea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Rexrode, K.M. A Review of Lipidomics of Cardiovascular Disease Highlights the Importance of Isolating Lipoproteins. Metabolites 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- German, J.B.; Gillies, L.A.; Smilowitz, J.T.; Zivkovic, A.M.; Watkins, S.M. Lipidomics and lipid profiling in metabolomics. Curr. Opin. Lipidol. 2007, 18, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Wong, G.; Barlow, C.K.; Kingwell, B.A. Lipidomics: Potential role in risk prediction and therapeutic monitoring for diabetes and cardiovascular disease. Pharmacol. Ther. 2014, 143, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, C.; Pechlaner, R.; Willeit, P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G.; et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 2014, 129, 1821–1831. [Google Scholar] [CrossRef]
- Bowman, E.; Funderburg, N.T. Lipidome abnormalities and cardiovascular disease risk in HIV infection. Curr. HIV/AIDS Rep. 2019, 16, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Trevillyan, J.M.; Fatou, B.; Cinel, M.; Weir, J.M.; Hoy, J.F.; Meikle, P.J. Plasma lipidomic profiling of treated HIV-positive individuals and the implications for cardiovascular risk prediction. PLoS ONE 2014, 9, e94810. [Google Scholar] [CrossRef]
- Zhang, E.; Chai, J.C.; Deik, A.A.; Hua, S.; Sharma, A.; Schneider, M.F.; Gustafson, D.; Hanna, D.B.; Lake, J.E.; Rubin, L.H.; et al. Plasma lipidomic profiles and risk of diabetes: 2 prospective cohorts of HIV-infected and HIV-uninfected individuals. J. Clin. Endocrinol. Metab. 2021, 106, e999–e1010. [Google Scholar] [CrossRef] [PubMed]
- Dirajlal-Fargo, S.; Sattar, A.; Yu, J.; Albar, Z.; Chaves, F.C.; Riedl, K.; Kityo, C.; Bowman, E.; McComsey, G.A.; Funderburg, N. Lipidome association with vascular disease and inflammation in HIV+ Ugandan children. AIDS 2021, 35, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.C.; Deik, A.A.; Hua, S.; Wang, T.; Hanna, D.B.; Xue, X.; Haberlen, S.A.; Shah, S.J.; Suh, Y.; Lazar, J.M.; et al. Association of lipidomic profiles with progression of carotid artery atherosclerosis in HIV infection. JAMA Cardiol. 2019, 4, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Van den Hof, M.; Ter Haar, A.M.; Scherpbier, H.J.; van der Lee, J.H.; Reiss, P.; Wit, F.W.; Oostrom, K.J.; Pajkrt, D. Neurocognitive development in perinatally human immunodeficiency virus–infected adolescents on long-term treatment, compared to healthy matched controls: A longitudinal study. Clin. Infect. Dis. 2020, 70, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, N.; Joshi, P.H. Lp (a): Addressing a target for cardiovascular disease prevention. Curr. Cardiol. Rep. 2019, 21, 102. [Google Scholar] [CrossRef]
- Reyes-Soffer, G.; Ginsberg, H.N.; Berglund, L.; Duell, P.B.; Heffron, S.P.; Kamstrup, P.R.; Lloyd-Jones, D.M.; Marcovina, S.M.; Yeang, C.; Koschinsky, M.L.; et al. Lipoprotein (a): A genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: A scientific statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e48–e60. [Google Scholar] [CrossRef] [PubMed]
- Vaz, F.M.; McDermott, J.H.; Alders, M.; Wortmann, S.B.; Kölker, S.; Pras-Raves, M.L.; Vervaart, M.A.T.; van Lenthe, H.; Luyf, A.C.M.; Elfrink, H.L.; et al. Mutations in PCYT2 disrupt etherlipid biosynthesis and cause a complex hereditary spastic paraplegia. Brain 2019, 142, 3382–3397. [Google Scholar] [CrossRef] [PubMed]
- Chouchane, O.; Schuurman, A.R.; Reijnders, T.D.; Peters-Sengers, H.; Butler, J.M.; Uhel, F.; Schultz, M.J.; Bonten, M.J.; Cremer, O.L.; Calfee, C.S.; et al. The Plasma Lipidomic Landscape in Patients with Sepsis due to Community-acquired Pneumonia. Am. J. Respir. Crit. Care Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Vaz, F.M.; Staps, P.; van Klinken, J.B.; van Lenthe, H.; Vervaart, M.; Wanders, R.J.; Pras-Raves, M.L.; van Weeghel, M.; Salomons, G.S.; Ferdinandusse, S.; et al. Discovery of novel diagnostic biomarkers for Sjögren-Larsson syndrome by untargeted lipidomics. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2024, 1869, 159447. [Google Scholar] [CrossRef] [PubMed]
- Mahieu, B.; Qannari, E.M.; Jaillais, B. Extension and significance testing of Variable Importance in Projection (VIP) indices in Partial Least Squares regression and Principal Components Analysis. Chemom. Intell. Lab. Syst. 2023, 242, 104986. [Google Scholar] [CrossRef]
- Olund Villumsen, S.; Benfeitas, R.; Knudsen, A.D.; Gelpi, M.; Høgh, J.; Thomsen, M.T.; Murray, D.; Ullum, H.; Neogi, U.; Nielsen, S.D. Integrative lipidomics and metabolomics for system-level understanding of the metabolic syndrome in long-term treated HIV-infected individuals. Front. Immunol. 2022, 12, 742736. [Google Scholar] [CrossRef] [PubMed]
- Trevillyan, J.M.; Wong, G.; Puls, R.; Petoumenos, K.; Emery, S.; Mellett, N.A.; Mundra, P.A.; Meikle, P.J.; Hoy, J.F.; ALTAIR Study Group. Changes in plasma lipidome following initiation of antiretroviral therapy. PLoS ONE 2018, 13, e0202944. [Google Scholar] [CrossRef] [PubMed]
- Curran, A.; Rull, A.; Navarro, J.; Vidal-González, J.; Martin-Castillo, M.; Burgos, J.; Falcó, V.; Ribera, E.; Torrella, A.; Planas, B.; et al. Lipidomics reveals reduced inflammatory lipid species and storage lipids after switching from EFV/FTC/TDF to RPV/FTC/TDF: A randomized open-label trial. J. Clin. Med. 2020, 9, 1246. [Google Scholar] [CrossRef] [PubMed]
- Belury, M.A.; Bowman, E.; Gabriel, J.; Snyder, B.; Kulkarni, M.; Palettas, M.; Mo, X.; Lake, J.E.; Zidar, D.; Sieg, S.F.; et al. Prospective analysis of lipid composition changes with antiretroviral therapy and immune activation in persons living with HIV. Pathog. Immun. 2017, 2, 376. [Google Scholar] [CrossRef] [PubMed]
- Akerele, O.A.; Cheema, S.K. Fatty acyl composition of lysophosphatidylcholine is important in atherosclerosis. Med. Hypotheses 2015, 85, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Feeney, E.R.; Mallon, P.W. HIV and HAART-associated dyslipidemia. Open Cardiovasc. Med. J. 2011, 5, 49. [Google Scholar] [CrossRef]
- Richmond, S.R.; Carper, M.J.; Lei, X.; Zhang, S.; Yarasheski, K.E.; Ramanadham, S. HIV-protease inhibitors suppress skeletal muscle fatty acid oxidation by reducing CD36 and CPT1 fatty acid transporters. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2010, 1801, 559–566. [Google Scholar] [CrossRef] [PubMed]
- El Hadri, K.; Glorian, M.; Monsempes, C.; Dieudonné, M.N.; Pecquery, R.; Giudicelli, Y.; Andreani, M.; Dugail, I.; Fève, B. In vitro suppression of the lipogenic pathway by the nonnucleoside reverse transcriptase inhibitor efavirenz in 3T3 and human preadipocytes or adipocytes. J. Biol. Chem. 2004, 279, 15130–15141. [Google Scholar] [CrossRef]
- Cai, X.; Perttula, K.; Pajouh, S.K.; Hubbard, A.; Nomura, D.K.; Rappaport, S.M. Untargeted lipidomic profiling of human plasma reveals differences due to race, gender and smoking status. Metabolomics 2014, 4, 1. [Google Scholar] [CrossRef]
- Eslabão, L.B.; Gubert, G.F.; Beltrame, L.C.; Mello, I.M.; Bruna-Romero, O.; Zárate-Bladés, C.R. Prophylactic treatment of undernourished mice with cotrimoxazole induces a different profile of dysbiosis with functional metabolic alterations. Cells 2022, 11, 2278. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.H.; Mukerji, S.S.; Russo, S.C.; Lo, J. Gastrointestinal dysfunction and HIV comorbidities. Curr. HIV/AIDS Rep. 2021, 18, 57–62. [Google Scholar] [CrossRef]
| PHIV (n = 20) | CONTROLS (n = 20) | ||||
|---|---|---|---|---|---|
| n | n | p | |||
| Age (years) | 20 | 17.5 (15.5–20.7) | 20 | 16.5 (15.7–19.8) | 0.191 Y |
| Female sex | 20 | 9 (45%) | 20 | 13 (65%) | 0.366 Z |
| Ethnic origin | 0.999 Z | ||||
| Black | 20 | 16 (80%) | 20 | 17 (85%) | |
| Other 1 | 4 (20%) | 3 (15%) | |||
| Height (m) | 19 | 1.66 (1.57–1.74) | 20 | 1.68 (1.63–1.77) | 0.278 Y |
| Weight (kg) | 19 | 56 (49–70) | 20 | 64 (56–78) | 0.084 Y |
| BMI (kg/m2) | 19 | 20.4 (19.2–22.3) | 20 | 22.8 (19.8–26.3) | 0.134 Y |
| Overweight (BMI ≥ 25 kg/m2) | 19 | 2 (11%) | 20 | 4 (20%) | 0.432 Z |
| Systolic BP (mmHg) Diastolic BP (mmHg) | 19 19 | 125 (115–133) 67 (59–76) | 18 | 119 (114–124) 64 (59–74) | 0.484 Y 0.843 Y |
| Smoking | 19 19 | 6 (30%) | 19 19 | 5 (26%) | 0.904 Z |
| Lipids | |||||
| Total cholesterol (mmol/L) | 3.89 (3.51–4.32) | 4.31 (3.83–4.56) | 0.158 Y | ||
| LDL (mmol/L) | 2.10 (1.75–2.67) | 2.33 (2.08–2.55) | 0.222 Y | ||
| HDL (mmol/L) | 1.33 (1.24–1.67) | 1.61 (1.23–1.81) | 0.5 Y | ||
| Triglycerides (mmol/L) | 0.83 (0.47–1.15) | 0.74 (0.42–0.68) | 0.259 Y | ||
| Age at HIV diagnosis (years) | 20 | 1.7 (0.8–4.2) | |||
| CDC | |||||
| NA | 8 (38%) | ||||
| B | 8 (38%) | ||||
| C | 4 (24%) | ||||
| Nadir CD4+ T-cell Z score (cells/µL) | 19 | 0.82 (0.61) | |||
| Zenith HIV viral load (log10 copies/mL) | 18 | 5.5 (4.9–5.8) | |||
| Age at cART initiation (years) | 18 | 2.5 (1.2–6.0) | |||
| Duration of cART (years) | 18 | 14.9 (9.5–19.6) | |||
| Undetectable viral load at assessment (<40 copies/mL) | 20 | 18 (90%) | |||
| cART regimen, No. (%) Backbone + INSTI Backbone + NNRTI Backbone + PI | 20 | 11 (55%) 5 (25%) 4 (20%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Post, J.; Guerra, T.E.J.; van den Hof, M.; Vaz, F.M.; Pajkrt, D.; van Genderen, J.G. Plasma Lipidomic Profiles in cART-Treated Adolescents with Perinatally Acquired HIV Compared to Matched Controls. Viruses 2024, 16, 580. https://doi.org/10.3390/v16040580
van der Post J, Guerra TEJ, van den Hof M, Vaz FM, Pajkrt D, van Genderen JG. Plasma Lipidomic Profiles in cART-Treated Adolescents with Perinatally Acquired HIV Compared to Matched Controls. Viruses. 2024; 16(4):580. https://doi.org/10.3390/v16040580
Chicago/Turabian Stylevan der Post, Julie, Thiara E. J. Guerra, Malon van den Hof, Frédéric M. Vaz, Dasja Pajkrt, and Jason G. van Genderen. 2024. "Plasma Lipidomic Profiles in cART-Treated Adolescents with Perinatally Acquired HIV Compared to Matched Controls" Viruses 16, no. 4: 580. https://doi.org/10.3390/v16040580
APA Stylevan der Post, J., Guerra, T. E. J., van den Hof, M., Vaz, F. M., Pajkrt, D., & van Genderen, J. G. (2024). Plasma Lipidomic Profiles in cART-Treated Adolescents with Perinatally Acquired HIV Compared to Matched Controls. Viruses, 16(4), 580. https://doi.org/10.3390/v16040580





