The W195 Residue of the Newcastle Disease Virus V Protein Is Critical for Multiple Aspects of Viral Self-Regulation through Interactions between V and Nucleoproteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cells, Virus and Antibody
2.3. Construction of the Minigenome (MG) System and Expression Plasmids
2.4. MG Assay
2.5. RT-qPCR
2.6. Coimmunoprecipitation (CO-IP)
2.7. Western Blot
2.8. Immunofluorescence Assay (IFA)
2.9. Construction of the F48E9 Strain Full-Length Clone
2.10. Rescue of rF48E9, rF48E9-eGFP and rF48E9-VW195R
2.11. Viral Growth Kinetics
2.12. Animal Experiments
2.13. Statistical Analysis
3. Results
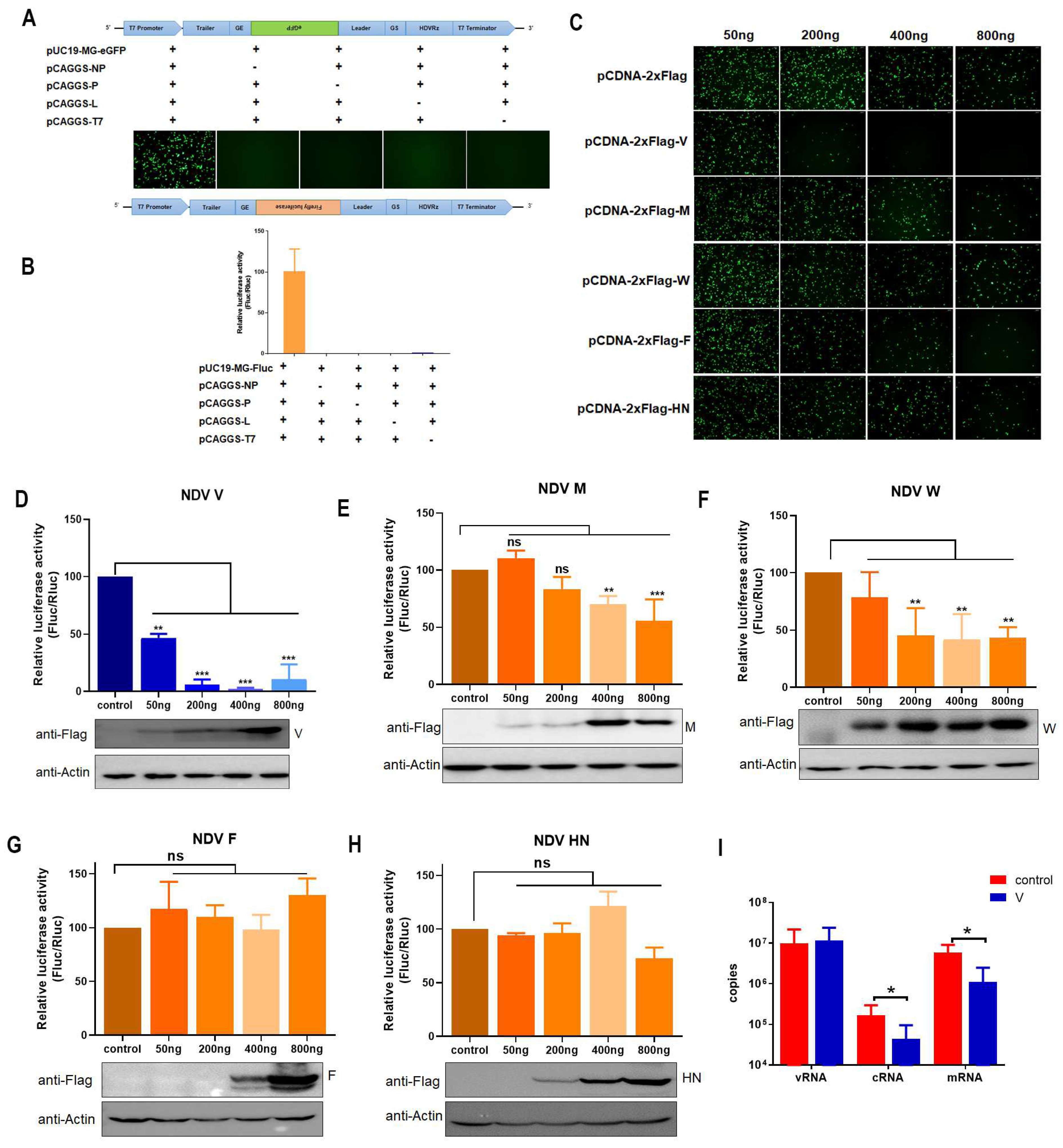
3.1. V Inhibits Expression of MG-Encoded Reporters
3.2. The Interaction of V with NP Reduces MG-Encoded Reporter Activity
3.3. The V Protein Alters IB Formation in Transfected Cells
3.4. Rescue of the Class II Genotype IX Strain F48E9
3.5. The W195 Mutation of V Increases Viral Replication and IB Formation in Infected Cells
3.6. The W195 Mutation of V Reduces Virulence and Pathogenicity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, C.C.; Rehman, Z.U.; Liu, K.C.; Qiu, X.S.; Tan, L.; Sun, Y.J.; Liao, Y.; Song, C.P.; Yu, S.Q.; Ding, Z.; et al. Potential of genotype VII Newcastle disease viruses to cause differential infections in chickens and ducks. Transbound. Emerg. Dis. 2018, 65, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, G.K.; Ayllon, M.A.; Bao, Y.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-Buschmann, A.; et al. Taxonomy of the order Mononegavirales: Update 2019. Arch. Virol. 2019, 164, 1967–1980. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Hamaguchi, M.; Toyoda, T. Molecular biology of Newcastle disease virus. Prog. Vet. Microbiol. Immunol. 1989, 5, 16–64. [Google Scholar]
- Ganar, K.; Das, M.; Sinha, S.; Kumar, S. Newcastle disease virus: Current status and our understanding. Virus Res. 2014, 184, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Nath, B.; Sharma, K.; Ahire, K.; Goyal, A.; Kumar, S. Structure analysis of the nucleoprotein of Newcastle disease virus: An insight towards its multimeric form in solution. Int. J. Biol. Macromol. 2020, 151, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.; Zhao, L.; Ledda, A.; Liu, W.; Ding, C.; Nair, V.; Ferretti, L. Patterns of RNA Editing in Newcastle Disease Virus Infections. Viruses 2020, 12, 1249. [Google Scholar] [CrossRef]
- Bloyet, L.M. The Nucleocapsid of Paramyxoviruses: Structure and Function of an Encapsidated Template. Viruses 2021, 13, 2465. [Google Scholar] [CrossRef]
- Calain, P.; Roux, L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J. Virol. 1993, 67, 4822–4830. [Google Scholar] [CrossRef] [PubMed]
- Emmerson, W.E.a.P.T. Assembly of recombinant Newcastle disease virus nucleocapsid protein into nucleocapsid-like structures is inhibited by the phosphoprotein. J. Gen. Virol. 1997, 78, 2335–2339. [Google Scholar]
- Jahanshiri, F.; Eshaghi, M.; Yusoff, K. Identification of phosphoprotein:phosphoprotein and phosphoprotein:nucleocapsid protein interaction domains of the Newcastle disease virus. Arch. Virol. 2005, 150, 611–618. [Google Scholar] [CrossRef]
- Nevers, Q.; Albertini, A.A.; Lagaudriere-Gesbert, C.; Gaudin, Y. Negri bodies and other virus membrane-less replication compartments. Biochim. Biophys. Acta Mol. Cell. Res. 2020, 1867, 118831. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Duan, L.; Wang, T.; Wang, W.; Han, Y.; Hu, R.; Hou, Q.; Liu, H.; Wang, J.; Wang, X.; et al. Newcastle disease virus forms inclusion bodies with features of liquid-liquid phase separation. Vet. Microbiol. 2023, 284, 109800. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Kubota, T.; Kita, S.; Nakatsu, Y.; Aoki, N.; Mori, Y.; Maenaka, K.; Takeda, M.; Kidokoro, M.; Lyles, D.S. Heat Shock Protein 70 Regulates Degradation of the Mumps Virus Phosphoprotein via the Ubiquitin-Proteasome Pathway. J. Virol. 2015, 89, 3188–3199. [Google Scholar] [CrossRef] [PubMed]
- Ringel, M.; Heiner, A.; Behner, L.; Halwe, S.; Sauerhering, L.; Becker, N.; Dietzel, E.; Sawatsky, B.; Kolesnikova, L.; Maisner, A. Nipah virus induces two inclusion body populations: Identification of novel inclusions at the plasma membrane. PLoS Pathog. 2019, 15, e1007733. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, Y.; Cheng, Q.; Zhong, Y.; Qin, Y.; Chen, M.; García-Sastre, A. Inclusion Body Fusion of Human Parainfluenza Virus Type 3 Regulated by Acetylated α-Tubulin Enhances Viral Replication. J. Virol. 2017, 91, e01802-16. [Google Scholar] [CrossRef] [PubMed]
- Mebatsion, T.; Verstegen, S.; De Vaan, L.T.; Romer-Oberdorfer, A.; Schrier, C.C. A recombinant newcastle disease virus with low-level V protein expression is immunogenic and lacks pathogenicity for chicken embryos. J. Virol. 2001, 75, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Childs, K.; Randall, R.; Goodbourn, S. Paramyxovirus V proteins interact with the RNA Helicase LGP2 to inhibit RIG-I-dependent interferon induction. J. Virol. 2012, 86, 3411–3421. [Google Scholar] [CrossRef] [PubMed]
- Childs, K.; Stock, N.; Ross, C.; Andrejeva, J.; Hilton, L.; Skinner, M.; Randall, R.; Goodbourn, S. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology 2007, 359, 190–200. [Google Scholar] [CrossRef]
- Park, M.S.; Garcia-Sastre, A.; Cros, J.F.; Basler, C.F.; Palese, P. Newcastle disease virus V protein is a determinant of host range restriction. J. Virol. 2003, 77, 9522–9532. [Google Scholar] [CrossRef]
- Yang, Y.; Zengel, J.; Sun, M.; Sleeman, K.; Timani, K.A.; Aligo, J.; Rota, P.; Wu, J.; He, B. Regulation of Viral RNA Synthesis by the V Protein of Parainfluenza Virus 5. J. Virol. 2015, 89, 11845–11857. [Google Scholar] [CrossRef]
- Witko, S.E.; Kotash, C.; Sidhu, M.S.; Udem, S.A.; Parks, C.L. Inhibition of measles virus minireplicon-encoded reporter gene expression by V protein. Virology 2006, 348, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, K.; Bankamp, B.; Hummel, K.B.; Lo, M.K.; Bellini, W.J.; Rota, P.A. The C, V and W proteins of Nipah virus inhibit minigenome replication. J. Gen. Virol. 2008, 89, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Sandra, M.; Horikami, S.S.; Sue, A. The Sendai Virus V Protein Interacts with the NP Protein to Regulate Viral Genome RNA Replication. Virology 1996, 222, 383–390. [Google Scholar]
- Lin, Y.; Horvath, F.; Aligo, J.A.; Wilson, R.; He, B. The role of simian virus 5 V protein on viral RNA synthesis. Virology 2005, 338, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Ohtsuka, J.; Tsurudome, M.; Nosaka, T.; Kolakofsky, D. Human parainfluenza virus type 2 V protein inhibits genome replication by binding to the L protein: Possible role in promoting viral fitness. J. Virol. 2008, 82, 6130–6138. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Noda, T.; Halfmann, P.; Jasenosky, L.; Kawaoka, Y. Ebola Virus (EBOV) VP24 Inhibits Transcription and Replication of the EBOV Genome. J. Infect. Dis. 2007, 196, S284–S290. [Google Scholar] [CrossRef] [PubMed]
- Hoenen, T.; Jung, S.; Herwig, A.; Groseth, A.; Becker, S. Both matrix proteins of Ebola virus contribute to the regulation of viral genome replication and transcription. Virology 2010, 403, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Huo, N.; Jin, Z.-Y.; Wei, Q.-L.; Wang, W.-B.; Mou, S.-J.; Li, Y.-S.; Chen, H.-J.; Xiao, S. Preparation and characterization of monoclonal antibodies againstHN protein of genotype VII Newcastle disease virus. Chin. J. Prev. Vet. Med. 2020, 42, 52–57. [Google Scholar]
- Su, J.; Dou, Y.; You, Y.; Cai, X. Application of minigenome technology in virology research of the Paramyxoviridae family. J. Microbiol. Immunol. Infect. 2015, 48, 123–129. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Yamaguchi, M.; Zhou, M.; Komatsu, T.; Nishio, M.; Sugiyama, T.; Takeuchi, K.; Itoh, M.; Gotoh, B. A tryptophan-rich motif in the human parainfluenza virus type 2 V protein is critical for the blockade of toll-like receptor 7 (TLR7)- and TLR9-dependent signaling. J. Virol. 2011, 85, 4606–4611. [Google Scholar] [CrossRef]
- Nishio, M.; Garcin, D.; Simonet, V.; Kolakofsky, D. The carboxyl segment of the mumps virus V protein associates with Stat proteins in vitro via a tryptophan-rich motif. Virology 2002, 300, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Murrieta, N.H.; Herrera-Camacho, I.; Palma-Ocampo, H.; Santos-López, G.; Reyes-Leyva, J. Interaction of mumps virus V protein variants with STAT1-STAT2 heterodimer: Experimental and theoretical studies. Virol. J. 2010, 7, 263. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, Q.; Luo, C.; Qin, Y.; Chen, M. Human Parainfluenza Virus Type 3 Matrix Protein Reduces Viral RNA Synthesis of HPIV3 by Regulating Inclusion Body Formation. Viruses 2018, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Peeters, B.P.; de Leeuw, O.S.; Koch, G.; Gielkens, A.L. Rescue of Newcastle disease virus from cloned cDNA: Evidence that cleavability of the fusion protein is a major determinant for virulence. J. Virol. 1999, 73, 5001–5009. [Google Scholar] [CrossRef] [PubMed]
- Molouki, A.; Peeters, B. Rescue of recombinant Newcastle disease virus: A short history of how it all started. Arch. Virol. 2017, 162, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Wan, H.Q.; Ni, X.X.; Wu, Y.T.; Liu, W.B. Pathotypical and genotypical characterization of strains of Newcastle disease virus isolated from outbreaks in chicken and goose flocks in some regions of China during 1985–2001. Arch. Virol. 2003, 148, 1387–1403. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Sun, Q.; Wu, S.; Dong, L.; Hu, S.; Meng, C.; Wu, Y.; Liu, X. Entire genome sequence analysis of genotype IX Newcastle disease viruses reveals their early-genotype phylogenetic position and recent-genotype genome size. Virol. J. 2011, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Shaw, M.L.; Munoz-Jordan, J.; Cros, J.F.; Nakaya, T.; Bouvier, N.; Palese, P.; Garcia-Sastre, A.; Basler, C.F. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J. Virol. 2003, 77, 1501–1511. [Google Scholar] [CrossRef]
- Wang, X.; Dang, R.; Yang, Z. The interferon antagonistic activities of the V proteins of NDV correlated with their virulence. Virus Genes 2019, 55, 233–237. [Google Scholar] [CrossRef]
- Nan, F.L.; Zhang, H.; Nan, W.L.; Xie, C.Z.; Ha, Z.; Chen, X.; Xu, X.H.; Qian, J.; Qiu, X.S.; Ge, J.Y.; et al. Lentogenic NDV V protein inhibits IFN responses and represses cell apoptosis. Vet. Microbiol. 2021, 261, 109181. [Google Scholar] [CrossRef]
- Jia, Y.Q.; Wang, X.W.; Chen, X.; Qiu, X.X.; Wang, X.L.; Yang, Z.Q. Characterization of chicken IFI35 and its antiviral activity against Newcastle disease virus. J. Vet. Med. Sci. 2022, 84, 473–483. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.; Wang, W.; Meng, F.; Wang, Y.; Wei, N.; Tian, J.; Li, H.; Hao, Q.; Zhou, Z.; Liu, H.; et al. The W195 Residue of the Newcastle Disease Virus V Protein Is Critical for Multiple Aspects of Viral Self-Regulation through Interactions between V and Nucleoproteins. Viruses 2024, 16, 584. https://doi.org/10.3390/v16040584
Wei Q, Wang W, Meng F, Wang Y, Wei N, Tian J, Li H, Hao Q, Zhou Z, Liu H, et al. The W195 Residue of the Newcastle Disease Virus V Protein Is Critical for Multiple Aspects of Viral Self-Regulation through Interactions between V and Nucleoproteins. Viruses. 2024; 16(4):584. https://doi.org/10.3390/v16040584
Chicago/Turabian StyleWei, Qiaolin, Wenbin Wang, Fanxing Meng, Ying Wang, Ning Wei, Jianxia Tian, Hanlue Li, Qiqi Hao, Zijie Zhou, Haijin Liu, and et al. 2024. "The W195 Residue of the Newcastle Disease Virus V Protein Is Critical for Multiple Aspects of Viral Self-Regulation through Interactions between V and Nucleoproteins" Viruses 16, no. 4: 584. https://doi.org/10.3390/v16040584





