Spatial Transmission Characteristics of the Bluetongue Virus Serotype 3 Epidemic in The Netherlands, 2023
Abstract
1. Introduction
2. Materials and Methods
Modelling
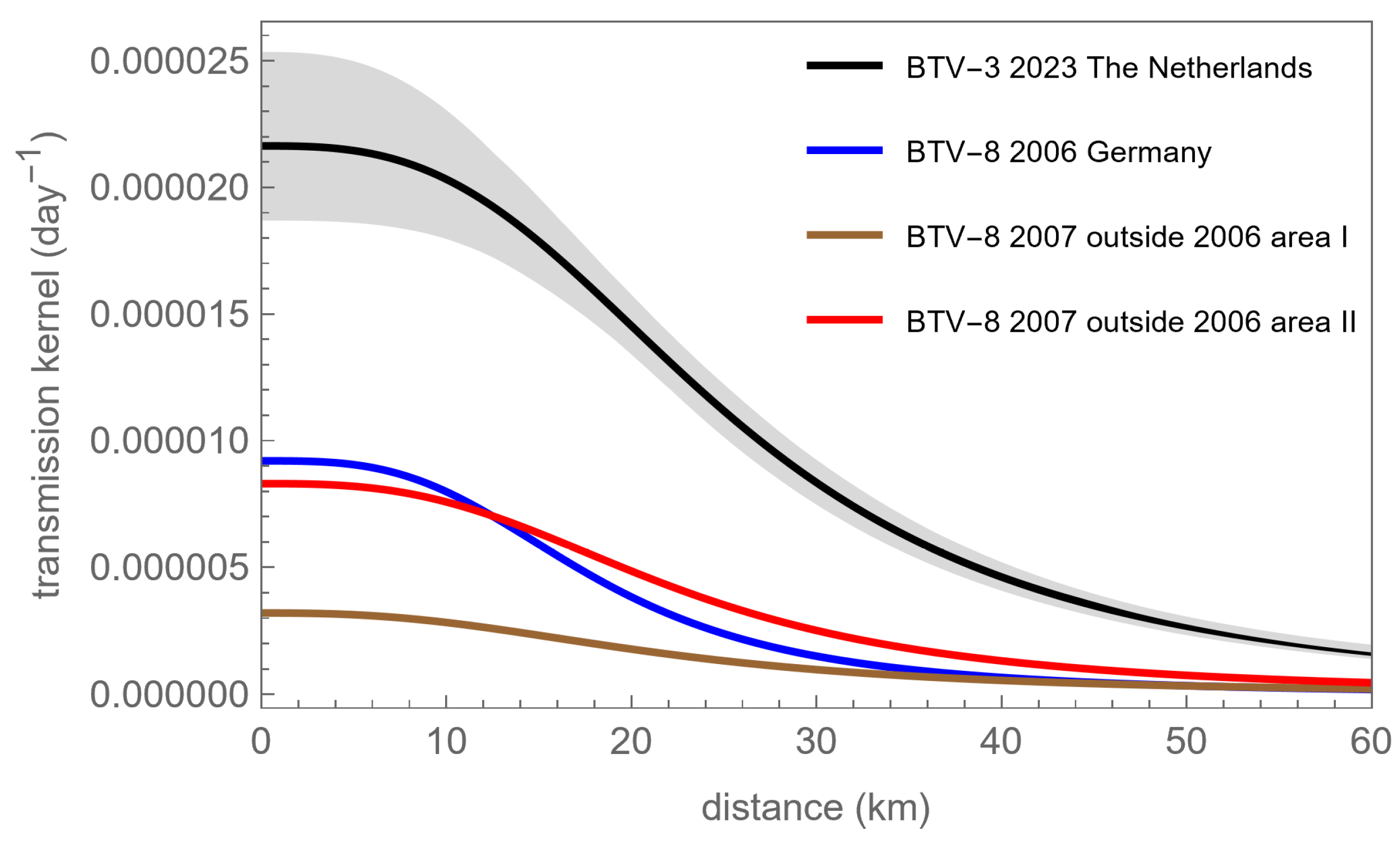
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sellers, R.F. Bluetongue in Africa, the Mediterranean region and the Near East—Disease, virus and vectors. Prev. Vet. Med. 1984, 2, 371–378. [Google Scholar] [CrossRef]
- Mellor, P.S.; Carpenter, S.; Harrup, L.; Baylis, M.; Mertens, P.P.C. Bluetongue in Europe and the Mediterranean Basin: History of occurrence prior to 2006. Prev. Vet. Med. 2008, 87, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Kundlacz, C.; Caignard, G.; Sailleau, C.; Viarouge, C.; Postic, L.; Vitour, D.; Zientara, S.; Breard, E. Bluetongue Virus in France: An Illustration of the European and Mediterranean Context since the 2000s. Viruses 2019, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Elbers, A.R.W.; Backx, A.; Meroc, E.; Gerbier, G.; Staubach, C.; Hendrickx, G.; van der Spek, A.; Mintiens, K. Field observations during the Bluetongue serotype 8 epidemic in 2006. I. Detection of first outbreaks and clinical signs in sheep and cattle in Belgium, France and The Netherlands. Prev. Vet. Med. 2008, 87, 21–30. [Google Scholar] [CrossRef]
- Boender, G.J.; Hagenaars, T.J.; Elbers, A.R.W.; Gethmann, J.M.; Meroc, E.; Guis, H.; de Koeijer, A.A. Confirmation of spatial patterns and temperature effects in Bluetongue virus serotype 8 transmission in NW-Europe from the 2007 reported case data. Vet. Res. 2014, 45, 75. [Google Scholar] [CrossRef]
- van Rijn, P.A.; Geurts, Y.; van der Spek, A.N.; Veldman, D.; van Gennip, R.G.P. Bluetongue virus serotype 6 in Europe in 2008—Emergence and disappearance of an unexpected non-virulent BTV. Vet. Microbiol. 2012, 158, 23–32. [Google Scholar] [CrossRef]
- Holwerda, M.; Santman-Berends, I.M.G.A.; Harders, F.; Engelsma, M.; Vloet, R.P.M.; Dijkstra, E.; van Gennip, R.G.P.; Mars, M.H.; Spierenburg, M.A.H.; Roos, L.; et al. Emergence of bluetongue virus serotype 3 in the Netherlands in September 2023. bioRxiv 2023. [Google Scholar] [CrossRef]
- UK Government. Research and Analysis—15 March 2024: Updated Outbreak Assessment for Bluetongue Virus in Europe. Available online: https://www.gov.uk/government/publications/bluetongue-virus-in-europe/15-march-2024-updated-outbreak-assessment-for-bluetongue-virus-in-europe (accessed on 11 April 2024).
- Golender, N.; Bumbarov, V.; Eldar, A.; Lorusso, A.; Kenigswald, G.; Varsano, J.S.; David, D.; Schainin, S.; Dagoni, I.; Gur, I.; et al. Bluetongue Serotype 3 in Israel 2013–2018: Clinical Manifestations of the Disease and Molecular Characterization of Israeli Strains. Front. Vet. Sci. 2020, 7, 112. [Google Scholar] [CrossRef]
- Cappai, S.; Rolesu, S.; Loi, F.; Liciardi, M.; Leone, A.; Marcacci, M.; Teodori, L.; Mangone, I.; Sghaier, S.; Portanti, O.; et al. Western Bluetongue virus serotype 3 in Sardinia, diagnosis and characterization. Transbound. Emerg. Dis. 2019, 66, 1426–1431. [Google Scholar] [CrossRef]
- Lorusso, A.; Guercio, A.; Purpari, G.; Cammà, C.; Calistri, P.; D’Alterio, N.; Hammami, S.; Sghaier, S.; Savini, G. Bluetongue virus serotype 3 in Western Sicily, November 2017. Vet. Ital. 2017, 53, 273–275. [Google Scholar] [CrossRef]
- Promed Mail, 2022. Bluetongue—Europe (06): Italy (SD) Sheep, St 3, Spread. Promed-Mail. November 1. Archive Number: 20221101.8706497. Available online: http://www.promedmail.org (accessed on 13 October 2023).
- Sghaier, S.; Lorusso, A.; Portanti, O.; Marcacci, M.; Orsini, M.; Barbria, M.E.; Mahmoud, A.S.; Hammami, S.; Petrini, A.; Savini, G. A novel Bluetongue virus serotype 3 strain in Tunisia, November 2016. Transbound. Emerg. Dis. 2017, 64, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Elbers, A.R.W.; Coenraadt, C.J.M.; Meiswinkel, R. Vector range of Culicoides biting midges and mosquitoes and influence of climate change. OIE Sci. Tech. Rev. 2015, 34, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Rigot, T.; Vercauteren Drubbel, M.; Delecolle, J.-C.; Gilbert, M. Farms, pastures and woodlands: The fine-scale distribution of Palearctic Culicoides spp. biting midges along an agro-ecological gradient. Med. Vet. Entomol. 2013, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Elbers, A.R.W.; Gonzales, J.L. Culicoides (Diptera: Ceratopogonidae) abundance is influenced by livestock host species and distance to hosts at the micro landscape scale. Insects 2023, 14, 637. [Google Scholar] [CrossRef] [PubMed]
- Szmaragd, C.; Wilson, A.J.; Carpenter, S.; Wood, J.L.N.; Mellor, P.S.; Gubbins, S. A modeling framework to describe the transmission of bluetongue virus within and between farms in Great Britain. PLoS ONE 2009, 4, e7741. [Google Scholar] [CrossRef] [PubMed]
- De Koeijer, A.A.; Boender, G.J.; Nodelijk, G.; Staubach, C.; Meroc, E.; Elbers, A.R.W. Quantitative analysis of transmission parameters for bluetongue virus serotype 8 in Western Europe in 2006. Vet. Res. 2011, 42, 53. [Google Scholar] [CrossRef] [PubMed]
- Chis Ster, I.; Ferguson, N.M. Transmission Parameters of the 2001 Foot and Mouth Epidemic in Great Britain. PLoS ONE 2007, 2, 502. [Google Scholar] [CrossRef]
- Backx, A.; Heutink, C.G.; van Rooij, E.M.A.; van Rijn, P.A. Clinical signs of bluetongue virus serotype 8 infection in sheep and goats. Vet. Rec. 2007, 161, 591–593. [Google Scholar] [CrossRef]
- Wolfram Research, Inc. Mathematica, Version 13.3; Wolfram Research, Inc.: Champaign, IL, USA, 2023.
- Elbers, A.R.W.; Meiswinkel, R.; van Weezep, E.; Sloet van Oldruitenborgh-Oosterbaan, M.M.; Kooi, E.A. Schmallenberg virus detected by RT-PCR in Culicoides biting midges captured during the 2011 epidemic in the Netherlands. Emerg. Infect. Dis. 2013, 19, 106–109. [Google Scholar] [CrossRef]
- Boender, G.J.; Hagenaars, T.J. Common features in spatial livestock disease transmission parameters. Sci. Rep. 2023, 13, 3550. [Google Scholar] [CrossRef]
- Courtejoie, N.; Cauchemez, S.; Zanella, G.; Durand, B. A network-based approach to modelling bluetongue spread in France. Prev. Vet. Med. 2019, 170, 104744. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Bowers, R.G.; Baylis, M. Modelling bluetongue virus transmission between farms using animal and vector movements. Sci. Rep. 2012, 2, 319. [Google Scholar] [CrossRef] [PubMed]

| BTV Epidemic Dataset | λ0 (10−6 day−1) | α | r0 (km) | Reference |
|---|---|---|---|---|
| BTV-3 Netherlands-2023 | 22 (19, 25) | 2.9 (2.7, 3.2) | 26 (22, 29) | This study |
| BTV-8 Germany-2006 | 9.2 (6.6, 13.4) | 3.2 (2.9, 3.7) | 18.0 (13.5, 23.0) | [18] |
| BTV-8 Europe-2007 without 2006 infected area | 3.2 (2.9, 3.8) | 2.6 (2.57, 2.62) | 21.8 (19.5, 24.3) | [5] |
| BTV-8 Europe-2007 without 2006 infected farm areas | 8.3 (7.2, 9.5) | 2.9 (2.8, 3.0) | 22.5 (20.2, 24.9) | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boender, G.-J.; Hagenaars, T.J.; Holwerda, M.; Spierenburg, M.A.H.; van Rijn, P.A.; van der Spek, A.N.; Elbers, A.R.W. Spatial Transmission Characteristics of the Bluetongue Virus Serotype 3 Epidemic in The Netherlands, 2023. Viruses 2024, 16, 625. https://doi.org/10.3390/v16040625
Boender G-J, Hagenaars TJ, Holwerda M, Spierenburg MAH, van Rijn PA, van der Spek AN, Elbers ARW. Spatial Transmission Characteristics of the Bluetongue Virus Serotype 3 Epidemic in The Netherlands, 2023. Viruses. 2024; 16(4):625. https://doi.org/10.3390/v16040625
Chicago/Turabian StyleBoender, Gert-Jan, Thomas J. Hagenaars, Melle Holwerda, Marcel A. H. Spierenburg, Piet A. van Rijn, Arco N. van der Spek, and Armin R. W. Elbers. 2024. "Spatial Transmission Characteristics of the Bluetongue Virus Serotype 3 Epidemic in The Netherlands, 2023" Viruses 16, no. 4: 625. https://doi.org/10.3390/v16040625
APA StyleBoender, G.-J., Hagenaars, T. J., Holwerda, M., Spierenburg, M. A. H., van Rijn, P. A., van der Spek, A. N., & Elbers, A. R. W. (2024). Spatial Transmission Characteristics of the Bluetongue Virus Serotype 3 Epidemic in The Netherlands, 2023. Viruses, 16(4), 625. https://doi.org/10.3390/v16040625






