Mechanisms by Which SARS-CoV-2 Invades and Damages the Central Nervous System: Apart from the Immune Response and Inflammatory Storm, What Else Do We Know?
Abstract
:1. Introduction
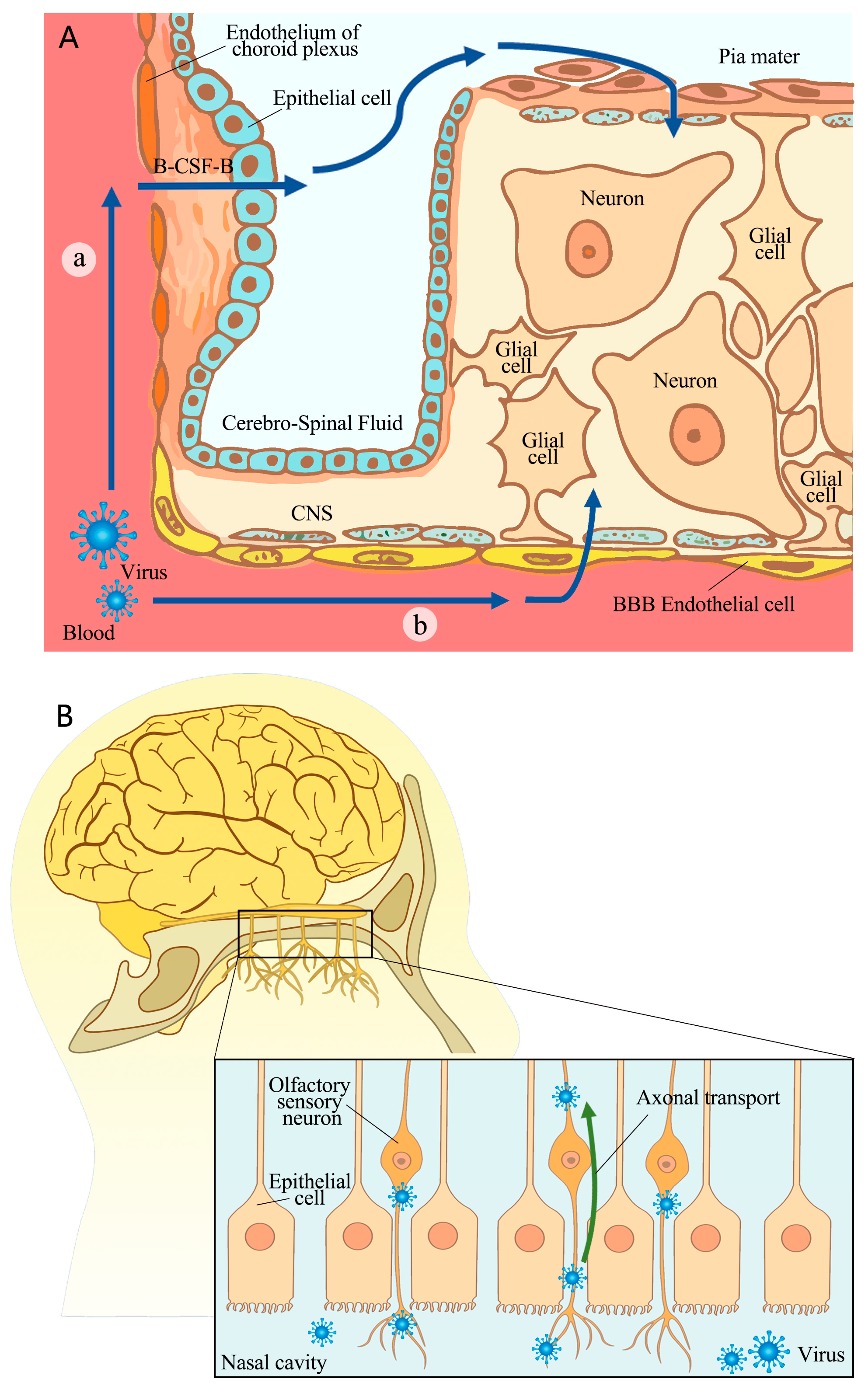
2. The Pathway of SARS-CoV-2’s Invasion of the CNS
2.1. Crossing BBB or B-CSF-B
2.2. Nerve Invasion
3. Mechanisms of SARS-CoV-2’s Impact and Damage on CNS
3.1. Direct Damage to Neurons and Glial Cells
3.1.1. Neurons
3.1.2. Glial Cells
3.2. Damage to the Olfactory System
3.3. Vascular and Pericyte Lesions
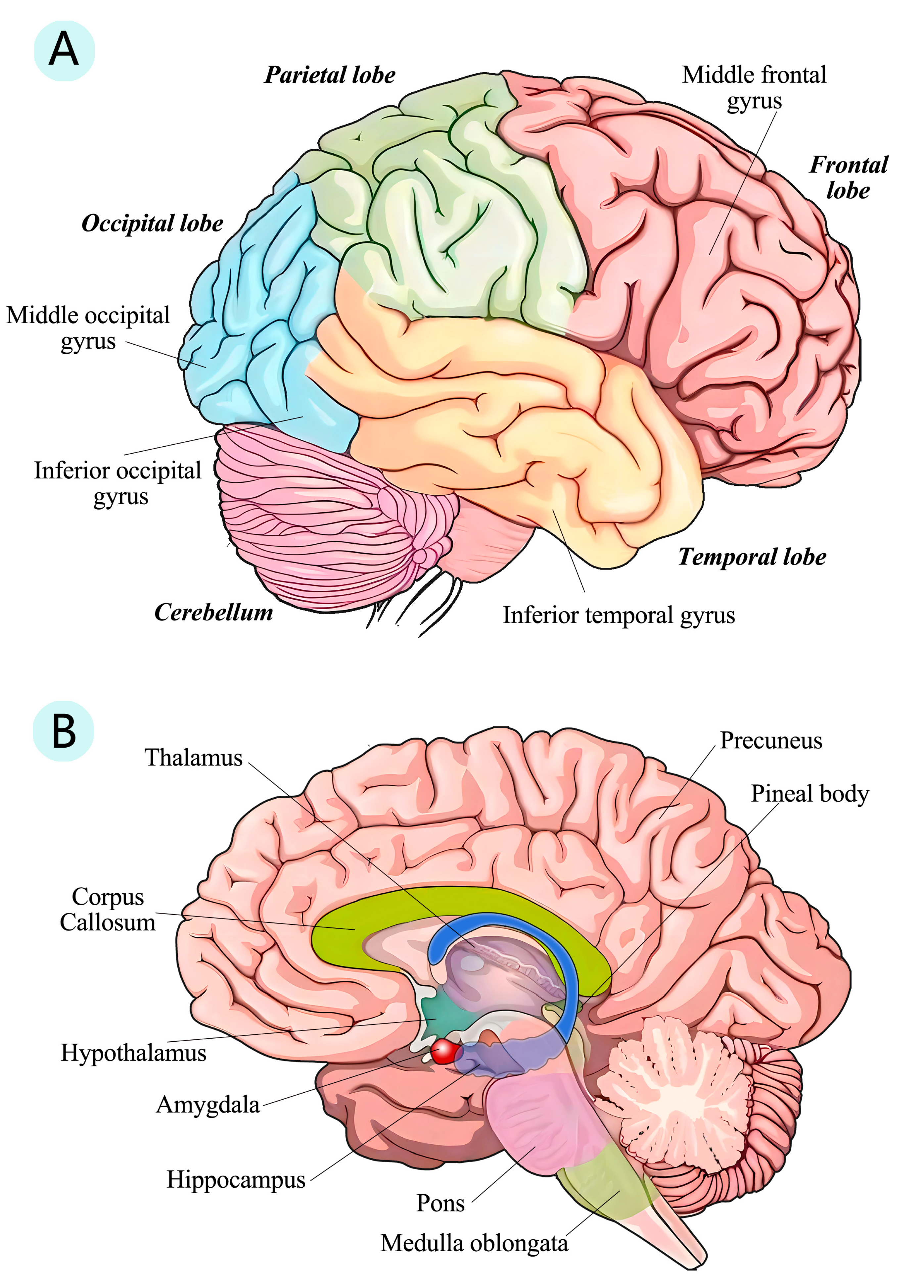
3.4. Structure Changes in Human Brain
3.5. Possible Indirect Affects
3.5.1. Lung
3.5.2. Gut and Intestinal Flora
4. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the Frequency and Variety of Persistent Symptoms Among Patients With COVID-19: A Systematic Review. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Takao, M.; Ohira, M. Neurological post-acute sequelae of SARS-CoV-2 infection. Psychiatry Clin. Neurosci. 2023, 77, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Schou, T.M.; Joca, S.; Wegener, G.; Bay-Richter, C. Psychiatric and neuropsychiatric sequelae of COVID-19—A systematic review. Brain Behav. Immun. 2021, 97, 328–348. [Google Scholar] [CrossRef] [PubMed]
- Netland, J.; Meyerholz, D.K.; Moore, S.; Cassell, M.; Perlman, S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008, 82, 7264–7275. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhong, S.; Liu, J.; Li, L.; Li, Y.; Wu, X.; Li, Z.; Deng, P.; Zhang, J.; Zhong, N.; et al. Detection of severe acute respiratory syndrome coronavirus in the brain: Potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005, 41, 1089–1096. [Google Scholar] [CrossRef]
- McCray, P.B., Jr.; Pewe, L.; Wohlford-Lenane, C.; Hickey, M.; Manzel, L.; Shi, L.; Netland, J.; Jia, H.P.; Halabi, C.; Sigmund, C.D.; et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007, 81, 813–821. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Harthi, A.; Hussein, J.; Bouchama, A.; Johani, S.; Hajeer, A.H.; Saeed, B.T.; Wahbi, A.; Saedy, A.; AlDabbagh, T.; et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV). Infection 2015, 43, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.; Müller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.; Zaki, A.; Fouchier, R.A.; et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef]
- Li, K.; Wohlford-Lenane, C.; Perlman, S.; Zhao, J.; Jewell, A.K.; Reznikov, L.R.; Gibson-Corley, K.N.; Meyerholz, D.K.; McCray, P.B., Jr. Middle East Respiratory Syndrome Coronavirus Causes Multiple Organ Damage and Lethal Disease in Mice Transgenic for Human Dipeptidyl Peptidase 4. J. Infect. Dis. 2016, 213, 712–722. [Google Scholar] [CrossRef]
- Arbour, N.; Day, R.; Newcombe, J.; Talbot, P.J. Neuroinvasion by human respiratory coronaviruses. J. Virol. 2000, 74, 8913–8921. [Google Scholar] [CrossRef] [PubMed]
- Meessen-Pinard, M.; Le Coupanec, A.; Desforges, M.; Talbot, P.J. Pivotal Role of Receptor-Interacting Protein Kinase 1 and Mixed Lineage Kinase Domain-Like in Neuronal Cell Death Induced by the Human Neuroinvasive Coronavirus OC43. J. Virol. 2017, 91, e01513-16. [Google Scholar] [CrossRef] [PubMed]
- Jacomy, H.; Fragoso, G.; Almazan, G.; Mushynski, W.E.; Talbot, P.J. Human coronavirus OC43 infection induces chronic encephalitis leading to disabilities in BALB/C mice. Virology 2006, 349, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Dubé, M.; Le Coupanec, A.; Wong, A.H.M.; Rini, J.M.; Desforges, M.; Talbot, P.J. Axonal Transport Enables Neuron-to-Neuron Propagation of Human Coronavirus OC43. J. Virol. 2018, 92, e00404-18. [Google Scholar] [CrossRef] [PubMed]
- Arbour, N.; Ekandé, S.; Côté, G.; Lachance, C.; Chagnon, F.; Tardieu, M.; Cashman, N.R.; Talbot, P.J. Persistent infection of human oligodendrocytic and neuroglial cell lines by human coronavirus 229E. J. Virol. 1999, 73, 3326–3337. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Spencer, P.S.; Reis, J.; Buguet, A.; Faris, M.E.A.; Katrak, S.M.; Láinez, M.; Medina, M.T.; Meshram, C.; Mizusawa, H.; et al. The neurology of COVID-19 revisited: A proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J. Neurol. Sci. 2020, 414, 116884. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Rao, Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat. Rev. Microbiol. 2021, 19, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Wu, L.A.; Wang, Q.; Qi, J.; Gao, G.F. Cell entry by SARS-CoV-2. Trends Biochem. Sci. 2021, 46, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Malik, J.R.; Acharya, A.; Avedissian, S.N.; Byrareddy, S.N.; Fletcher, C.V.; Podany, A.T.; Dyavar, S.R. ACE-2, TMPRSS2, and Neuropilin-1 Receptor Expression on Human Brain Astrocytes and Pericytes and SARS-CoV-2 Infection Kinetics. Int. J. Mol. Sci. 2023, 24, 8622. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Ariño, H.; Heartshorne, R.; Michael, B.D.; Nicholson, T.R.; Vincent, A.; Pollak, T.A.; Vogrig, A. Neuroimmune disorders in COVID-19. J. Neurol. 2022, 269, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.D.; Liu, M.Q.; Chen, Y.; Shan, C.; Zhou, Y.W.; Shen, X.R.; Li, Q.; Zhang, L.; Zhu, Y.; Si, H.R.; et al. Pathogenesis of SARS-CoV-2 in Transgenic Mice Expressing Human Angiotensin-Converting Enzyme 2. Cell 2020, 182, 50–58.e58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, L.; Bao, L.; Liu, J.; Zhu, H.; Lv, Q.; Liu, R.; Chen, W.; Tong, W.; Wei, Q.; et al. SARS-CoV-2 crosses the blood-brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct. Target. Ther. 2021, 6, 337. [Google Scholar] [CrossRef]
- Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.J.; Raber, J.; Banks, W.A.; Erickson, M.A. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat. Neurosci. 2021, 24, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Krasemann, S.; Haferkamp, U.; Pfefferle, S.; Woo, M.S.; Heinrich, F.; Schweizer, M.; Appelt-Menzel, A.; Cubukova, A.; Barenberg, J.; Leu, J.; et al. The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Rep. 2022, 17, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.; Albecka, A.; Mallery, D.L.; Kellner, M.J.; Paul, D.; Carter, A.P.; James, L.C.; Lancaster, M.A. SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids. Cell Stem Cell 2020, 27, 951–961.e955. [Google Scholar] [CrossRef]
- Stüdle, C.; Nishihara, H.; Wischnewski, S.; Kulsvehagen, L.; Perriot, S.; Ishikawa, H.; Schroten, H.; Frank, S.; Deigendesch, N.; Du Pasquier, R.; et al. SARS-CoV-2 infects epithelial cells of the blood-cerebrospinal fluid barrier rather than endothelial cells or pericytes of the blood-brain barrier. Fluids Barriers CNS 2023, 20, 76. [Google Scholar] [CrossRef]
- Erickson, M.A.; Rhea, E.M.; Knopp, R.C.; Banks, W.A. Interactions of SARS-CoV-2 with the Blood-Brain Barrier. Int. J. Mol. Sci. 2021, 22, 2681. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Vidal, E.; López-Figueroa, C.; Rodon, J.; Pérez, M.; Brustolin, M.; Cantero, G.; Guallar, V.; Izquierdo-Useros, N.; Carrillo, J.; Blanco, J.; et al. Chronological brain lesions after SARS-CoV-2 infection in hACE2-transgenic mice. Vet. Pathol. 2022, 59, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Bulfamante, G.; Chiumello, D.; Canevini, M.P.; Priori, A.; Mazzanti, M.; Centanni, S.; Felisati, G. First ultrastructural autoptic findings of SARS -Cov-2 in olfactory pathways and brainstem. Minerva Anestesiol. 2020, 86, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Vitale-Cross, L.; Szalayova, I.; Scoggins, A.; Palkovits, M.; Mezey, E. SARS-CoV-2 entry sites are present in all structural elements of the human glossopharyngeal and vagal nerves: Clinical implications. EBioMedicine 2022, 78, 103981. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chen, C.; Wu, Y.; Wu, M.; Lin, L. Advances in miR-132-Based Biomarker and Therapeutic Potential in the Cardiovascular System. Front. Pharmacol. 2021, 12, 751487. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Clijsters, M.; Choi, S.; Backaert, W.; Claerhout, M.; Couvreur, F.; Van Breda, L.; Bourgeois, F.; Speleman, K.; Klein, S.; et al. Anatomical barriers against SARS-CoV-2 neuroinvasion at vulnerable interfaces visualized in deceased COVID-19 patients. Neuron 2022, 110, 3919–3935.e3916. [Google Scholar] [CrossRef] [PubMed]
- McGavern, D.B.; Kang, S.S. Illuminating viral infections in the nervous system. Nat. Rev. Immunol. 2011, 11, 318–329. [Google Scholar] [CrossRef]
- Klein, R.S. Mechanisms of coronavirus infectious disease 2019-related neurologic diseases. Curr. Opin. Neurol. 2022, 35, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Frontera, J.; Placantonakis, D.G.; Lighter, J.; Galetta, S.; Balcer, L.; Melmed, K.R. Cerebrospinal fluid in COVID-19: A systematic review of the literature. J. Neurol. Sci. 2021, 421, 117316. [Google Scholar] [CrossRef]
- Plantone, D.; Locci, S.; Bergantini, L.; Manco, C.; Cortese, R.; Meocci, M.; Cavallaro, D.; d’Alessandro, M.; Bargagli, E.; De Stefano, N. Brain neuronal and glial damage during acute COVID-19 infection in absence of clinical neurological manifestations. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1343–1348. [Google Scholar] [CrossRef]
- Mesci, P.; de Souza, J.S.; Martin-Sancho, L.; Macia, A.; Saleh, A.; Yin, X.; Snethlage, C.; Adams, J.W.; Avansini, S.H.; Herai, R.H.; et al. SARS-CoV-2 infects human brain organoids causing cell death and loss of synapses that can be rescued by treatment with Sofosbuvir. PLoS Biol. 2022, 20, e3001845. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef] [PubMed]
- Giordano-Santini, R.; Linton, C.; Hilliard, M.A. Cell-cell fusion in the nervous system: Alternative mechanisms of development, injury, and repair. Semin. Cell Dev. Biol. 2016, 60, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mármol, R.; Giordano-Santini, R.; Kaulich, E.; Cho, A.N.; Przybyla, M.; Riyadh, M.A.; Robinson, E.; Chew, K.Y.; Amor, R.; Meunier, F.A.; et al. SARS-CoV-2 infection and viral fusogens cause neuronal and glial fusion that compromises neuronal activity. Sci. Adv. 2023, 9, eadg2248. [Google Scholar] [CrossRef]
- Maury, A.; Lyoubi, A.; Peiffer-Smadja, N.; de Broucker, T.; Meppiel, E. Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: A narrative review for clinicians. Rev. Neurol. 2021, 177, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Crunfli, F.; Carregari, V.C.; Veras, F.P.; Silva, L.S.; Nogueira, M.H.; Antunes, A.; Vendramini, P.H.; Valença, A.G.F.; Brandão-Teles, C.; Zuccoli, G.D.S.; et al. Morphological, cellular, and molecular basis of brain infection in COVID-19 patients. Proc. Natl. Acad. Sci. USA 2022, 119, e2200960119. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Montano, M.; Corley, M.J.; Helmy, E.; Kobayashi, H.; Kinisu, M.; Suryawanshi, R.; Luo, X.; Royer, L.A.; Roan, N.R.; et al. Neuropilin-1 Mediates SARS-CoV-2 Infection of Astrocytes in Brain Organoids, Inducing Inflammation Leading to Dysfunction and Death of Neurons. mBio 2022, 13, e0230822. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Jin, S.Y.; Yang, J.M.; Gao, T.M. The Memory Orchestra: Contribution of Astrocytes. Neurosci. Bull. 2023, 39, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Aceti, A.; Margarucci, L.M.; Scaramucci, E.; Orsini, M.; Salerno, G.; Di Sante, G.; Gianfranceschi, G.; Di Liddo, R.; Valeriani, F.; Ria, F.; et al. Serum S100B protein as a marker of severity in COVID-19 patients. Sci. Rep. 2020, 10, 18665. [Google Scholar] [CrossRef]
- Fernández-Castañeda, A.; Lu, P.; Geraghty, A.C.; Song, E.; Lee, M.H.; Wood, J.; O’Dea, M.R.; Dutton, S.; Shamardani, K.; Nwangwu, K.; et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 2022, 185, 2452–2468.e2416. [Google Scholar] [CrossRef]
- Tsilioni, I.; Theoharides, T.C. Recombinant SARS-CoV-2 Spike Protein and Its Receptor Binding Domain Stimulate Release of Different Pro-Inflammatory Mediators via Activation of Distinct Receptors on Human Microglia Cells. Mol. Neurobiol. 2023, 60, 6704–6714. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, E.A.; Amarilla, A.A.; Modhiran, N.; Parker, S.; Li, X.X.; Wijesundara, D.K.; Aguado, J.; Zamora, A.P.; McMillan, C.L.D.; Liang, B.; et al. SARS-CoV-2 drives NLRP3 inflammasome activation in human microglia through spike protein. Mol. Psychiatry 2023, 28, 2878–2893. [Google Scholar] [CrossRef] [PubMed]
- Schwabenland, M.; Salié, H.; Tanevski, J.; Killmer, S.; Lago, M.S.; Schlaak, A.E.; Mayer, L.; Matschke, J.; Püschel, K.; Fitzek, A.; et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity 2021, 54, 1594–1610.e1511. [Google Scholar] [CrossRef] [PubMed]
- Kase, Y.; Sonn, I.; Goto, M.; Murakami, R.; Sato, T.; Okano, H. The original strain of SARS-CoV-2, the Delta variant, and the Omicron variant infect microglia efficiently, in contrast to their inability to infect neurons: Analysis using 2D and 3D cultures. Exp. Neurol. 2023, 363, 114379. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.U.; Lyu, J.; Kim, K.D.; Chung, Y.C.; Yoon, G.Y.; Lee, S.; Hwang, I.; Shin, W.H.; Ko, J.; Lee, J.Y.; et al. SARS-CoV-2 Infection of Microglia Elicits Proinflammatory Activation and Apoptotic Cell Death. Microbiol. Spectr. 2022, 10, e0109122. [Google Scholar] [CrossRef] [PubMed]
- Samudyata; Oliveira, A.O.; Malwade, S.; Rufino de Sousa, N.; Goparaju, S.K.; Gracias, J.; Orhan, F.; Steponaviciute, L.; Schalling, M.; Sheridan, S.D.; et al. SARS-CoV-2 promotes microglial synapse elimination in human brain organoids. Mol. Psychiatry 2022, 27, 3939–3950. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.K.; Cruz, N.F.; Ball, K.K.; Dienel, G.A. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. J. Neurochem. 2009, 111, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Brandebura, A.N.; Paumier, A.; Onur, T.S.; Allen, N.J. Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat. Rev. Neurosci. 2023, 24, 23–39. [Google Scholar] [CrossRef]
- Kriaučiūnaitė, K.; Kaušylė, A.; Pajarskienė, J.; Tunaitis, V.; Lim, D.; Verkhratsky, A.; Pivoriūnas, A. Immortalised Hippocampal Astrocytes from 3xTG-AD Mice Fail to Support BBB Integrity In Vitro: Role of Extracellular Vesicles in Glial-Endothelial Communication. Cell. Mol. Neurobiol. 2021, 41, 551–562. [Google Scholar] [CrossRef]
- Bercury, K.K.; Macklin, W.B. Dynamics and mechanisms of CNS myelination. Dev. Cell 2015, 32, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Khodanovich, M.Y.; Kamaeva, D.A.; Naumova, A.V. Role of Demyelination in the Persistence of Neurological and Mental Impairments after COVID-19. Int. J. Mol. Sci. 2022, 23, 1291. [Google Scholar] [CrossRef] [PubMed]
- Steadman, P.E.; Xia, F.; Ahmed, M.; Mocle, A.J.; Penning, A.R.A.; Geraghty, A.C.; Steenland, H.W.; Monje, M.; Josselyn, S.A.; Frankland, P.W. Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice. Neuron 2020, 105, 150–164.e156. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.S.; Santos, S.; Leite-Aguiar, R.; Paiva-Pereira, E.; Dos Reis, R.R.; Calazans, M.L.; Fernandes, G.G.; Antônio, L.S.; de Lima, E.V.; Kurtenbach, E.; et al. SARS-CoV-2 Spike protein alters microglial purinergic signaling. Front. Immunol. 2023, 14, 1158460. [Google Scholar] [CrossRef] [PubMed]
- Kanberg, N.; Simrén, J.; Edén, A.; Andersson, L.M.; Nilsson, S.; Ashton, N.J.; Sundvall, P.D.; Nellgård, B.; Blennow, K.; Zetterberg, H.; et al. Neurochemical signs of astrocytic and neuronal injury in acute COVID-19 normalizes during long-term follow-up. EBioMedicine 2021, 70, 103512. [Google Scholar] [CrossRef] [PubMed]
- Spinato, G.; Fabbris, C.; Polesel, J.; Cazzador, D.; Borsetto, D.; Hopkins, C.; Boscolo-Rizzo, P. Alterations in Smell or Taste in Mildly Symptomatic Outpatients With SARS-CoV-2 Infection. JAMA 2020, 323, 2089–2090. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Yoo, S.J.; Clijsters, M.; Backaert, W.; Vanstapel, A.; Speleman, K.; Lietaer, C.; Choi, S.; Hether, T.D.; Marcelis, L.; et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell 2021, 184, 5932–5949.e5915. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.C.; Chou, H.J.; Fletcher, R.B.; et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020, 6, eabc5801. [Google Scholar] [CrossRef]
- Zheng, J.; Wong, L.R.; Li, K.; Verma, A.K.; Ortiz, M.E.; Wohlford-Lenane, C.; Leidinger, M.R.; Knudson, C.M.; Meyerholz, D.K.; McCray, P.B., Jr.; et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 2021, 589, 603–607. [Google Scholar] [CrossRef]
- Zazhytska, M.; Kodra, A.; Hoagland, D.A.; Frere, J.; Fullard, J.F.; Shayya, H.; McArthur, N.G.; Moeller, R.; Uhl, S.; Omer, A.D.; et al. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell 2022, 185, 1052–1064.e1012. [Google Scholar] [CrossRef]
- Clowney, E.J.; LeGros, M.A.; Mosley, C.P.; Clowney, F.G.; Markenskoff-Papadimitriou, E.C.; Myllys, M.; Barnea, G.; Larabell, C.A.; Lomvardas, S. Nuclear aggregation of olfactory receptor genes governs their monogenic expression. Cell 2012, 151, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Vaira, L.A.; Saussez, S. Prevalence and 24-month recovery of olfactory dysfunction in COVID-19 patients: A multicentre prospective study. J. Intern. Med. 2023, 293, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef] [PubMed]
- D’Ascanio, L.; Pandolfini, M.; Cingolani, C.; Latini, G.; Gradoni, P.; Capalbo, M.; Frausini, G.; Maranzano, M.; Brenner, M.J.; Di Stadio, A. Olfactory Dysfunction in COVID-19 Patients: Prevalence and Prognosis for Recovering Sense of Smell. Otolaryngol. --Head Neck Surg. 2021, 164, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.S.; Aldhahi, M.I.; Alqahtani, F.; Altamimi, M.; Alshehri, M.M. Impact of the loss of smell on the sleep quality and fatigue level in COVID-19 survivors. Eur. Arch. Otorhinolaryngol. 2022, 279, 4443–4449. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.Y.; Chen, Z.K.; Ni, J.; Wang, T.X.; Jiang, S.Y.; Dong, H.; Qu, W.M.; Huang, Z.L.; Li, R.X. Ablation of olfactory bulb glutamatergic neurons induces depressive-like behaviors and sleep disturbances in mice. Psychopharmacology 2020, 237, 2517–2530. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Lüscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado Del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913.e907. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Lee, M.H.; Perl, D.P.; Steiner, J.; Pasternack, N.; Li, W.; Maric, D.; Safavi, F.; Horkayne-Szakaly, I.; Jones, R.; Stram, M.N.; et al. Neurovascular injury with complement activation and inflammation in COVID-19. Brain 2022, 145, 2555–2568. [Google Scholar] [CrossRef]
- Campello, E.; Bulato, C.; Spiezia, L.; Boscolo, A.; Poletto, F.; Cola, M.; Gavasso, S.; Simion, C.; Radu, C.M.; Cattelan, A.; et al. Thrombin generation in patients with COVID-19 with and without thromboprophylaxis. Clin. Chem. Lab. Med. 2021, 59, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Wang, X.; Yang, L.; Li, H.; Wang, Y.; Liu, M.; Zhao, X.; Xie, Y.; Yang, Y.; et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Liu, F.; Blair, R.; Wang, C.; Yang, H.; Mudd, J.; Currey, J.M.; Iwanaga, N.; He, J.; Mi, R.; et al. Endothelial cell infection and dysfunction, immune activation in severe COVID-19. Theranostics 2021, 11, 8076–8091. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ji, W.; Yang, H.; Chen, S.; Zhang, W.; Duan, G. Endothelial activation and dysfunction in COVID-19: From basic mechanisms to potential therapeutic approaches. Signal Transduct. Target. Ther. 2020, 5, 293. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.; Lampe, J.; Müller-Fielitz, H.; Schuster, R.; Zille, M.; Müller, K.; Krohn, M.; Körbelin, J.; Zhang, L.; Özorhan, Ü.; et al. The SARS-CoV-2 main protease M(pro) causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat. Neurosci. 2021, 24, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.R. A review of string vessels or collapsed, empty basement membrane tubes. J. Alzheimers Dis. 2010, 21, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Challa, V.R.; Thore, C.R.; Moody, D.M.; Anstrom, J.A.; Brown, W.R. Increase of white matter string vessels in Alzheimer’s disease. J. Alzheimers Dis. 2004, 6, 379–383; discussion 443–449. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Pavlovic, D.; Waldvogel, H.; Dragunow, M.; Synek, B.; Turner, C.; Faull, R.; Guan, J. String Vessel Formation is Increased in the Brain of Parkinson Disease. J. Park. Dis. 2015, 5, 821–836. [Google Scholar] [CrossRef]
- Fisher, M. Pericyte signaling in the neurovascular unit. Stroke 2009, 40 (Suppl. S3), S13–S15. [Google Scholar] [CrossRef]
- Khaddaj-Mallat, R.; Aldib, N.; Bernard, M.; Paquette, A.S.; Ferreira, A.; Lecordier, S.; Saghatelyan, A.; Flamand, L.; ElAli, A. SARS-CoV-2 deregulates the vascular and immune functions of brain pericytes via Spike protein. Neurobiol. Dis. 2021, 161, 105561. [Google Scholar] [CrossRef]
- Hirunpattarasilp, C.; James, G.; Kwanthongdee, J.; Freitas, F.; Huo, J.; Sethi, H.; Kittler, J.T.; Owens, R.J.; McCoy, L.E.; Attwell, D. SARS-CoV-2 triggers pericyte-mediated cerebral capillary constriction. Brain 2023, 146, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Secomb, T.W.; Pries, A.R. Blood viscosity in microvessels: Experiment and theory. C R. Phys. 2013, 14, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Sagris, D.; Papanikolaou, A.; Kvernland, A.; Korompoki, E.; Frontera, J.A.; Troxel, A.B.; Gavriatopoulou, M.; Milionis, H.; Lip, G.Y.H.; Michel, P.; et al. COVID-19 and ischemic stroke. Eur. J. Neurol. 2021, 28, 3826–3836. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Liu, X.; Bao, K.; Huang, C. Ischemic stroke associated with COVID-19: A systematic review and meta-analysis. J. Neurol. 2022, 269, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, G.; Zhang, X.; Zhang, M.; Lu, J.; Li, H. Altered intrinsic brain activity and functional connectivity in COVID-19 hospitalized patients at 6-month follow-up. BMC Infect. Dis. 2023, 23, 521. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, J.; Bauer, A.; Huang, R.; Wen, H.; Li, M.; Wang, T.; Xia, L.; Jiang, G. Reduced Integrity of Right Lateralized White Matter in Patients with Primary Insomnia: A Diffusion-Tensor Imaging Study. Radiology 2016, 280, 520–528. [Google Scholar] [CrossRef]
- Guo, W.; Liu, F.; Liu, Z.; Gao, K.; Xiao, C.; Chen, H.; Zhao, J. Right lateralized white matter abnormalities in first-episode, drug-naive paranoid schizophrenia. Neurosci. Lett. 2012, 531, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Mendoza-Quiñones, R.; Cabrera Muñoz, A.; Iglesias-Fuster, J.; Virués, A.V.; Zvyagintsev, M.; Edgar, J.C.; Zweerings, J.; Mathiak, K. Transdiagnostic alterations in neural emotion regulation circuits—Neural substrates of cognitive reappraisal in patients with depression and post-traumatic stress disorder. BMC Psychiatry 2022, 22, 173. [Google Scholar] [CrossRef]
- Falconer, E.; Bryant, R.; Felmingham, K.L.; Kemp, A.H.; Gordon, E.; Peduto, A.; Olivieri, G.; Williams, L.M. The neural networks of inhibitory control in posttraumatic stress disorder. J. Psychiatry Neurosci. 2008, 33, 413–422. [Google Scholar]
- Díez-Cirarda, M.; Yus, M.; Gómez-Ruiz, N.; Polidura, C.; Gil-Martínez, L.; Delgado-Alonso, C.; Jorquera, M.; Gómez-Pinedo, U.; Matias-Guiu, J.; Arrazola, J.; et al. Multimodal neuroimaging in post-COVID syndrome and correlation with cognition. Brain 2023, 146, 2142–2152. [Google Scholar] [CrossRef]
- Hillary, F.G.; Roman, C.A.; Venkatesan, U.; Rajtmajer, S.M.; Bajo, R.; Castellanos, N.D. Hyperconnectivity is a fundamental response to neurological disruption. Neuropsychology 2015, 29, 59–75. [Google Scholar] [CrossRef]
- Hillary, F.G.; Grafman, J.H. Injured Brains and Adaptive Networks: The Benefits and Costs of Hyperconnectivity. Trends Cogn. Sci. 2017, 21, 385–401. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, X.; Zhao, W.; Du, Y.; Yang, D.; Huang, Y.; Chen, Y.; Zhang, H.; Yang, G.; Liu, J.; et al. Dynamic white matter changes in recovered COVID-19 patients: A two-year follow-up study. Theranostics 2023, 13, 724–735. [Google Scholar] [CrossRef]
- Bispo, D.D.C.; Brandão, P.R.P.; Pereira, D.A.; Maluf, F.B.; Dias, B.A.; Paranhos, H.R.; von Glehn, F.; de Oliveira, A.C.P.; Regattieri, N.A.T.; Silva, L.S.; et al. Brain microstructural changes and fatigue after COVID-19. Front. Neurol. 2022, 13, 1029302. [Google Scholar] [CrossRef]
- Besteher, B.; Machnik, M.; Troll, M.; Toepffer, A.; Zerekidze, A.; Rocktäschel, T.; Heller, C.; Kikinis, Z.; Brodoehl, S.; Finke, K.; et al. Larger gray matter volumes in neuropsychiatric long-COVID syndrome. Psychiatry Res. 2022, 317, 114836. [Google Scholar] [CrossRef]
- Díez-Cirarda, M.; Yus-Fuertes, M.; Sanchez-Sanchez, R.; Gonzalez-Rosa, J.J.; Gonzalez-Escamilla, G.; Gil-Martínez, L.; Delgado-Alonso, C.; Gil-Moreno, M.J.; Valles-Salgado, M.; Cano-Cano, F.; et al. Hippocampal subfield abnormalities and biomarkers of pathologic brain changes: From SARS-CoV-2 acute infection to post-COVID syndrome. EBioMedicine 2023, 94, 104711. [Google Scholar] [CrossRef]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—Linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Deng, W.; Aimone, J.B.; Gage, F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010, 11, 339–350. [Google Scholar] [CrossRef]
- Oh, J.; Cho, W.H.; Barcelon, E.; Kim, K.H.; Hong, J.; Lee, S.J. SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death. Sci. Rep. 2022, 12, 5496. [Google Scholar] [CrossRef]
- Stępień, T.; Tarka, S.; Chmura, N.; Grzegorczyk, M.; Acewicz, A.; Felczak, P.; Wierzba-Bobrowicz, T. Influence of SARS-CoV-2 on Adult Human Neurogenesis. Cells 2023, 12, 244. [Google Scholar] [CrossRef]
- Bayat, A.H.; Azimi, H.; Hassani Moghaddam, M.; Ebrahimi, V.; Fathi, M.; Vakili, K.; Mahmoudiasl, G.R.; Forouzesh, M.; Boroujeni, M.E.; Nariman, Z.; et al. COVID-19 causes neuronal degeneration and reduces neurogenesis in human hippocampus. Apoptosis 2022, 27, 852–868. [Google Scholar] [CrossRef]
- Seighali, N.; Abdollahi, A.; Shafiee, A.; Amini, M.J.; Teymouri Athar, M.M.; Safari, O.; Faghfouri, P.; Eskandari, A.; Rostaii, O.; Salehi, A.H.; et al. The global prevalence of depression, anxiety, and sleep disorder among patients coping with Post COVID-19 syndrome (long COVID): A systematic review and meta-analysis. BMC Psychiatry 2024, 24, 105. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, Y.; Cui, X.; Cao, G.; Li, D.; Yin, H. Negative emotions and brain: Negative emotions mediates the association between structural and functional variations in emotional-related brain regions and sleep quality. Sleep. Med. 2022, 94, 8–16. [Google Scholar] [CrossRef]
- Besteher, B.; Gaser, C.; Nenadić, I. Brain Structure and Subclinical Symptoms: A Dimensional Perspective of Psychopathology in the Depression and Anxiety Spectrum. Neuropsychobiology 2020, 79, 270–283. [Google Scholar] [CrossRef]
- Taquet, M.; Sillett, R.; Zhu, L.; Mendel, J.; Camplisson, I.; Dercon, Q.; Harrison, P.J. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: An analysis of 2-year retrospective cohort studies including 1,284,437 patients. Lancet. Psychiatry 2022, 9, 815–827. [Google Scholar] [CrossRef]
- Ahmed, S.; Paramasivam, P.; Kamath, M.; Sharma, A.; Rome, S.; Murugesan, R. Genetic Exchange of Lung-Derived Exosome to Brain Causing Neuronal Changes on COVID-19 Infection. Mol. Neurobiol. 2021, 58, 5356–5368. [Google Scholar] [CrossRef]
- Bulfamante, G.; Bocci, T.; Falleni, M.; Campiglio, L.; Coppola, S.; Tosi, D.; Chiumello, D.; Priori, A. Brainstem neuropathology in two cases of COVID-19: SARS-CoV-2 trafficking between brain and lung. J. Neurol. 2021, 268, 4486–4491. [Google Scholar] [CrossRef]
- Adingupu, D.D.; Soroush, A.; Hansen, A.; Twomey, R.; Dunn, J.F. Brain hypoxia, neurocognitive impairment, and quality of life in people post-COVID-19. J. Neurol. 2023, 270, 3303–3314. [Google Scholar] [CrossRef]
- Waldrop, G.; Safavynia, S.A.; Barra, M.E.; Agarwal, S.; Berlin, D.A.; Boehme, A.K.; Brodie, D.; Choi, J.M.; Doyle, K.; Fins, J.J.; et al. Prolonged Unconsciousness is Common in COVID-19 and Associated with Hypoxemia. Ann. Neurol. 2022, 91, 740–755. [Google Scholar] [CrossRef]
- Perisetti, A.; Goyal, H.; Gajendran, M.; Boregowda, U.; Mann, R.; Sharma, N. Prevalence, Mechanisms, and Implications of Gastrointestinal Symptoms in COVID-19. Front. Med. 2020, 7, 588711. [Google Scholar] [CrossRef]
- Ancona, G.; Alagna, L.; Alteri, C.; Palomba, E.; Tonizzo, A.; Pastena, A.; Muscatello, A.; Gori, A.; Bandera, A. Gut and airway microbiota dysbiosis and their role in COVID-19 and long-COVID. Front. Immunol. 2023, 14, 1080043. [Google Scholar] [CrossRef] [PubMed]
- He, L.H.; Ren, L.F.; Li, J.F.; Wu, Y.N.; Li, X.; Zhang, L. Intestinal Flora as a Potential Strategy to Fight SARS-CoV-2 Infection. Front. Microbiol. 2020, 11, 1388. [Google Scholar] [CrossRef] [PubMed]
- Manosso, L.M.; Arent, C.O.; Borba, L.A.; Ceretta, L.B.; Quevedo, J.; Réus, G.Z. Microbiota-Gut-Brain Communication in the SARS-CoV-2 Infection. Cells 2021, 10, 1993. [Google Scholar] [CrossRef] [PubMed]
- Vakili, K.; Fathi, M.; Yaghoobpoor, S.; Sayehmiri, F.; Nazerian, Y.; Nazerian, A.; Mohamadkhani, A.; Khodabakhsh, P.; Réus, G.Z.; Hajibeygi, R.; et al. The contribution of gut-brain axis to development of neurological symptoms in COVID-19 recovered patients: A hypothesis and review of literature. Front. Cell. Infect. Microbiol. 2022, 12, 983089. [Google Scholar] [CrossRef]
- Deffner, F.; Scharr, M.; Klingenstein, S.; Klingenstein, M.; Milazzo, A.; Scherer, S.; Wagner, A.; Hirt, B.; Mack, A.F.; Neckel, P.H. Histological Evidence for the Enteric Nervous System and the Choroid Plexus as Alternative Routes of Neuroinvasion by SARS-CoV2. Front. Neuroanat. 2020, 14, 596439. [Google Scholar] [CrossRef]
| Glial Cell Type | Receptor Has Been Identified | Directly Infect Cell | Impact of SARS-CoV-2 Infection | Refs. |
|---|---|---|---|---|
| Astrocyte | NRP1 | √ | Disrupts interferon signaling and proinflammatory chemokines/cytokines Oxidative metabolism ↑ Neuronal supporting function ↓ Soluble factors releasing Affects memory formation BBB permeability ↑ | [46,47,48,49] |
| Oligodendrocyte | No direct evidence | Brain demyelination and myelin destruction Compromising neural circuits and axonal health | [50] | |
| Microglia | TLR-4 (Toll-like receptor 4), ACE2 | √ | Activation of neuroinflammatory responses Synaptic density ↓ (through phagocytosis) Purinergic receptor expression ↑ | [51,52,53,54,55,56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Shi, C.; Jin, L. Mechanisms by Which SARS-CoV-2 Invades and Damages the Central Nervous System: Apart from the Immune Response and Inflammatory Storm, What Else Do We Know? Viruses 2024, 16, 663. https://doi.org/10.3390/v16050663
Sun Z, Shi C, Jin L. Mechanisms by Which SARS-CoV-2 Invades and Damages the Central Nervous System: Apart from the Immune Response and Inflammatory Storm, What Else Do We Know? Viruses. 2024; 16(5):663. https://doi.org/10.3390/v16050663
Chicago/Turabian StyleSun, Zihan, Chunying Shi, and Lixin Jin. 2024. "Mechanisms by Which SARS-CoV-2 Invades and Damages the Central Nervous System: Apart from the Immune Response and Inflammatory Storm, What Else Do We Know?" Viruses 16, no. 5: 663. https://doi.org/10.3390/v16050663




