Phylogenomic Characterization of Ranavirus Isolated from Wild Smallmouth Bass (Micropterus dolomieu)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Histopathology
2.3. Nucleic Acid Extraction and PCR Amplification
2.4. Virus Isolation
2.5. Whole-Genome Sequencing and Phylogenetic and Genetic Analyses
3. Results
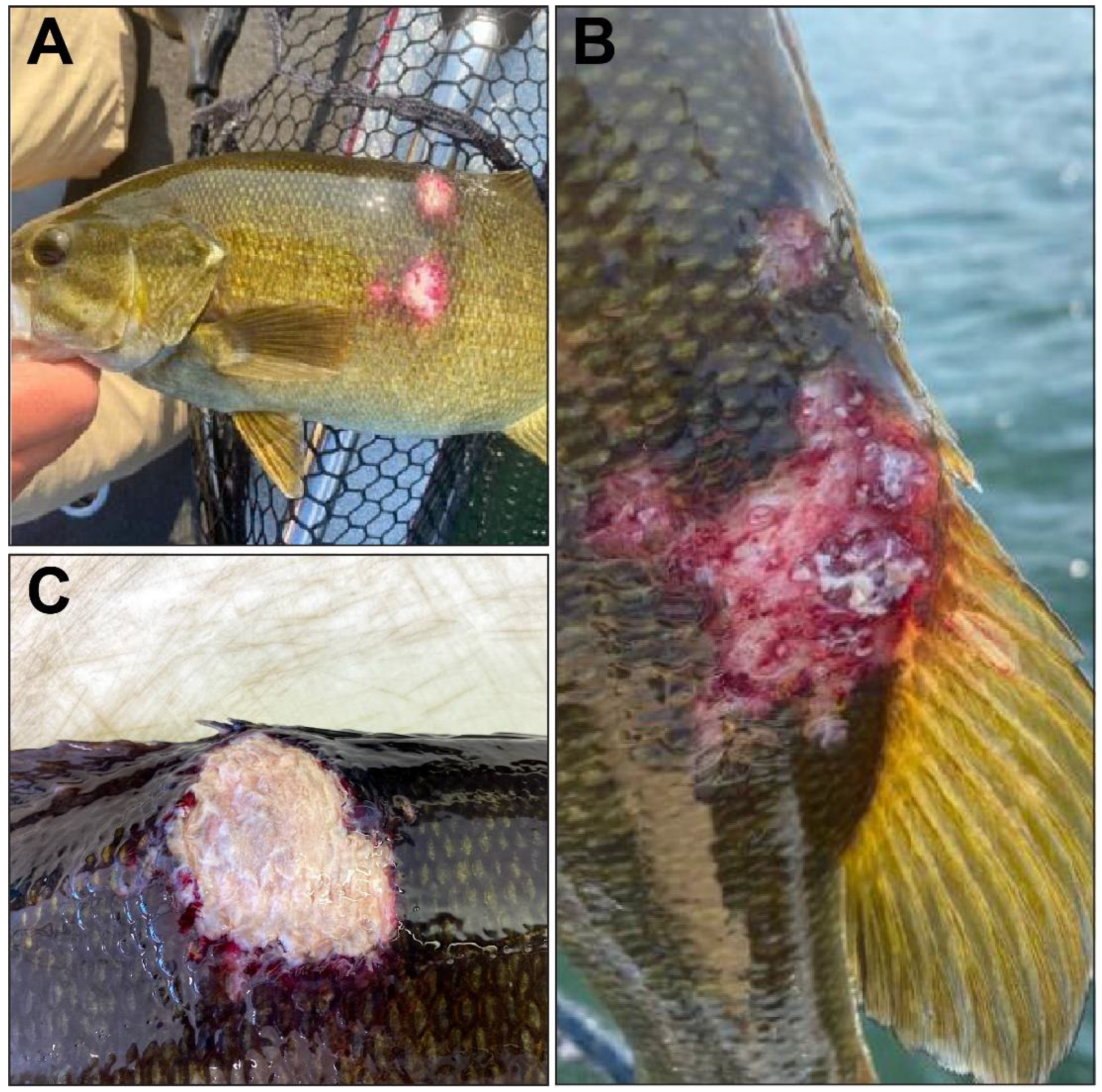
3.1. Gross Pathology and Bacteriology
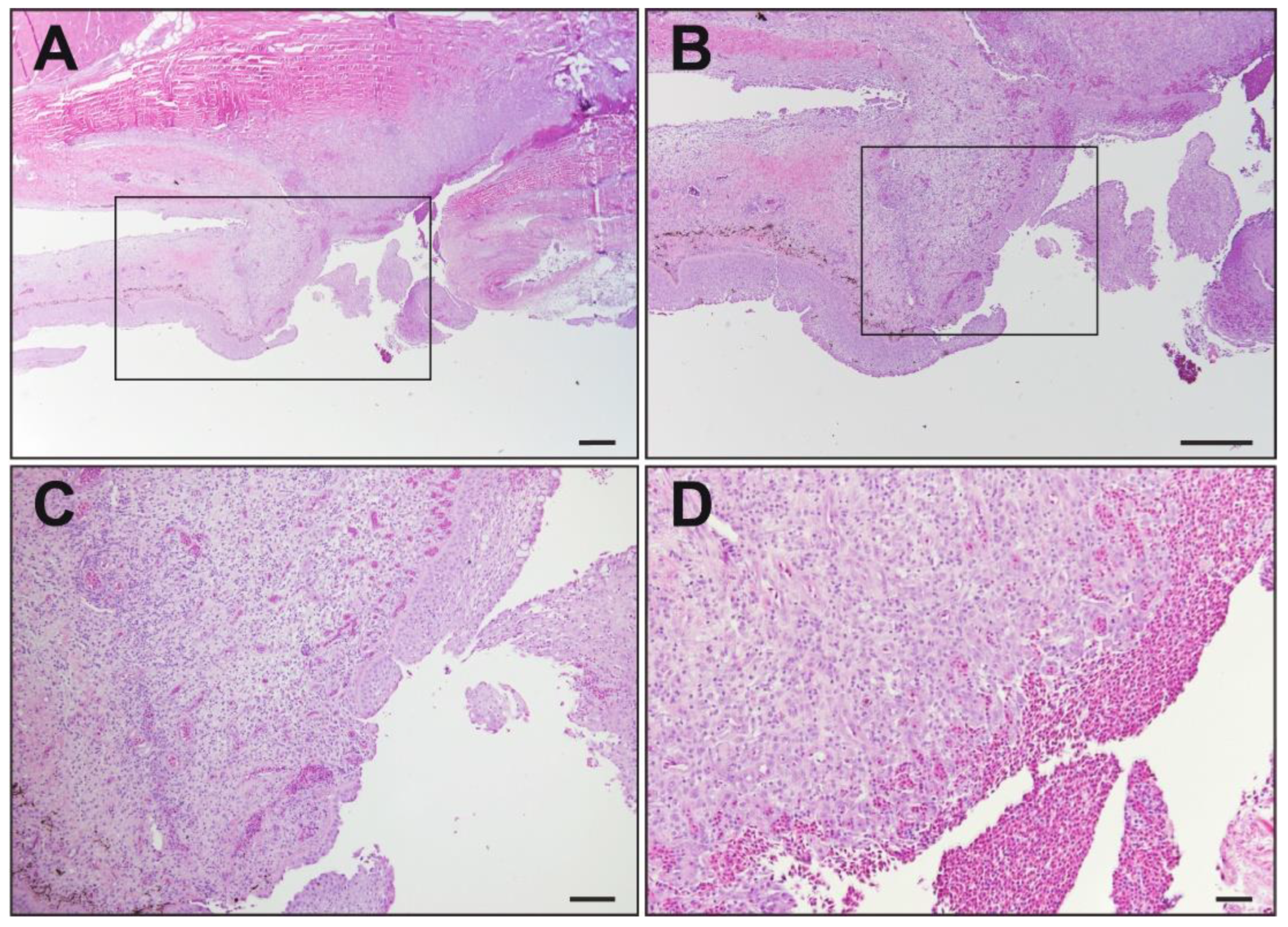
3.2. Histopathology
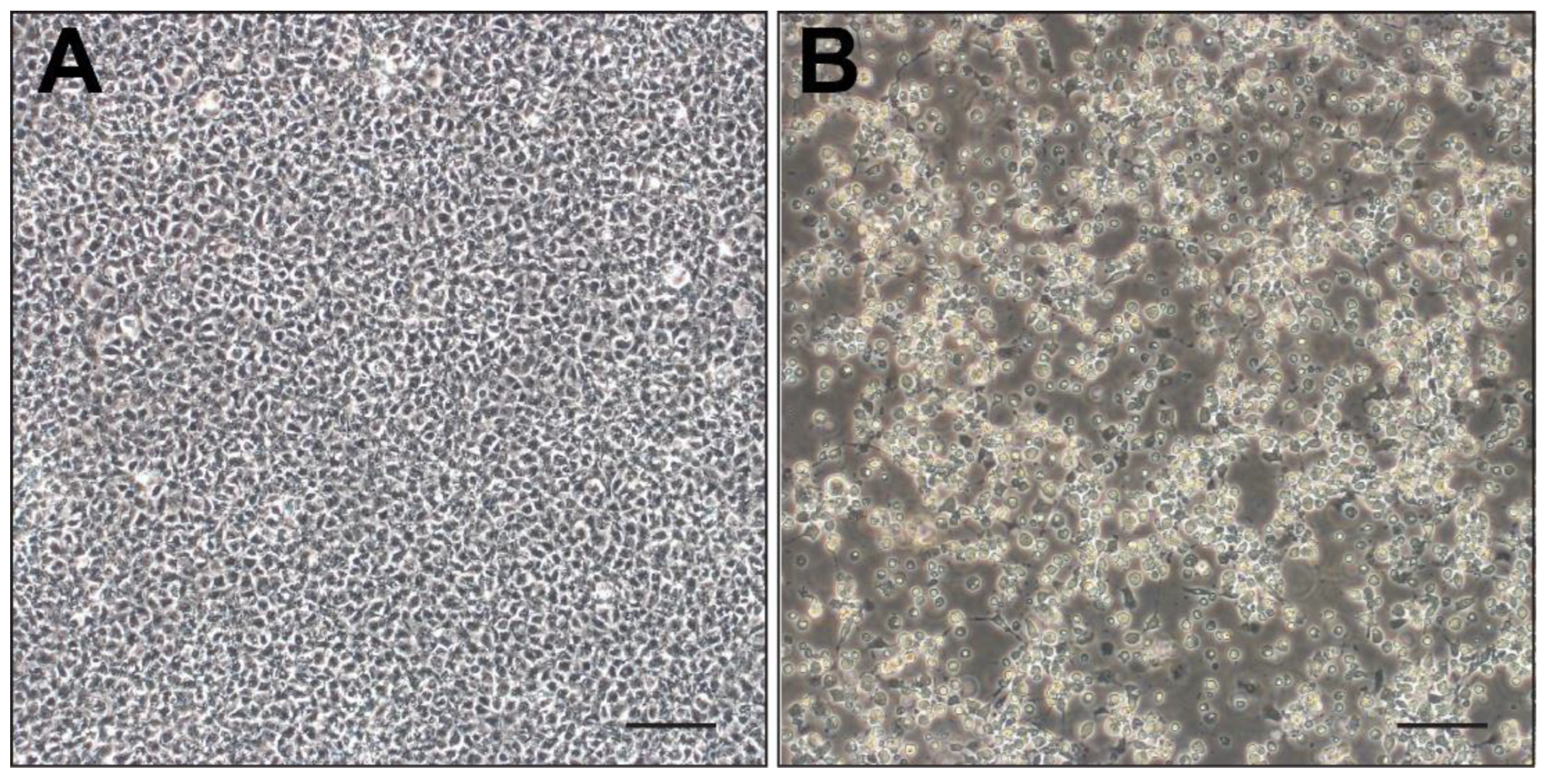
3.3. PCR Amplification and Virus Isolation
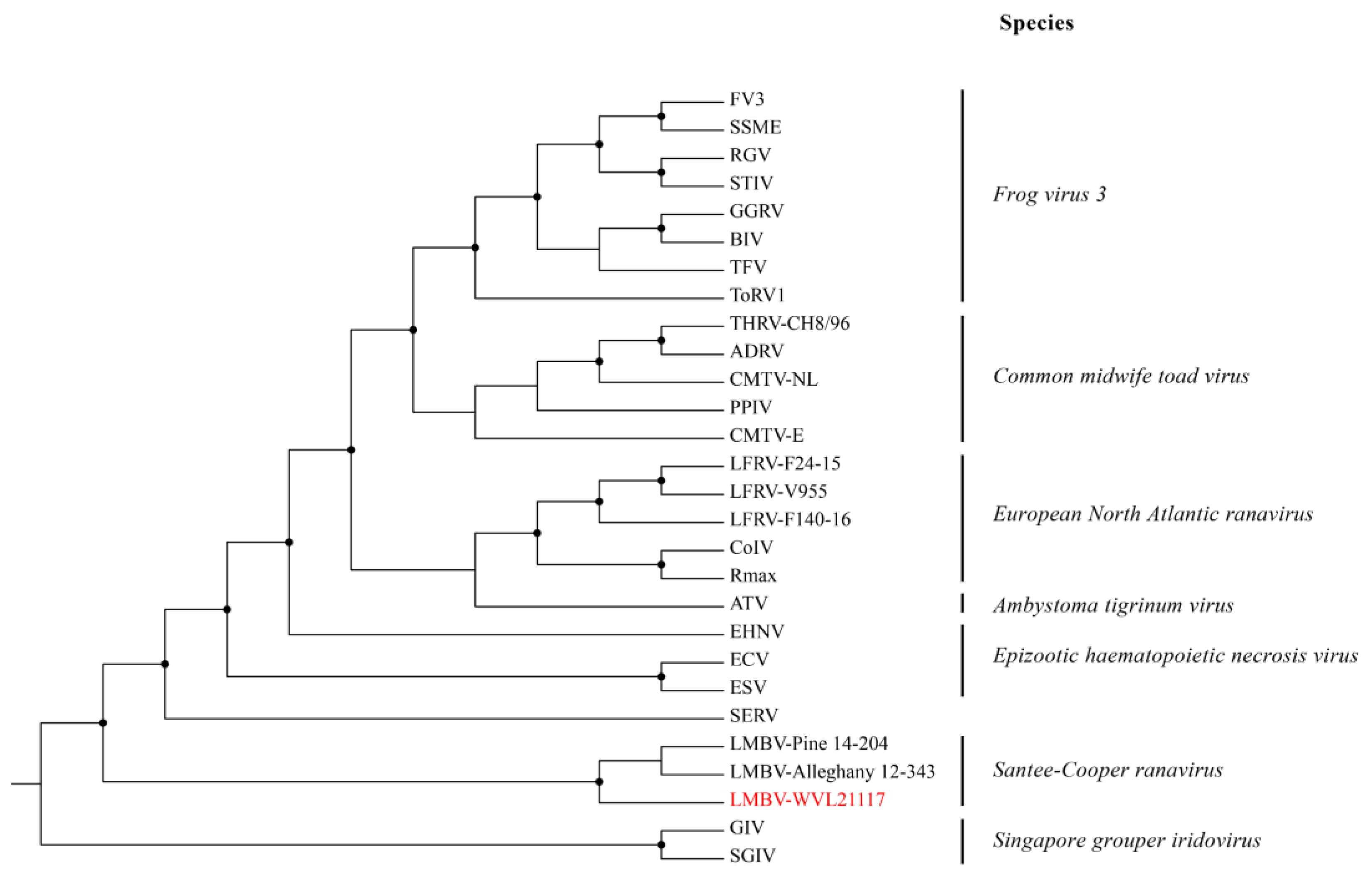
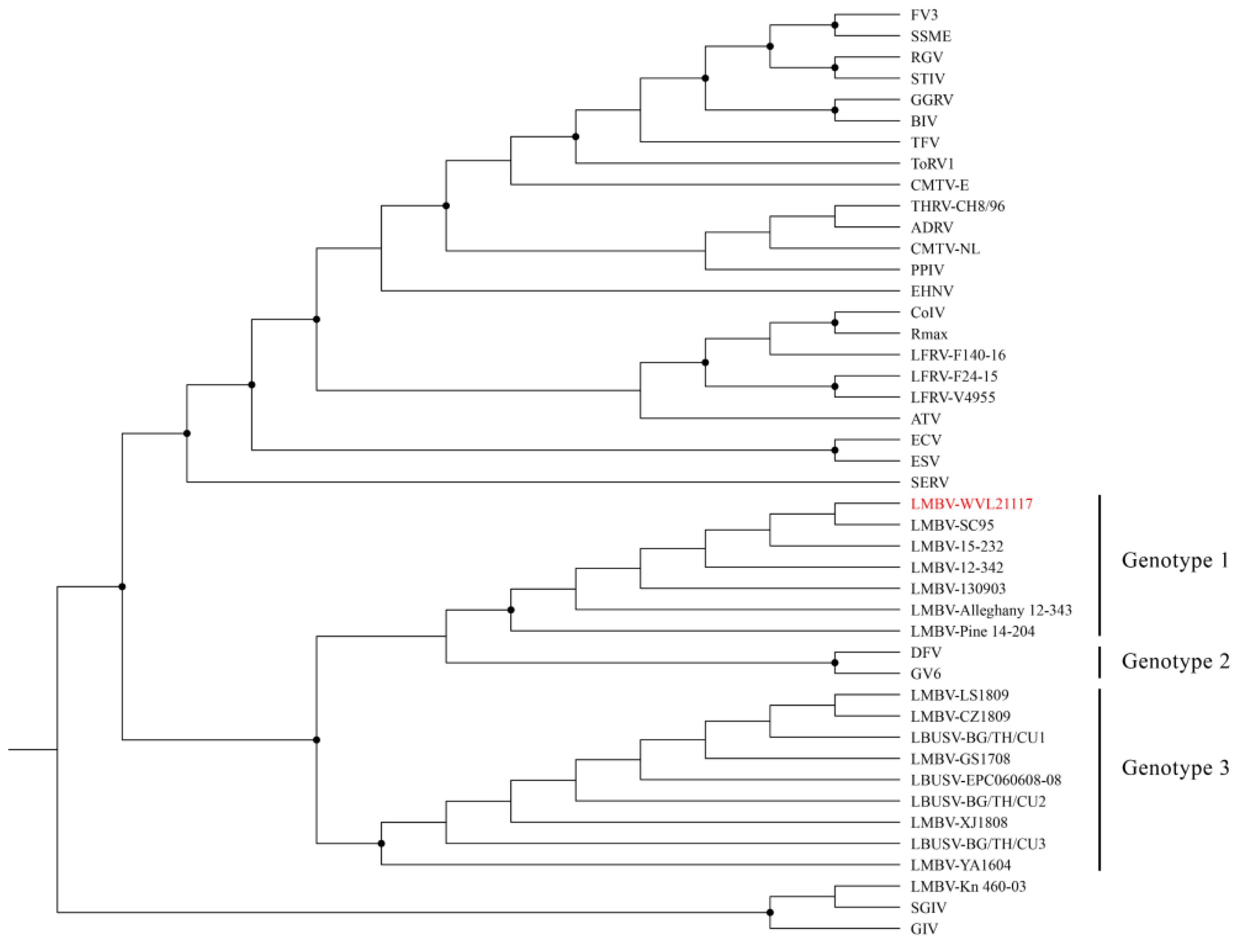
3.4. Whole-Genome Assembly and Phylogenetic and Genetic Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duffus, A.L.; Waltzek, T.B.; Stöhr, A.C.; Allender, M.C.; Gotesman, M.; Whittington, R.J.; Hick, P.; Hines, M.K.; Marschang, R.E. Distribution and host range of ranaviruses. In Ranaviruses, 1st ed.; Gray, M.J., Chinchar, V.G., Eds.; Springer: Cham, Switzerland; New York, NY, USA, 2015; pp. 9–57. [Google Scholar]
- Chinchar, V.G.; Hick, P.; Ince, I.A.; Jancovich, J.K.; Marschang, R.; Qin, Q.; Subramaniam, K.; Waltzek, T.B.; Whittington, R.; Williams, T.; et al. ICTV Virus Taxonomy Profile: Iridoviridae. J. Gen. Virol. 2017, 98, 890–891. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses. 2020. Available online: https://ictv.global/report/chapter/iridoviridae/iridoviridae/ranavirus (accessed on 28 April 2023).
- Mao, J.; Wang, J.; Chinchar, G.D.; Chinchar, V.G. Molecular characterization of a ranavirus isolated from largemouth bass Micropterus salmoides. Dis. Aquat. Org. 1999, 37, 107–114. [Google Scholar] [CrossRef]
- Hedrick, R.P.; McDowell, T.S. Properties of iridoviruses from ornamental fish. Vet. Res. 1995, 26, 423–427. [Google Scholar]
- Grizzle, J.M.; Altinok, I.; Fraser, W.A.; Francis-Floyd, R. First isolation of largemouth bass virus. Dis. Aquat. Org. 2002, 50, 233–235. [Google Scholar] [CrossRef]
- Plumb, J.A.; Grizzle, J.M.; Young, H.E.; Noyes Andrew, D.; Lamprecht, S. An Iridovirus Isolated from Wild Largemouth Bass. J. Aquat. Anim. Health 1996, 8, 265–270. [Google Scholar] [CrossRef]
- Steed, E. Micropterus salmoides (American Black Bass). Animal Diversity Web. 2015. Available online: https://animaldiversity.org/accounts/Micropterus_salmoides/#:~:text=Ecosystem%20Roles,control%20of%20the%20food%20web (accessed on 28 April 2023).
- Li, R.; Wen, Z.Y.; Zou, Y.C.; Qin, C.J.; Yuan, D.Y. Largemouth Bass Pond Culture in China: A Review. Int. J. Vet. Sci. Res. 2017, 3, 014–017. [Google Scholar] [CrossRef]
- Stepzinski, T. Researchers Study Huge Bass That Thrill Anglers in Clay County’s Kingsley Lake. The Florida Times-Union. 2016. Available online: https://www.jacksonville.com/story/news/2016/05/27/researchers-study-huge-bass-clay-county-learn-why-lake-provides-anglers/15711811007/ (accessed on 28 April 2023).
- Woodland, J.E.; Noyes, A.D.; Grizzle, J.M. A survey to detect largemouth bass virus among fish from hatcheries in the southeastern USA. Trans. Am. Fish. Soc. 2002, 131, 308–311. [Google Scholar] [CrossRef]
- Yi, W.; Zhang, X.; Zeng, K.; Xie, D.; Song, C.; Tam, K.; Liu, Z.; Zhou, T.; Li, W. Construction of a DNA vaccine and its protective effect on largemouth bass (Micropterus salmoides) challenged with largemouth bass virus (LMBV). Fish Shellfish. Immunol. 2020, 106, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Zilberg, D.; Grizzle, J.M.; Plumb, J.A. Preliminary description of lesions in juvenile largemouth bass injected with largemouth bass virus. Dis. Aquat. Org. 2000, 39, 143–146. [Google Scholar]
- Boonthai, T.; Loch, T.P.; Yamashita, C.J.; Smith, G.D.; Winters, A.D.; Kiupel, M.; Brenden, T.O.; Faisal, M. Laboratory investigation into the role of largemouth bass virus (Ranavirus, Iridoviridae) in smallmouth bass mortality events in Pennsylvania rivers. BMC Vet. Res. 2018, 14, 62. [Google Scholar] [CrossRef]
- Plumb, J.A.; Zilberg, D. The lethal dose of largemouth bass virus in juvenile largemouth bass and the comparative susceptibility of striped bass. J. Aquat. Anim. Health 1999, 11, 246–252. [Google Scholar] [CrossRef]
- Woodland, J.E.; Brunner, C.J.; Noyes, A.D.; Grizzle, J.M. Experimental oral transmission of largemouth bass virus. J. Fish Dis. 2002, 25, 669–672. [Google Scholar] [CrossRef]
- Hanson, L.A.; Petrie-Hanson, L.; Meals, K.O.; Chinchar, V.G.; Rudis, M. Persistence of largemouth bass virus infection in a northern Mississippi reservoir after a die-off. J. Aquat. Anim. Health 2001, 13, 27–34. [Google Scholar] [CrossRef]
- Fisheries and Oceans Canada, Communications Branch. 2021. Available online: https://www.dfo-mpo.gc.ca/species-especes/profiles-profils/smallmouthbass-achiganpetitebouche-eng.html (accessed on 28 April 2023).
- Blazer, V.S.; Iwanowicz, L.R.; Starliper, C.E.; Iwanowicz, D.D.; Barbash, P.; Hedrick, J.D.; Reeser, S.J.; Mullican, J.E.; Zaugg, S.D.; Burkhardt, M.R.; et al. Mortality of centrarchid fishes in the Potomac drainage: Survey results and overview of potential contributing factors. J. Aquat. Anim. Health 2010, 22, 190–218. [Google Scholar] [CrossRef]
- AFS-FHS (American Fisheries Society-Fish Health Section). FHS Blue Book: Suggested Procedures for the Detection and Identification of Certain Finfish and Shellfish Pathogens, 2020th ed.; AFS-FHS (American Fisheries Society-Fish Health Section): Corvallis, OR, USA, 2014. [Google Scholar]
- Grizzle, J.M.; Altinok, I.; Noyes, A.D. PCR method for detection of largemouth bass virus. Dis. Aquat. Org. 2003, 54, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Fijan, N.; Sulimanović, D.; Bearzotti, M.; Muzinić, D.; Zwillenberg, L.O.; Chilmonczyk, S.; Vautherot, J.F.; de Kinkelin, P. Some properties of the Epithelioma papulosum cyprini (EPC) cell line from carp cyprinus carpio. Ann. Inst. Pasteur Virol. 1983, 134, 207–220. [Google Scholar] [CrossRef]
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast Metagenomic Sequence Classification Using Exact Alignments. Genome Biol. 2014, 15, R46. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Besemer, J.; Lomsadze, A.; Borodovsky, M. GeneMarkS: A self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 2001, 29, 2607–2618. [Google Scholar] [CrossRef]
- Sriwanayos, P.; Subramaniam, K.; Stilwell, N.K.; Imnoi, K.; Popov, V.L.; Kanchanakhan, S.; Polchana, J.; Waltzek, T.B. Phylogenomic characterization of ranaviruses isolated from cultured fish and amphibians in Thailand. Facets 2020, 5, 963–979. [Google Scholar] [CrossRef]
- Waltzek, T.B.; Subramaniam, K.; Jancovich, J.K. Ranavirus Taxonomy and Phylogeny; University of Mississippi Medical Center (Department of Microbiology): Jackson, MS, USA, 2025; Book chapter in preparation. [Google Scholar]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.R. A simple procedure for identification of Cytophaga columnaris. J. Aquat. Anim. Health 1992, 4, 63–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quail, H.; Viadanna, P.H.O.; Vann, J.A.; Hsu, H.-M.; Pohly, A.; Smith, W.; Hansen, S.; Nietlisbach, N.; Godard, D.; Waltzek, T.B.; et al. Phylogenomic Characterization of Ranavirus Isolated from Wild Smallmouth Bass (Micropterus dolomieu). Viruses 2024, 16, 715. https://doi.org/10.3390/v16050715
Quail H, Viadanna PHO, Vann JA, Hsu H-M, Pohly A, Smith W, Hansen S, Nietlisbach N, Godard D, Waltzek TB, et al. Phylogenomic Characterization of Ranavirus Isolated from Wild Smallmouth Bass (Micropterus dolomieu). Viruses. 2024; 16(5):715. https://doi.org/10.3390/v16050715
Chicago/Turabian StyleQuail, Hannah, Pedro H. O. Viadanna, Jordan A. Vann, Hui-Min Hsu, Andrea Pohly, Willow Smith, Scott Hansen, Nicole Nietlisbach, Danielle Godard, Thomas B. Waltzek, and et al. 2024. "Phylogenomic Characterization of Ranavirus Isolated from Wild Smallmouth Bass (Micropterus dolomieu)" Viruses 16, no. 5: 715. https://doi.org/10.3390/v16050715
APA StyleQuail, H., Viadanna, P. H. O., Vann, J. A., Hsu, H.-M., Pohly, A., Smith, W., Hansen, S., Nietlisbach, N., Godard, D., Waltzek, T. B., & Subramaniam, K. (2024). Phylogenomic Characterization of Ranavirus Isolated from Wild Smallmouth Bass (Micropterus dolomieu). Viruses, 16(5), 715. https://doi.org/10.3390/v16050715





