First Characterization of Acinetobacter baumannii-Specific Filamentous Phages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. In Silico Identification and Classification of Filamentous Prophages in A. baumannii Strains
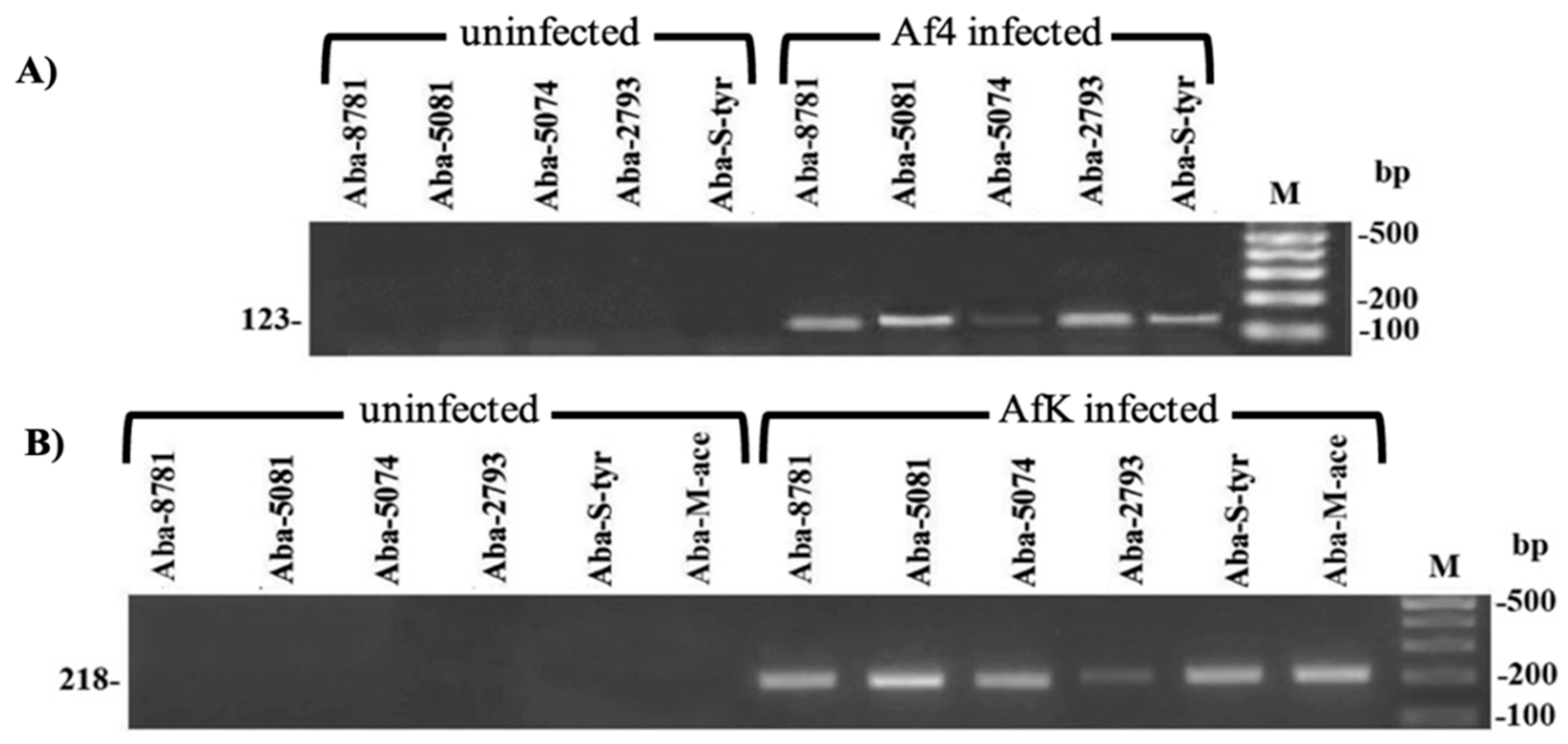
2.3. PCR Identification of Filamentous Phages in Different A. baumannii Strains
2.4. Propagation and Purification of A. baumannii-Specific Filamentous Phages
2.5. Transmission Electron Microscopy (TEM) Imaging of Isolated A. baumannii-Specific Filamentous Phages
2.6. Protein Characterization of A. baumannii-Specific Filamentous Phages
2.7. Viral DNA Isolation and Treatment with Different Enzymes
2.8. Assessment of Filamentous Phage Plaque Formation by SPOT Methods
2.9. A. baumannii-Specific Filamentous Phage Infection of Selected Strains
2.10. A. baumannii-Specific Filamentous Phages Influence on Host Phenotype
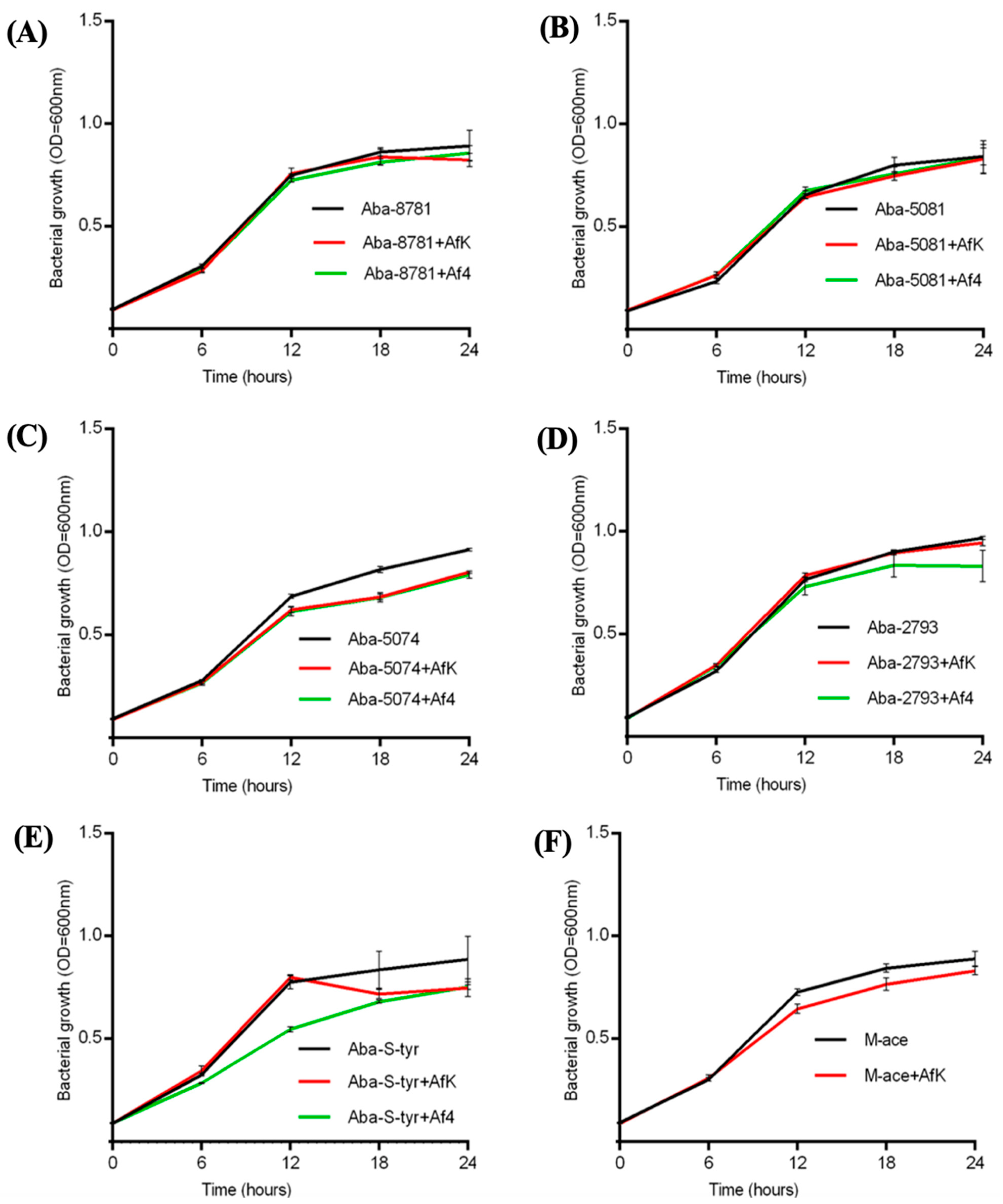
2.10.1. Growth Kinetics
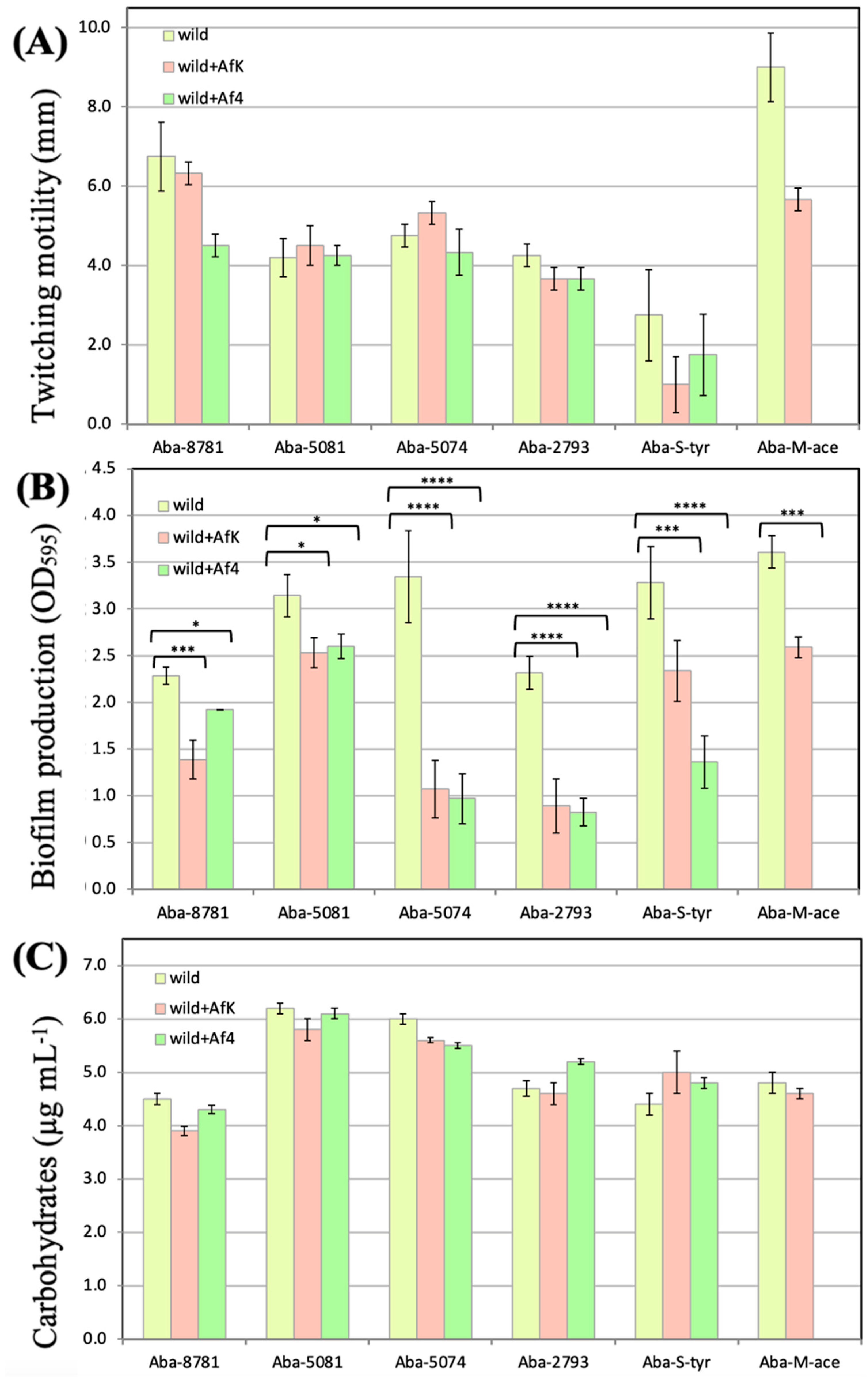
2.10.2. Twitching Motility
2.10.3. Biofilm Formation on Polystyrene Surface
2.10.4. Carbohydrate Production
2.10.5. Antibiotic Susceptibility
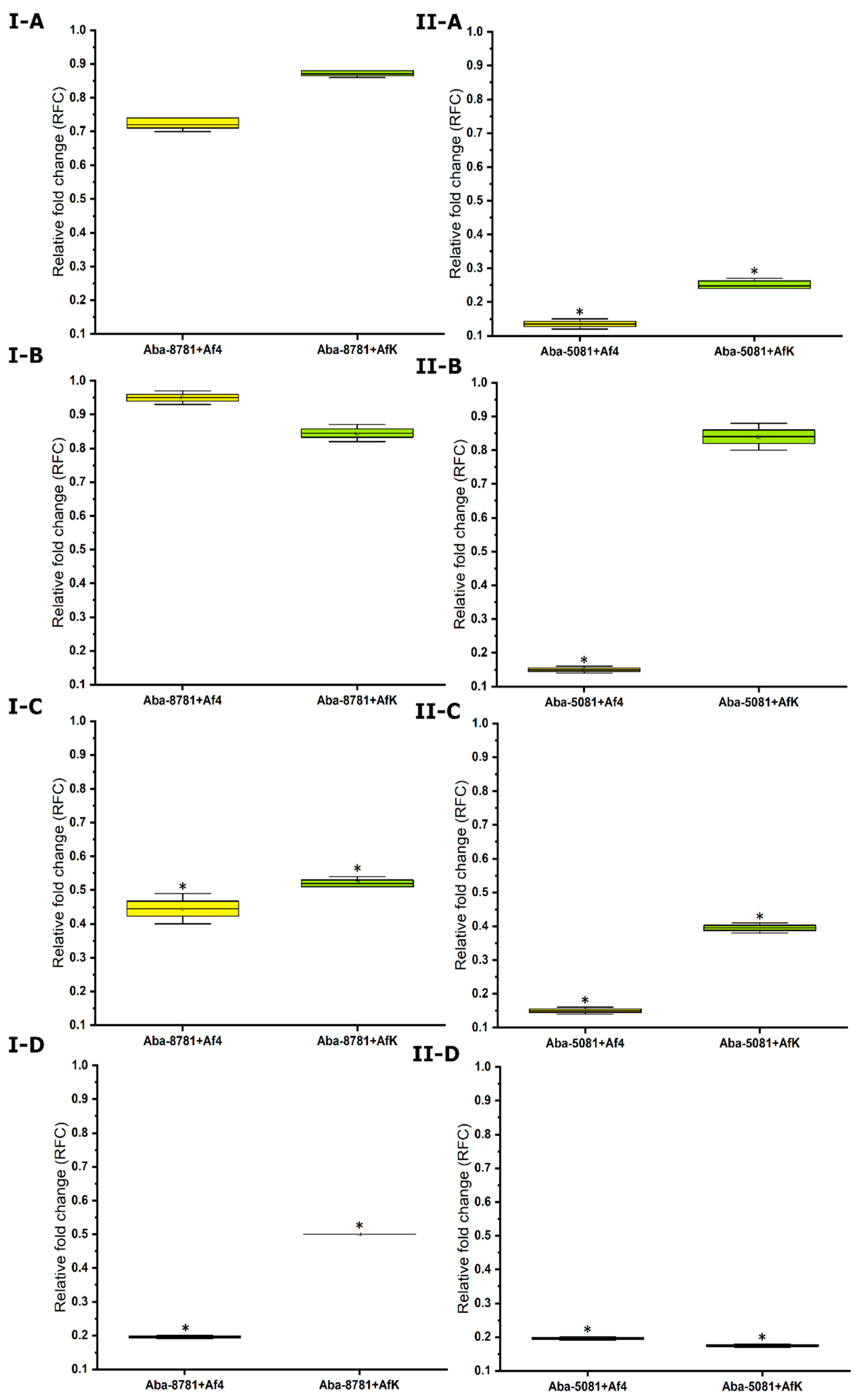
2.10.6. Expression of Efflux Pumps
3. Results
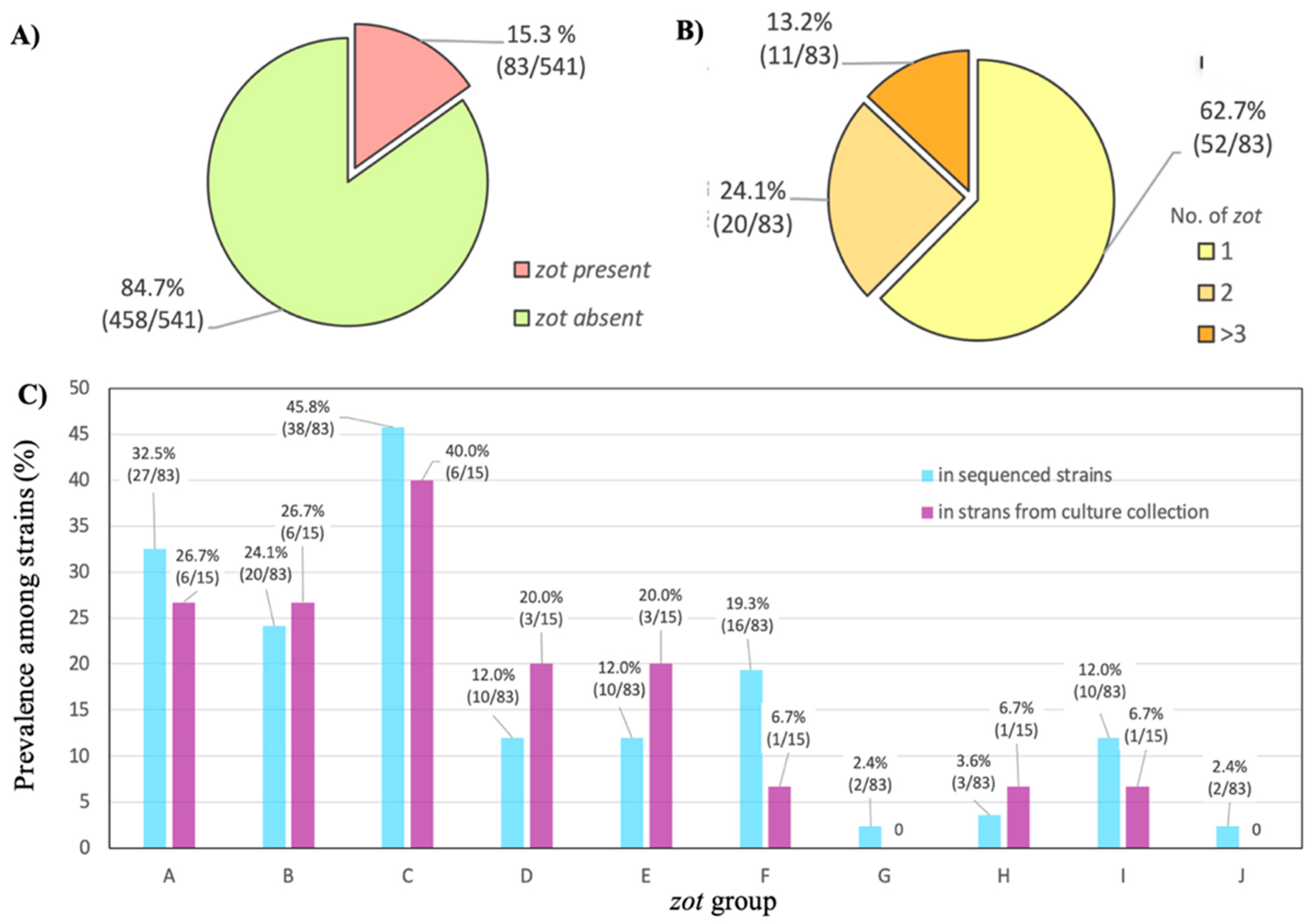
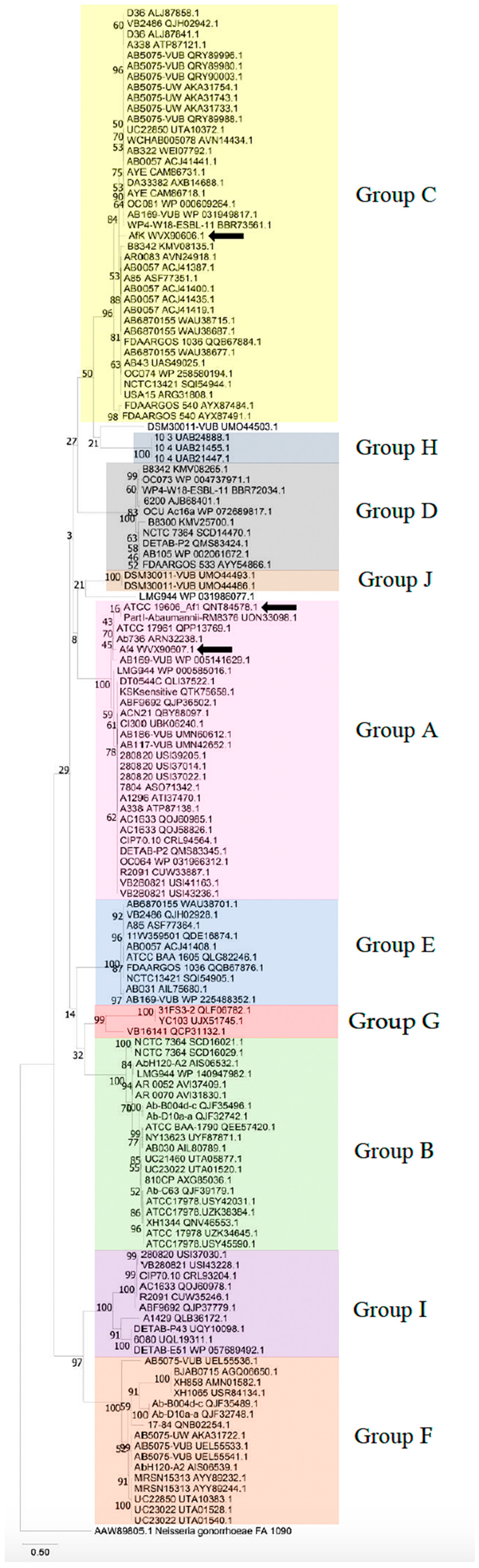
3.1. Identification and Classification of Filamentous Prophages in A. baumannii Strains
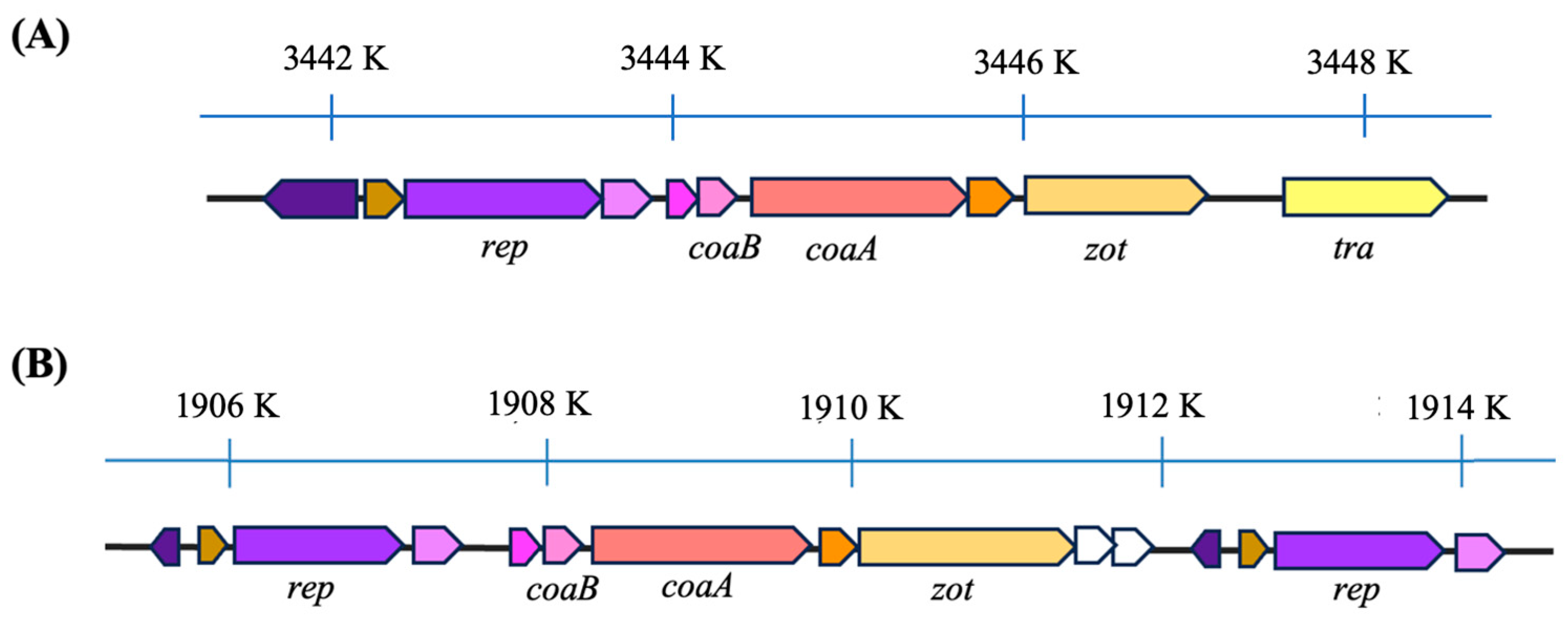
3.2. Genome Organization of Af
3.3. Morphology of Af1
3.4. Protein Profile of Af Filamentous Phages
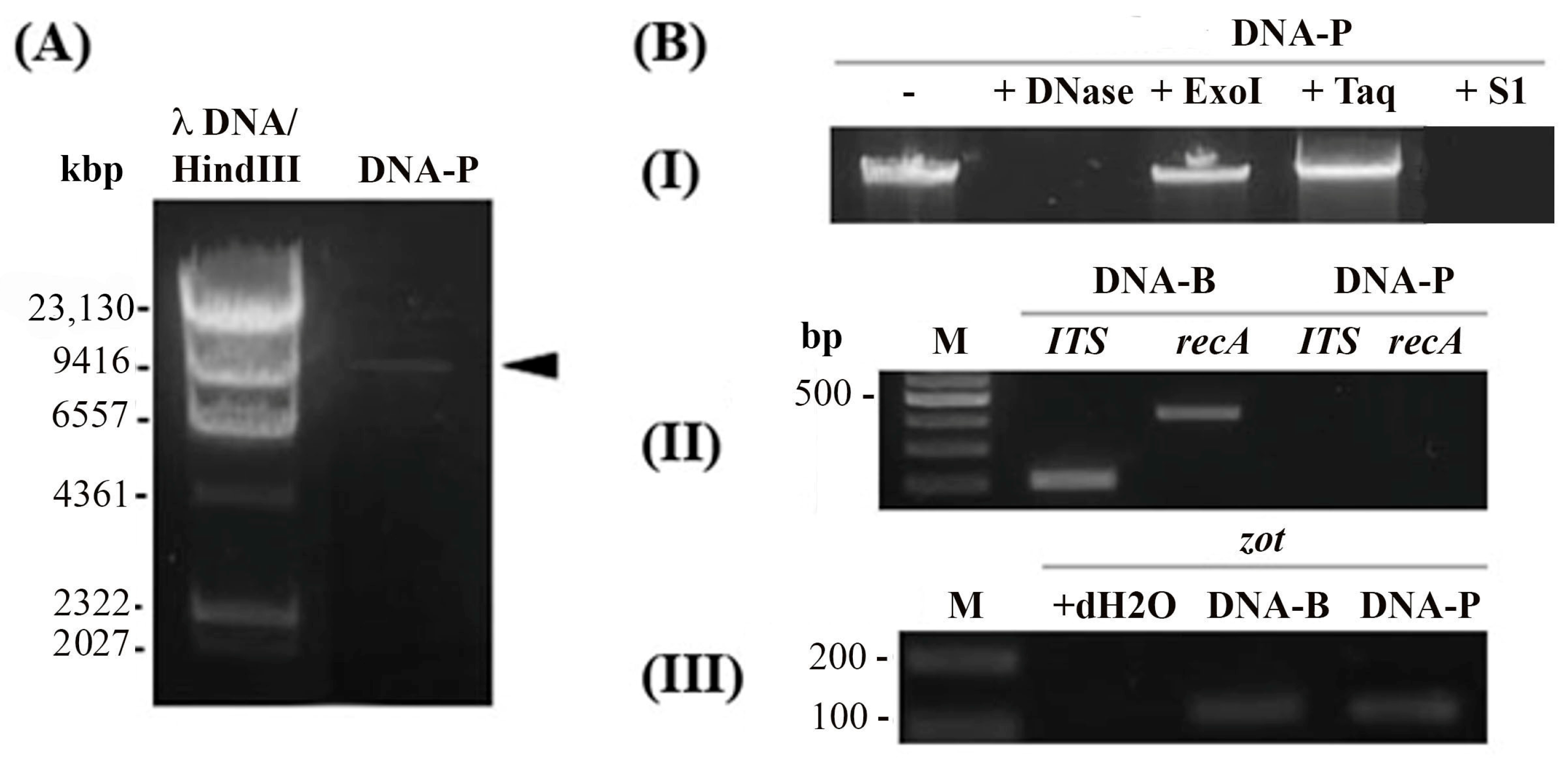
3.5. Af1 Produces Virions with ssDNA
3.6. Plaque Formation Experiments
3.7. A. baumannii Infection with Af Phages
3.8. The Influence of Afphages on the Host Phenotype
3.8.1. Growth Kinetics
3.8.2. Twitching Motility
3.8.3. Biofilm Formation on Polystyrene Surface
3.8.4. LOS Production
3.8.5. Antibiotics Susceptibility
3.8.6. Expression of Efflux Pumps
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knezevic, P.; Adriaenssens, E.M.; ICTV Report Consortium. ICTV Virus Taxonomy Profile: Inoviridae. J. Gen. Virol. 2021, 102, 001614. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Kim, W.G.; Kim, C.; Park, G.T.; Heo, J.; Yoo, S.Y.; Oh, J.W. M13 Bacteriophage-Based Self-Assembly Structures and Their Functional Capabilities. Mini Rev. Org. Chem. 2015, 12, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.R.; Dowell, C.E. Replication of coliphage M-13 I. Effects on host cell after synchronized infection. J. Virol. 1968, 2, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.T.; Tan, M.S.; Su, M.T.; Yang, M.K. Complete nucleotide sequence of filamentous phage Cf1c from Xanthomonas campestris pv. citri Nucleic Acids Res. 1991, 19, 2498. [Google Scholar] [CrossRef] [PubMed]
- Askora, A.; Kawasaki, T.; Usami, S.; Fujie, M.; Yamada, T. Host recognition and integration of filamentous phage phiRSM in the phytopathogen, Ralstonia solanacearum. Virology 2009, 384, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.C.; Chen, X.L.; Shen, Q.T.; Zhao, D.L.; Tang, B.L.; Su, H.N.; Zhang, Y.Z. Filamentous phages prevalent in Pseudoalteromonas spp. confer properties advantageous to host survival in Arctic sea ice. ISME J. 2015, 9, 871–881. [Google Scholar] [CrossRef]
- Addy, H.S.; Askora, A.; Kawasaki, T.; Fujie, M.; Yamada, T. The filamentous phage φrSS1 enhances virulence of phytopathogenic Ralstonia solanacearum on tomato. Phytopathology 2012, 102, 244–251. [Google Scholar] [CrossRef]
- Chen, J.; Su, Z.; Liu, Y.; Wang, S.; Dai, X.; Li, Y.; Peng, S.; Shao, Q.; Zhang, H.; Wen, P.; et al. Identification and characterization of class 1 integrons among Pseudomonas aeruginosa isolates from patients in Zhenjiang. China Int. J. Infect. Dis. 2009, 13, 717–721. [Google Scholar] [CrossRef]
- Wang, Q.; Kan, B.; Wang, R. Isolation and characterization of the new mosaic filamentous phage VFJΦ of Vibrio cholerae. PLoS ONE 2013, 8, e70934. [Google Scholar] [CrossRef]
- Ahmad, A.; Askora, A.; Kawasaki, T.; Efujie, M.; Eyamada, T. The filamentous phage XacF1 causes loss of virulence in Xanthomonas axonopodis pv. citri, the causative agent of citrus canker disease. Front. Microbiol. 2014, 5, 321. [Google Scholar] [CrossRef]
- Ahmad, A.; Fadel, F.; Sebban-Kreuzer, C.; Ba, M.; Pélissier, G.D.; Bornet, O.; Vincent, F. Structural and functional insights into the periplasmic detector domain of the GacS histidine kinase controlling biofilm formation in Pseudomonas aeruginosa. Sci. Rep. 2017, 7, 11262. [Google Scholar] [CrossRef] [PubMed]
- Secor, P.; Michaels, L.; Smigiel, K.; Rohani, M.; Jennings, L.; Hisert, K.; Arrigoni, A.; Braun, K.; Birkland, T.; Lai, Y.; et al. Filamentous Bacteriophage Produced by Pseudomonas aeruginosa Alters the Inflammatory Response and Promotes Noninvasive Infection In Vivo. Infect. Immun. 2016, 85, e00648-16. [Google Scholar] [CrossRef]
- Gavric, D.; Knezevic, P. Filamentous Pseudomonas Phage Pf4 in the Context of Therapy-Inducibility, Infectivity, Lysogenic Conversion, and Potential Application. Viruses 2022, 14, 1261. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Baudry, B.; Pumplin, D.W.; Wasserman, S.S.; Tall, B.D.; Ketley, J.M.; Kaper, J.B. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. USA 1991, 88, 5242–5246. [Google Scholar] [CrossRef] [PubMed]
- Trucksis, M.; Galen, J.E.; Michalski, J.; Fasano, A.; Kaper, J.B. Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette. Proc. Natl. Acad. Sci. USA 1993, 90, 5267–5271. [Google Scholar] [CrossRef] [PubMed]
- Waldor, M.K.; Mekalanos, J.J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 1996, 272, 1910–1914. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses: I.C.T.V. Available online: https://ictv.global/report/chapter/inoviridae/inoviridae/inovirus (accessed on 25 December 2023).
- Knezevic, P.; Voet, M.; Lavigne, R. Prevalence of Pf1-like (pro)phage genetic elements among Pseudomonas aeruginosa isolates. Virology 2015, 483, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Roux, S.; Adriaenssens, E.M.; Dutilh, B.E.; Koonin, E.V.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Lavigne, R.; Brister, J.R.; Varsani, A.; et al. Minimum Information about an Uncultivated Virus Genome (MIUViG). Nat. Biotechnol. 2019, 37, 29–37. [Google Scholar] [CrossRef]
- Espinal, P.; Martí, S.; Vila, J. Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J. Hosp. Infect. 2012, 80, 56–60. [Google Scholar] [CrossRef]
- Brenner, D.J.; Krieg, N.R.; Staley, J.T.; Garrity, G. Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Volume 2: Proteobacteria; Part B: The Gammaproteobacteria; Garrity, G.M., Ed.; Springer: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Joly-Guillou, M.L. Clinical impact and pathogenicity of Acinetobacter. Clin. Microbiol. Infect. 2005, 11, 868–873. [Google Scholar] [CrossRef]
- Vashist, J.; Tiwari, V.; Das, R.; Kapil, A.; Rajeswari, M.R. Analysis of penicillin-binding proteins (PBPs) in carbapenem resistant Acinetobacter baumannii. Indian J. Med. Res. 2011, 133, 332–338. [Google Scholar]
- Falagas, M.E.; Koletsi, P.K.; Bliziotis, I.A. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J. Med. Microbiol. 2006, 55, 1619–1629. [Google Scholar] [CrossRef]
- Kuo, L.C.; Yu, C.J.; Lee, L.N.; Wang, J.L.; Wang, H.C.; Hsueh, P.R.; Yang, P.C. Clinical features of pandrug-resistant Acinetobacter baumannii bacteremia at a university hospital in Taiwan. J. Formos. Med. Assoc. 2003, 102, 601–606. [Google Scholar]
- Kuo, L.C.; Teng, L.J.; Yu, C.J.; Ho, S.W.; Hsueh, P.R. Dissemination of a clone of unusual phenotype of pandrug-resistant Acinetobacter baumannii at a university hospital in Taiwan. J. Clin. Microbiol. 2004, 442, 1759–1763. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Sheng, W.H.; Chang, Y.Y.; Wang, L.H.; Lin, H.C.; Chen, M.L.; Pan, H.J.; Ko, W.J.; Chang, S.C.; Lin, F.J. Healthcare-associated outbreak due to pan-drug resistant Acinetobacter baumannii in a surgical intensive care unit. J. Hosp. Inf. 2003, 53, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Pour, N.K.; Dusane, D.H.; Dhakephalkar, P.K.; Zamin, F.R.; Zinjarde, S.S.; Chopade, B.A. Biofilm formation by Acinetobacter baumannii strains isolated from urinary tract infection and urinary catheters. FEMS Immunol. Med. Microbiol. 2011, 62, 328–338. [Google Scholar] [CrossRef]
- McConnell, M.J.; Actis, L.; Pachón, J. Acinetobacter baumannii: Human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol. Rev. 2013, 37, 130–155. [Google Scholar] [CrossRef] [PubMed]
- Aleksic Sabo, V.; Gavric, D.; Pejic, J.; Knezevic, P. Acinetobacter calcoaceticus-A. baumannii complex: Isolation, identification and characterisation of environmental and clinical strains. Biol. Serb. 2022, 44, 3–17. [Google Scholar] [CrossRef]
- Aleksic, V.; Mimica-Dukic, N.; Simin, N.; Nedeljkovic, N.S.; Knezevic, P. Synergistic effect of Myrtus communis L. essential oils and conventional antibiotics against multi-drug resistant Acinetobacter baumannii wound isolates. Phytomedicine 2014, 21, 1666–1674. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef]
- GenBank. National Institute of Health. Available online: https://www.ncbi.nlm.nih.gov/genome/browse/#!/prokaryotes/403/ (accessed on 14 March 2023).
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA 11: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- OlygoAnalyzer Tool. Available online: https://www.idtdna.com/calc/analyzer (accessed on 12 December 2023).
- Sequence Manipulation Suite. Available online: http://www.bioinformatics.org/sms2/pcr_primer_stats.html (accessed on 12 December 2023).
- Gavric, D.; Knezevic, P. Optimized Method for Pseudomonas aeruginosa Integrative Filamentous Bacteriophage Propagation. Front. Microbiol. 2022, 12, 707815. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, P.; Kostanjsek, R.; Obreht, D.; Petrovic, O. Isolation of Pseudomonas aeruginosa Specific Phages with Broad Activity Spectra. Curr. Microbiol. 2009, 59, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Bozzola, J.J.; Russell, L.D. Electron Microscopy—Principles and Techniques for Biology, 2nd ed.; Jones and Bartlett Publishers Book News, Inc.: Portland, OR, USA, 1998; p. 670. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Bradford, M. A Rapid and Sensitive Method for the Quantification of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- SignalP 5.0 Server. Available online: https://services.healthtech.dtu.dk/services/SignalP-5.0/ (accessed on 12 December 2023).
- WebCutter. Available online: https://heimanlab.com/cut2.html (accessed on 11 May 2024).
- Vijayakumar, S.; Rajenderan, S.; Laishram, S.; Anandan, S.; Balaji, V.; Biswas, I. Biofilm Formation and Motility Depend on the Nature of the Acinetobacter baumannii Clinical Isolates. Front. Public Health 2016, 4, 105. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, P.; Petrovic, O. A colorimetric microtiter plate method for assessment of phage effect on Pseudomonas aeruginosa biofilm. J. Microbiol. Methods 2008, 74, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Fekrirad, Z.; Darabpour, E.; Kashef, N. Eradication of Acinetobacter baumannii Planktonic and Biofilm Cells Through Erythrosine-Mediated Photodynamic Inactivation Augmented by Acetic Acid and Chitosan. Curr. Microbiol. 2021, 78, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 8, 350–356. [Google Scholar] [CrossRef]
- Kon, H.; Schwartz, D.; Temkin, E.; Carmeli, Y.; Lellouche, J. Rapid identification of capsulated Acinetobacter baumannii using a density-dependent gradient test. BMC Microbiol. 2020, 20, 285. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. CLSI supplement M100. In Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Siguier, P.; Gourbeyre, E.; Chandler, M. Bacterial insertion sequences: Their genomic impact and diversity. FEMS Microbiol. Rev. 2014, 38, 865–891. [Google Scholar] [CrossRef] [PubMed]
- Makałowski, W.; Gotea, V.; Pande, A.; Makałowska, I. Transposable Elements: Classification, Identification, and Their Use As a Tool For Comparative Genomics. Methods Mol. Biol. 2019, 1910, 177–207. [Google Scholar] [CrossRef]
- Kawai, M.; Uchiyama, I.; Kobayashi, I. In silico genome comparison suggested that filamentous bacteriophages are integrated to Neisseria genomes by their own transposase. DNA Res. 2005, 12, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.E.; Waldor, M.K. Filamentous phage integration requires the host recombinases XerC and XerD. Nature 2002, 417, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Lesterlin, C.; Barre, F.X.; Cornet, F. Genetic recombination and the cell cycle: What we have learned from chromosome dimers. Mol. Microbiol. 2004, 54, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Bischerour, J.; Spangenberg, C.; Barre, F.X. Holliday junction affinity of the base excision repair factor Endo III contributes to cholera toxin phage integration. EMBO J. 2012, 31, 3757–3767. [Google Scholar] [CrossRef]
- Derbise, A.; Carniel, E. YpfΦ: A filamentous phage acquired by Yersinia pestis. Front. Microbiol. 2014, 15, 701. [Google Scholar] [CrossRef]
- Chouikha, I.; Charrier, L.; Filali, S.; Derbise, A.; Carniel, E. Insights into the infective properties of YpfΦ, the Yersinia pestis filamentous phage. Virology 2010, 407, 43–52. [Google Scholar] [CrossRef]
- Müller, M.G.; Ing, J.Y.; Cheng, M.K.; Flitter, B.A.; Moe, G.R. Identification of a phage-encoded Ig-binding protein from invasive Neisseria meningitidis. J. Immunol. 2013, 191, 3287–3296. [Google Scholar] [CrossRef]
- Yamada, T.; Kawasaki, T.; Nagata, S.; Fujiwara, A.; Usami, S.; Fujie, M. New bacteriophages that infect the phytopathogen Ralstonia solanacearum. Microbiology 2007, 153, 2630–2639. [Google Scholar] [CrossRef] [PubMed]
- Kamiunten, H.; Wakimoto, S. Effect of the Phage Infection on the with Filamentous of Properties Xanthomonas campestris. Ann. Phytopathol. Soc. Jpn. 1981, 47, 627–636. [Google Scholar] [CrossRef]
- Wan, Z.; Goddard, N. Competition Between Conjugation and M13 Phage Infection in Escherichia coli in the Absence of Selection Pressure: A Kinetic Study. G3 2012, 2, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.T.; Lin, Y.H.; Huang, C.M.; Chang, S.F.; Dai, H.; Feng, T.J. The lysogenic cycle of the filamentous phage Cflt from Xanthomonas campestris pv. citri. Virology 1987, 156, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Loh, B.; Kuhn, A.; Leptihn, S. The fascinating biology behind phage display: Filamentous phage assembly. Mol. Microbiol. 2019, 111, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Hay, I.D.; Lithgow, T. Filamentous phages: Masters of a microbial sharing economy. EMBO Rep. 2019, 20, 47427. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.E. A function of Pseudomonas aeruginosa PAO polar pili: Twitching motility. Can. J. Microbiol. 1980, 26, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.K.; Fitzpatrick, A.D.; Schwartzkopf, C.M.; Faith, D.R.; Jennings, L.K.; Coluccio, A.; Hunt, D.J.; Michaels, L.A.; Hargil, A.; Chen, Q.; et al. A Filamentous Bacteriophage Protein Inhibits Type IV Pili To Prevent Superinfection of Pseudomonas aeruginosa. mBio 2022, 13, e0244121. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.K.; Adams, F.G.; Brown, M.H. Diversity and function of capsular polysaccharide in Acinetobacter baumannii. Front. Microbiol. 2019, 9, 3301. [Google Scholar] [CrossRef]
- Weber, B.S.; Harding, C.M.; Feldman, M.F. Pathogenic Acinetobacter: From the cell surface to infinity and beyond. J. Bacteriol. 2016, 198, 880–887. [Google Scholar] [CrossRef]
- Geisinger, E.; Isberg, R.R. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog. 2015, 11, e1004691. [Google Scholar] [CrossRef] [PubMed]
- Amankwah, S.; Abdella, K.; Kassa, T. Bacterial biofilm destruction: A focused review on the recent use of phage-based strategies with other antibiofilm agents. Nanotechnol. Sci. Appl. 2021, 14, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Su, P.W.; Moi, S.H.; Chuang, L.Y. Biofilm Formation in Acinetobacter baumannii: Genotype-Phenotype Correlation. Molecules 2019, 24, 1849. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T. Filamentous phages of Ralstonia solanacearum: Double-edged swords for pathogenic bacteria. Front. Microbiol. 2013, 4, 325. [Google Scholar] [CrossRef] [PubMed]
- May, T.; Tsuruta, K.; Okabe, S. Exposure of conjugative plasmid carrying Escherichia coli biofilms to male-specific bacteriophages. ISME J. 2011, 5, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.S.; Thompson, L.S.; James, S.; Charlton, T.; Tolker-Nielsen, T.; Koch, B.; Givskov, M.; Kjelleberg, S. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 2003, 185, 4585–4592. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.; Tan, C.; Mikkelsen, P. The biofilm life cycle and virulence of Pseudomonas aeruginosa are dependent on a filamentous prophage. ISME J. 2009, 3, 271–282. [Google Scholar] [CrossRef]
- Secor, P.R.; Sweere, J.M.; Michaels, L.A.; Malkovskiy, A.V.; Lazzareschi, D.; Katznelson, E.; Rajadas, J.; Birnbaum, M.E.; Arrigoni, A.; Braun, K.R. Filamentous bacteriophage promote biofilm assembly and function. Cell Host Microbe 2015, 18, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, M.; Bangera, M.G.; Bumgarner, R.E.; Parsek, M.R.; Teitzel, G.M.; Lory, S.; Greenberg, E.P. Gene expression in Pseudomonas aeruginosa biofilms. Nature 2001, 413, 860–864. [Google Scholar] [CrossRef]
- Addy, H.S.; Askora, A.; Kawasaki, T.; Fujie, M.; Yamada, T. Utilization of filamentous phage phi RSM3 to control bacterial wilt caused by Ralstonia solanacearum. Plant Dis. 2012, 96, 1204–1209. [Google Scholar] [CrossRef]
- Hagens, S.; Habel, A.; Bläsi, U. Augmentation of the Antimicrobial Efficacy of Antibiotics by Filamentous Phage. Microb. Drug Resist. 2006, 12, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Coyne, S.; Guigon, G.; Courvalin, P.; Périchon, B. Screening and quantification of the expression of antibiotic resistance genes in Acinetobacter baumannii with a microarray. Antimicrob. Agents Chemother. 2010, 54, 333–340. [Google Scholar] [CrossRef] [PubMed]

| Strain | Ceftriaxone (µg mL−1) | Tobramycin (µg mL−1) | Ciprofloxacin (µg mL−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Uninfected | +AfK | +Af4 | Uninfected | +AfK | +Af4 | Uninfected | +AfK | +Af4 | |
| Aba-8781 | 32 (I) * | 16 (I) | 8 (S) | 64 (R) | 1 (S) | 1 (S) | 0.25 (S) | ≤0.125 (S) | ≤0.125 (S) |
| Aba-5081 | >512 (R) | >512 (R) | 512 (R) | 64 (R) | 32 (R) | 64 (R) | 128 (R) | 32 (R) | 32 (R) |
| Aba-5074 | >512 (R) | >512 (R) | >512 (R) | >512 (R) | >512 (R) | >512 (R) | 64 (R) | 32 (R) | 64 (R) |
| Aba-2793 | >512 (R) | 128 (R) | 128 (R) | 1 (S) | 1 (S) | 1 (S) | 0.25 (S) | ≤0.125 (S) | ≤0.125 (S) |
| Aba-S-tyr | 128 (R) | 128 (R) | 128 (R) | 256 (R) | 64 (R) | 64 (R) | 32 (R) | 32 (R) | 32 (R) |
| Aba-M-ace | 128 (R) | 128 (R) | - | 1 (S) | 0.5 (S) | - | 16 (R) | 4 (R) | - |
| E. coli ATCC 25922 | 0.25 (S) | - | - | 0.5 (S) | - | - | <0.125 (S) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narancic, J.; Gavric, D.; Kostanjsek, R.; Knezevic, P. First Characterization of Acinetobacter baumannii-Specific Filamentous Phages. Viruses 2024, 16, 857. https://doi.org/10.3390/v16060857
Narancic J, Gavric D, Kostanjsek R, Knezevic P. First Characterization of Acinetobacter baumannii-Specific Filamentous Phages. Viruses. 2024; 16(6):857. https://doi.org/10.3390/v16060857
Chicago/Turabian StyleNarancic, Jelena, Damir Gavric, Rok Kostanjsek, and Petar Knezevic. 2024. "First Characterization of Acinetobacter baumannii-Specific Filamentous Phages" Viruses 16, no. 6: 857. https://doi.org/10.3390/v16060857






