The Impact of Time between Booster Doses on Humoral Immune Response in Solid Organ Transplant Recipients Vaccinated with BNT162b2 Vaccines
Abstract
1. Introduction
2. Methods
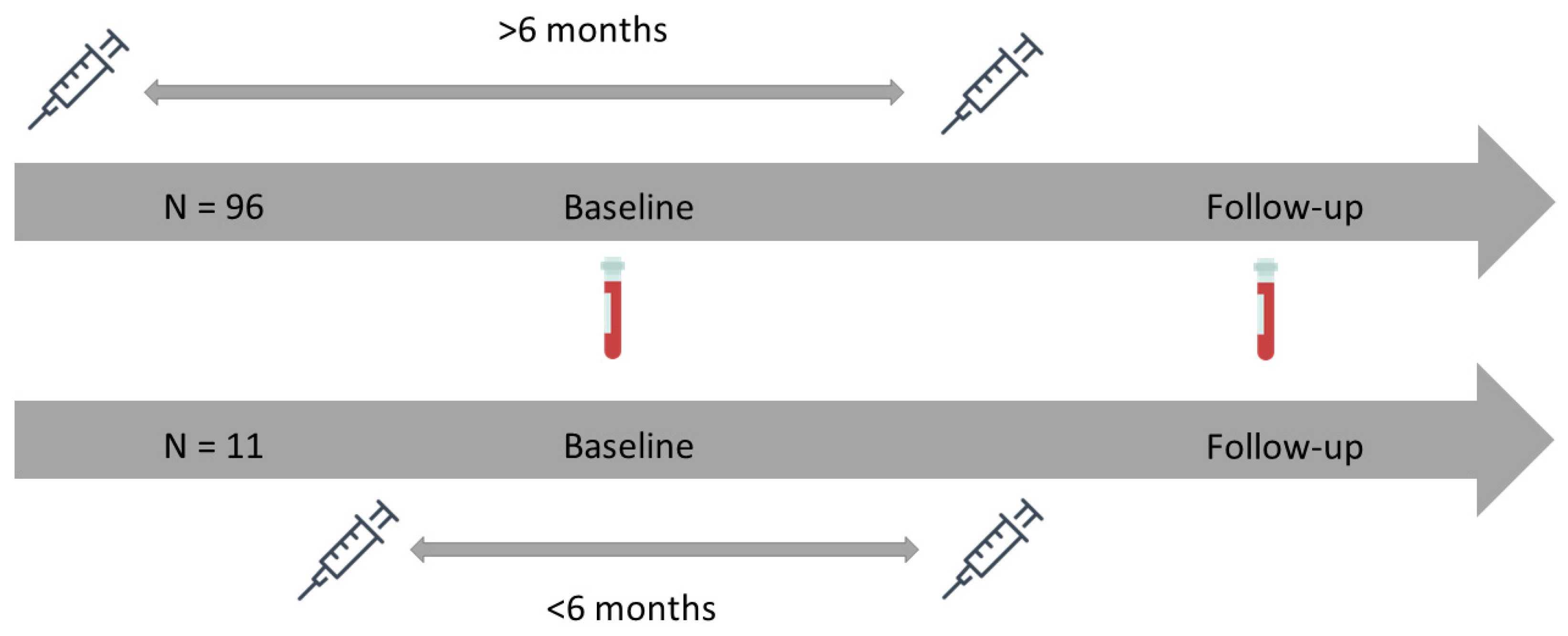
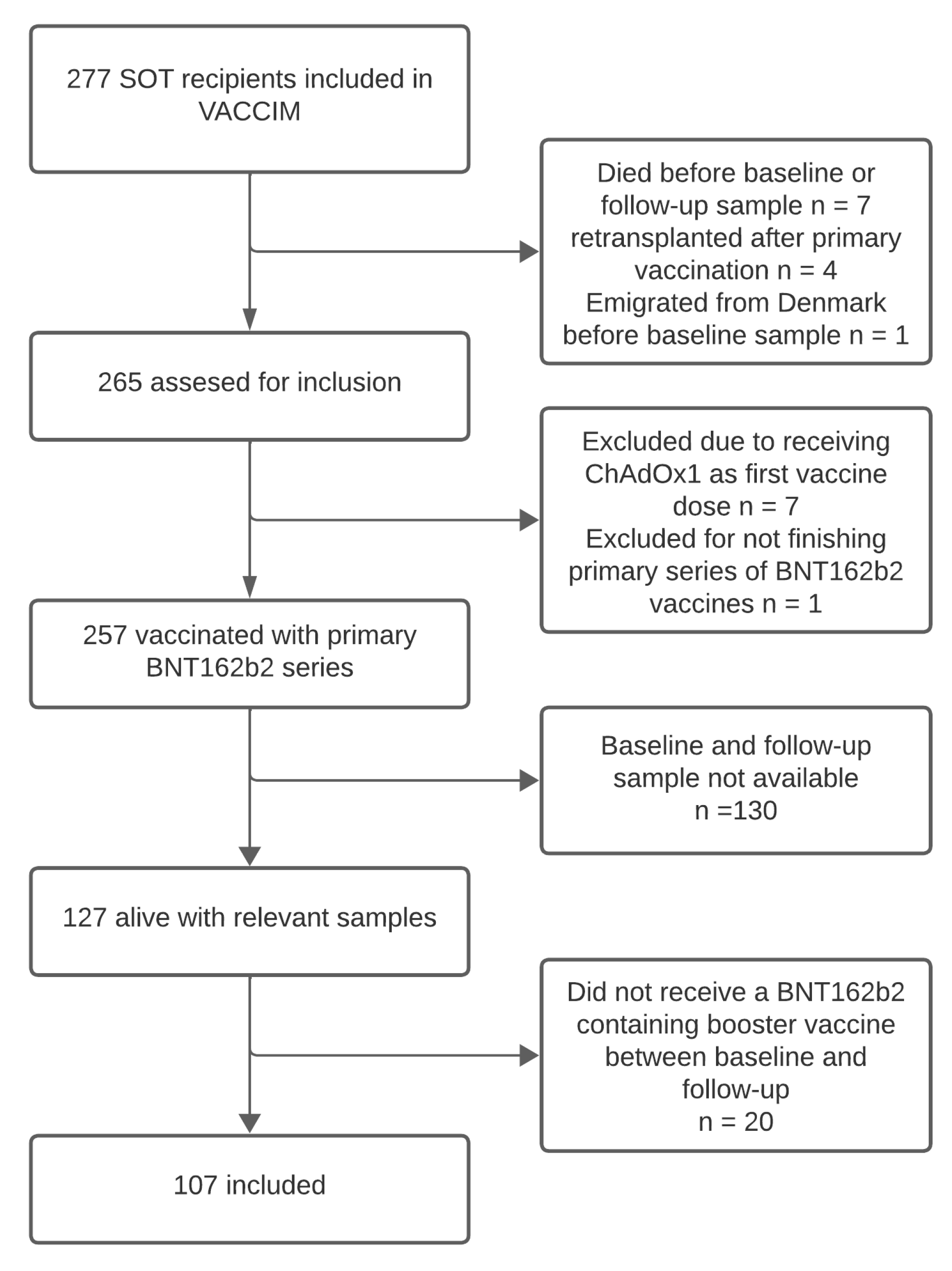
2.1. Study Design
2.2. Clinical Information
2.3. Definitions
2.4. Antibody Quantification
2.5. Statistics
3. Results
3.1. Cohort Characteristics
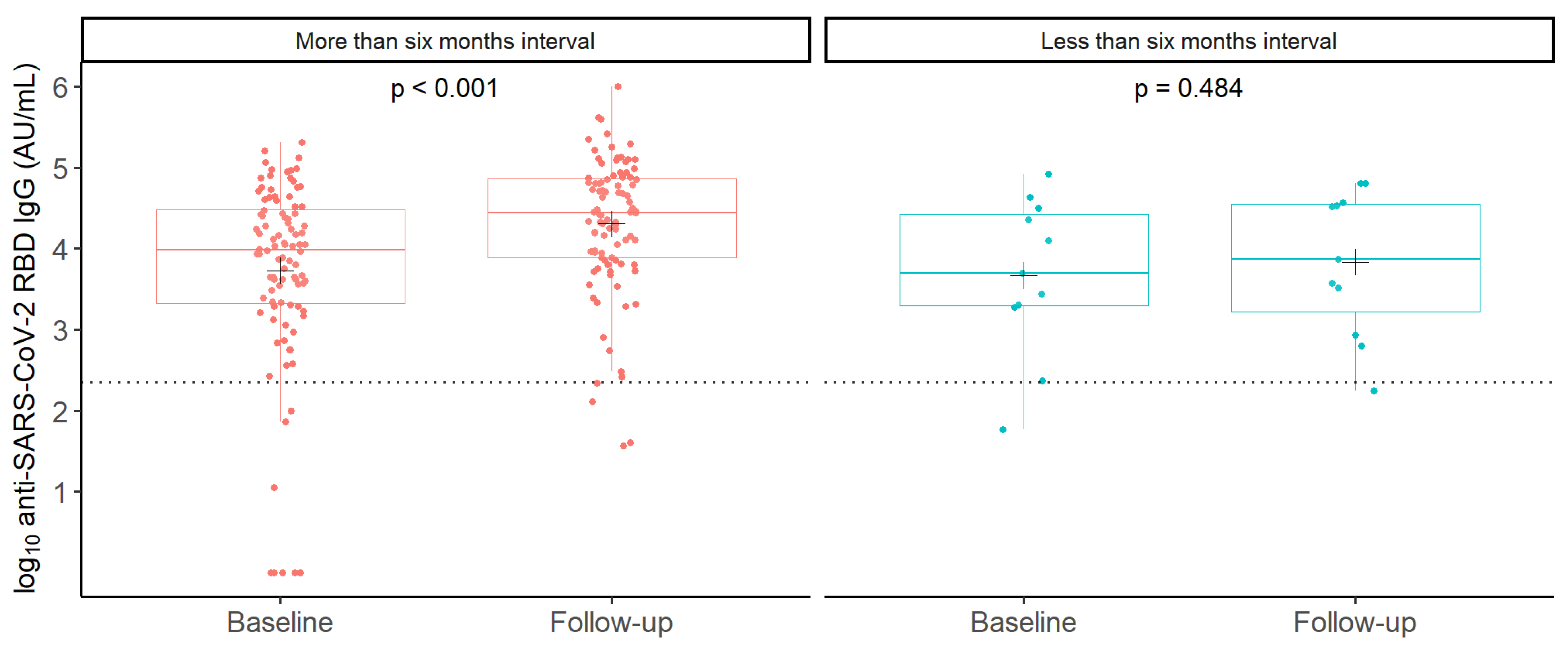
3.2. GMC of Anti-SARS-CoV-2 RBD IgG
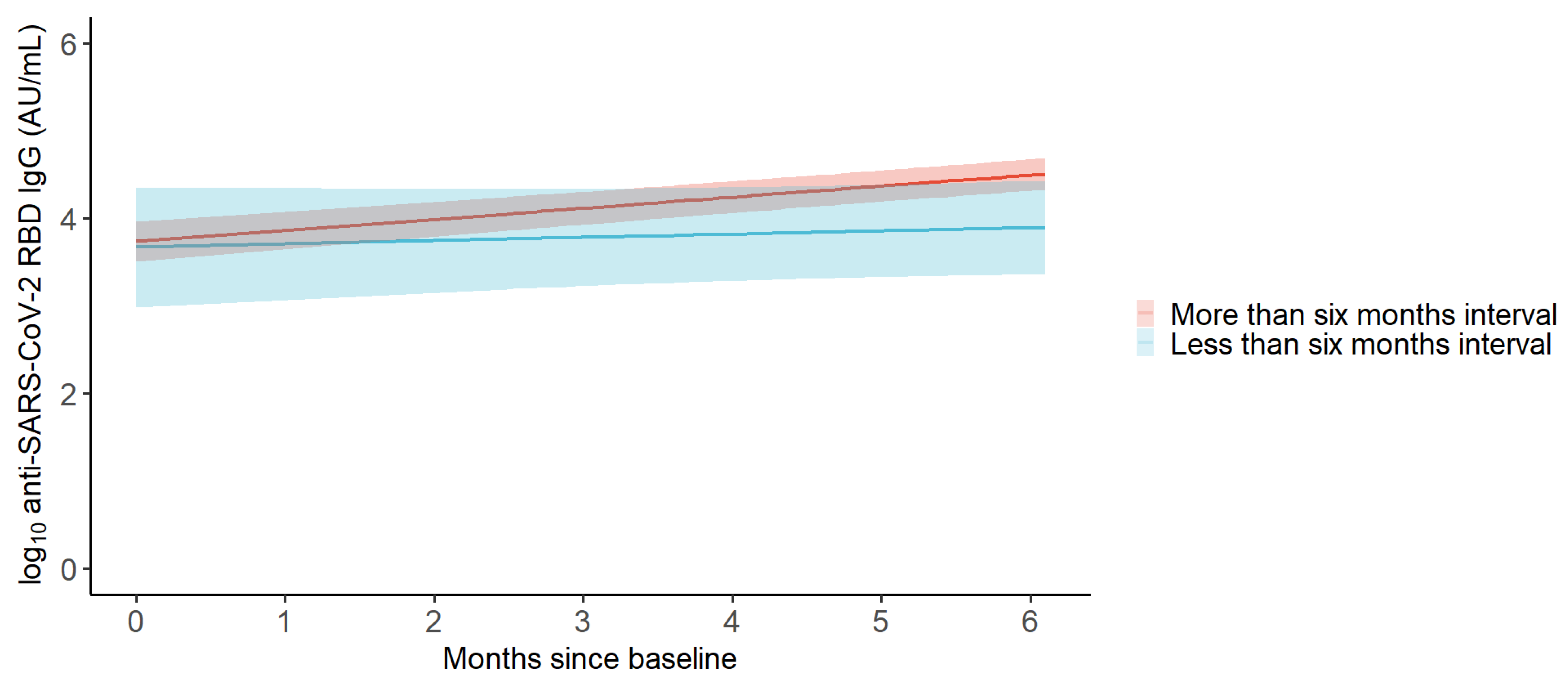
3.3. Monthly Change in the GMC of Anti-SARS-CoV-2 RBD IgG
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiang, T.P.Y.; Abedon, A.T.; Alejo, J.L.; Segev, D.L.; Massie, A.B.; Werbel, W.A. Incident COVID-19 and Hospitalizations by Variant Era among Vaccinated Solid Organ Transplant Recipients. JAMA Netw. Open. 2023, 6, E2329736. [Google Scholar] [CrossRef] [PubMed]
- Solera, J.T.; Árbol, B.G.; Mittal, A.; Hall, V.; Marinelli, T.; Bahinskaya, I.; Selzner, N.; McDonald, M.; Schiff, J.; Sidhu, A.; et al. Longitudinal Outcomes of COVID-19 in Solid Organ Transplant Recipients from 2020 to 2023. Am. J. Transplant. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Yamanaga, S.; Shimata, K.; Ohfuji, S.; Yoshikawa, M.; Natori, Y.; Hibi, T.; Yuzawa, K.; Egawa, H. Excess mortality in COVID-19-affected solid organ transplant recipients across the pandemic. Am. J. Transplant. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Balsby, D.; Nilsson, A.C.; Petersen, I.; Lindvig, S.O.; Davidsen, J.R.; Abazi, R.; Poulsen, M.K.; Holden, I.K.; Justesen, U.S.; Bistrup, C.; et al. Humoral immune response following a third SARS-CoV-2 mRNA vaccine dose in solid organ transplant recipients compared with matched controls. Front. Immunol. 2022, 13, 1039245. [Google Scholar] [CrossRef] [PubMed]
- Hamm, S.R.; Møller, D.L.; Pérez-Alós, L.; Hansen, C.B.; Pries-Heje, M.M.; Heftdal, L.D.; Hasselbalch, R.B.; Fogh, K.; Madsen, J.R.; Almagro Armenteros, J.J.; et al. Decline in Antibody Concentration 6 Months After Two Doses of SARS-CoV-2 BNT162b2 Vaccine in Solid Organ Transplant Recipients and Healthy Controls. Front. Immunol. 2022, 13, 832501. [Google Scholar] [CrossRef]
- Rezahosseini, O.; Hamm, S.R.; Heftdal, L.D.; Pérez-Alós, L.; Møller, D.L.; Perch, M.; Madsen, J.R.; Hald, A.; Hansen, C.B.; Armenteros, J.J.A.; et al. Humoral and T-cell response 12 months after the first BNT162b2 vaccination in solid organ transplant recipients and controls: Kinetics, associated factors, and role of SARS-CoV-2 infection. Front. Immunol. 2023, 13, 1075423. [Google Scholar] [CrossRef] [PubMed]
- Giannella, M.; Righi, E.; Pascale, R.; Rinaldi, M.; Caroccia, N.; Gamberini, C.; Palacios-Baena, Z.R.; Caponcello, G.; Morelli, M.C.; Tamè, M.; et al. Evaluation of the Kinetics of Antibody Response to COVID-19 Vaccine in Solid Organ Transplant Recipients: The Prospective Multicenter ORCHESTRA Cohort. Microorganisms 2022, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Naylor, K.L.; Knoll, G.A.; Smith, G.; McArthur, E.; Kwong, J.C.; Dixon, S.N.; Treleaven, D.; Kim, S.J. Effectiveness of a Fourth COVID-19 mRNA Vaccine Dose Against the Omicron Variant in Solid Organ Transplant Recipients. Transplantation 2024, 108, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Huh, K.; Kang, M.; Kim, Y.-E.; Choi, Y.; An, S.J.; Seong, J.; Go, M.J.; Kang, J.-M.; Jung, J. Risk of Severe COVID-19 and Protective Effectiveness of Vaccination Among Solid Organ Transplant Recipients. J. Infect. Dis. 2023, 229, 1026–1034. [Google Scholar] [CrossRef]
- Kabbani, D.; Yotis, D.M.; Ferreira, V.H.; Shalhoub, S.; Belga, S.; Tyagi, V.; Ierullo, M.; Kulasingam, V.; Hébert, M.J.; West, L.; et al. Immunogenicity, Safety, and Breakthrough Severe Acute Respiratory Syndrome Coronavirus 2 Infections After Coronavirus Disease 2019 Vaccination in Organ Transplant Recipients: A Prospective Multicenter Canadian Study. Open Forum Infect Dis. 2023, 10, ofad200. [Google Scholar] [CrossRef]
- Solera, J.T.; Árbol, B.G.; Alshahrani, A.; Bahinskaya, I.; Marks, N.; Humar, A.; Kumar, D. Impact of Vaccination and Early Monoclonal Antibody Therapy on Coronavirus Disease 2019 Outcomes in Organ Transplant Recipients during the Omicron Wave. Clin. Infect. Dis. 2022, 75, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Vinson, A.J.; Anzalone, A.J.; Sun, J.; Dai, R.; Agarwal, G.; Lee, S.B.; French, E.; Olex, A.; Ison, M.G.; Mannon, R.B.; et al. The Risk and Consequences of Breakthrough SARS-CoV-2 Infection in Solid Organ Transplant Recipients Relative to Non-Immunosuppressed Controls. Am. J. Transplant. 2022, 22, 2418–2432. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, C.J.; Curtis, R.M.K.; Mumford, L.; Whitaker, H.; Pettigrew, G.; Gardiner, D.; Marson, L.; Thorburn, D.; White, S.; Parmar, J.; et al. Vaccine Effectiveness Against the SARS-CoV-2 B.1.1.529 Omicron Variant in Solid Organ and Islet Transplant Recipients in England: A National Retrospective Cohort Study. Transplantation 2023, 107, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Naylor, K.L.; Kim, S.J.; Smith, G.; McArthur, E.; Kwong, J.C.; Dixon, S.N.; Treleaven, D.; Knoll, G.A. Effectiveness of first, second, and third COVID-19 vaccine doses in solid organ transplant recipients: A population-based cohort study from Canada. Am. J. Transplant. 2022, 22, 2228–2236. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Kemlin, D.; Gemander, N.; Depickère, S.; Olislagers, V.; Georges, D.; Waegemans, A.; Pannus, P.; Lemy, A.; Goossens, M.E.; Desombere, I.; et al. Humoral and cellular immune correlates of protection against COVID-19 in kidney transplant recipients. Am. J. Transplant. 2023, 23, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.G.; Ferreira, V.H.; Ku, T.; Ierullo, M.; Majchrzak-Kita, B.; Chaparro, C.; Selzner, N.; Schiff, J.; McDonald, M.; Tomlinson, G.; et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N. Engl. J. Med. 2021, 385, 1244–1246. [Google Scholar] [CrossRef] [PubMed]
- Karaba, A.H.; Zhu, X.; Liang, T.; Wang, K.H.; Rittenhouse, A.G.; Akinde, O.; Eby, Y.; Ruff, J.E.; Blankson, J.N.; Abedon, A.T.; et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am. J. Transplant. 2022, 22, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; Del Bello, A. Three Doses of an mRNA COVID-19 Vaccine in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef]
- Mitchell, J.; Alejo, J.L.; Chiang, T.P.Y.; Kim, J.; Chang, A.; Abedon, A.T.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Levan, M.L.; et al. Antibody Response to a Fourth Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: An Update. Transplantation 2022, 106, E338–E340. [Google Scholar] [CrossRef]
- Karaba, A.H.; Johnston, T.S.; Aytenfisu, T.Y.; Akinde, O.; Eby, Y.; Ruff, J.E.; Abedon, A.T.; Alejo, J.L.; Blankson, J.N.; Cox, A.L.; et al. A Fourth Dose of COVID-19 Vaccine Does Not Induce Neutralization of the Omicron Variant Among Solid Organ Transplant Recipients With Suboptimal Vaccine Response. Transplantation 2022, 106, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Perrier, Q.; Lupo, J.; Gerster, T.; Augier, C.; Falque, L.; Rostaing, L.; Pelletier, L.; Bedouch, P.; Blanc, M.; Saint-Raymond, C.; et al. SARS-CoV-2 anti-spike antibodies after a fourth dose of COVID-19 vaccine in adult solid-organ transplant recipients. Vaccine 2022, 40, 6404. [Google Scholar] [CrossRef]
- Del Bello, A.; Kamar, N.; Marion, O.; Izopet, J.; Abravanel, F. Humoral and Cellular Responses to a Delayed Fourth SARS-CoV-2 mRNA-Based Vaccine in Weak Responders to 3 Doses Kidney Transplant Recipients. Vaccines 2022, 10, 1439. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Chiang, T.P.Y.; Alejo, J.L.; Kim, J.D.; Chang, A.; Abedon, A.T.; Avery, R.K.; Tobian, A.A.R.; Levan, M.L.; Warren, D.S.; et al. 6-month antibody kinetics and durability after four doses of a SARS-CoV-2 vaccine in solid organ transplant recipients. Clin. Transplant. 2023, 37, e14868. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccines Advice. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice (accessed on 24 April 2024).
- COVID-19 Vaccine Advice and Recommendations for 2024 | Australian Government Department of Health and Aged Care. Available online: https://www.health.gov.au/our-work/covid-19-vaccines/getting-your-vaccination (accessed on 16 May 2024).
- COVID-19 Vaccines for People Who Are Moderately or Severely Immunocompromised |, C.D.C. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html (accessed on 16 May 2024).
- Hall, V.G.; Ferreira, V.H.; Wood, H.; Ierullo, M.; Majchrzak-Kita, B.; Manguiat, K.; Robinson, A.; Kulasingam, V.; Humar, A.; Kumar, D. Delayed-interval BNT162b2 mRNA COVID-19 vaccination enhances humoral immunity and induces robust T cell responses. Nat. Immunol. 2022, 23, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.P.; Longet, S.; Austin, J.A.; Skelly, D.T.; Dejnirattisai, W.; Adele, S.; Meardon, N.; Faustini, S.; Al-Taei, S.; Moore, S.C.; et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021, 184, 5699. [Google Scholar] [CrossRef] [PubMed]
- Parry, H.; Bruton, R.; Stephens, C.; Parry, H.; Bruton, R.; Stephens, C.; Bentley, C.; Brown, K.; Amirthalingam, G.; Hallis, B.; et al. Extended interval BNT162b2 vaccination enhances peak antibody generation. NPJ Vaccines 2022, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.H.; Liu, X.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Effect of priming interval on reactogenicity, peak immunological response, and waning after homologous and heterologous COVID-19 vaccine schedules: Exploratory analyses of Com-COV, a randomised control trial. Lancet Respir Med. 2022, 10, 1049–1060. [Google Scholar] [CrossRef]
- Prusinkiewicz, M.A.; Sediqi, S.; Li, Y.J.; Goldfarb, D.M.; Asamoah-Boaheng, M.; Wall, N.; Lavoie, P.M.; Grunau, B. Effect of vaccine dosing intervals on Omicron surrogate neutralization after three doses of BNT162b2. Heliyon 2023, 9, e17259. [Google Scholar] [CrossRef]
- Grove Krause, T.; Jakobsen, S.; Haarh, M.; Mølbak, K. The Danish vaccination register. Eurosurveillance 2012, 17, 2. [Google Scholar] [CrossRef]
- Voldstedlund, M.; Haarh, M.; Mølbak, K. The Danish Microbiology Database (MiBa) 2010 to 2013. Eurosurveillance 2014, 19, 20667. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.B.; Jarlhelt, I.; Pérez-Alós, L.; Hummelshøj Landsy, L.; Loftager, M.; Rosbjerg, A.; Helgstrand, C.; Bjelke, J.R.; Egebjerg, T.; Jardine, J.G.; et al. SARS-CoV-2 Antibody Responses Are Correlated to Disease Severity in COVID-19 Convalescent Individuals. J. Immunol. 2021, 206, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alós, L.; Armenteros, J.J.A.; Madsen, J.R.; Hansen, C.B.; Jarlhelt, I.; Hamm, S.R.; Heftdal, L.D.; Pries-Heje, M.M.; Møller, D.L.; Fogh, K.; et al. Modeling of waning immunity after SARS-CoV-2 vaccination and influencing factors. Nat. Commun. 2022, 13, 1614. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 24 May 2024).
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Nicolas, A.; Sannier, G.; Dubé, M.; Nayrac, M.; Tauzin, A.; Painter, M.M.; Goel, R.R.; Laporte, M.; Gendron-Lepage, G.; Medjahed, H.; et al. An extended SARS-CoV-2 mRNA vaccine prime-boost interval enhances B cell immunity with limited impact on T cells. iScience 2023, 26, 105904. [Google Scholar] [CrossRef]
- Buckner, C.M.; Kardava, L.; El Merhebi, O.; Narpala, S.R.; Serebryannyy, L.; Lin, B.C.; Wang, W.; Zhang, X.; Lopes de Assis, F.; Kelly, S.E.M.; et al. Interval between prior SARS-CoV-2 infection and booster vaccination impacts magnitude and quality of antibody and B cell responses. Cell 2022, 185, 4333–4346.e14. [Google Scholar] [CrossRef]
| n = 107 | |
|---|---|
| Age at baseline, median [IQR] in years | 61 [54, 67] |
| Male sex, n (%) | 66 (61.7) |
| Time from transplantation to baseline, median [IQR] in years | 7 [4, 12] |
| Transplant type, n (%) | |
| Kidney | 50 (46.7) |
| Liver | 38 (35.5) |
| Lung | 19 (17.8) |
| Re-transplantation, n (%) | 19 (17.8) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 24 (22.4) |
| Cardiovascular disease | 81 (75.7) |
| Dialysis | 2 (1.9) |
| Immunosuppressive maintenance therapy, n (%) | |
| Tacrolimus * | 72 (67.3) |
| Ciclosporin * | 23 (21.5) |
| mTOR inhibitor * | 17 (15.9) |
| Corticosteroids | 73 (68.2) |
| Antimetabolites | |
| None | 15 (14.0) |
| Azathioprine | 14 (13.1) |
| Mycophenolate | 78 (72.9) |
| Time from baseline to follow-up sample, median [IQR] in months | 4.5 [3.9, 4.9] |
| Time from baseline sample to vaccination, median [IQR] in days | 35 [21, 52] |
| Time from vaccination to follow-up, median [IQR] in days | 98 [80, 114] |
| Number of vaccine doses at baseline, n (%) | |
| 3 | 10 (9.3) |
| 4 | 92 (86.0) |
| 5 | 5 (4.7) |
| Type of latest COVID-19 vaccine at follow-up, n (%) | |
| Monovalent BNT162b2 | 5 (4.7) |
| Bivalent BNT162b2/BA.1 | 34 (31.8) |
| Bivalent BNT162b2/BA.4-5 | 68 (63.6) |
| Time between last two doses of COVID-19 vaccine, median [IQR] in days | 253 [241, 261] |
| Time between last two doses of COVID-19 vaccine, n (%) | |
| More than six months | 96 (89.7) |
| Less than six months | 11 (10.3) |
| Time of latest SARS-CoV-2 infection, n (%) | |
| Never infected | 30 (28.0) |
| Before baseline | 60 (56.1) |
| During follow-up | 17 (15.9) |
| Monoclonal anti-SARS-CoV-2 antibody within six months before baseline, n (%) | 7 (6.5) |
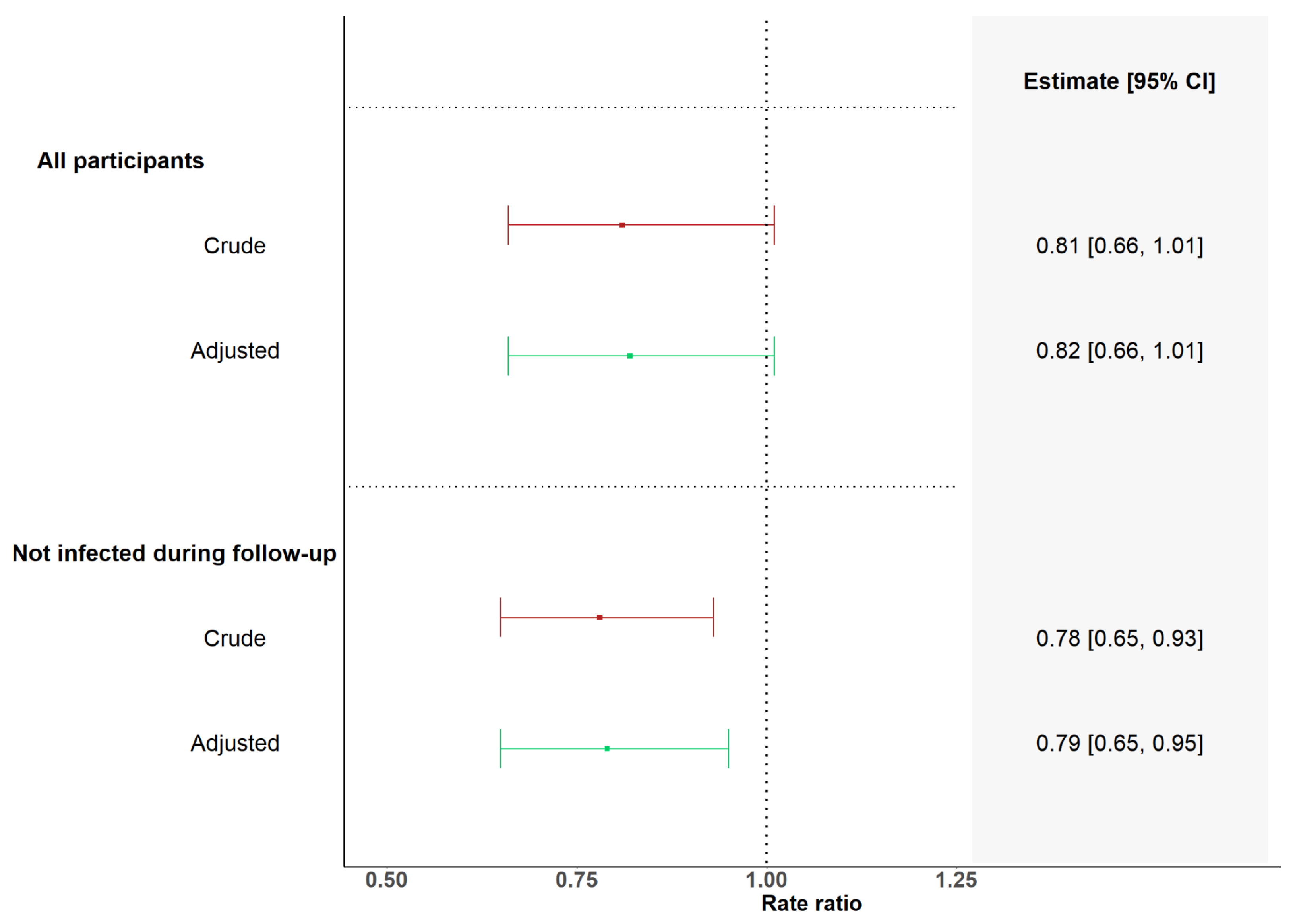
| Vaccinated with Less than Six Months Interval | Vaccinated with More than Six Months Interval | Rate Ratio of Monthly Fold Change in GMCs between SOT Recipients Vaccinated with Less than 6 Months Interval and More than 6 Months Interval | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Fold Change in GMCs per Month | 95% CI | n | Fold Change in GMCs per Month | 95% CI | Rate Ratio | 95% CI | p-Value | |
| All | 11 | 96 | |||||||
| Crude | 1.09 | 0.89–1.33 | 1.34 | 1.25–1.44 | 0.81 | 0.66–1.01 | 0.059 | ||
| Adjusted | 1.09 | 0.89–1.34 | 1.34 | 1.25–1.44 | 0.82 | 0.66–1.01 | 0.063 | ||
| No infection during follow-up | 10 | 80 | |||||||
| Crude | 0.97 | 0.82–1.15 | 1.24 | 1.17–1.32 | 0.78 | 0.65–0.93 | 0.007 | ||
| Adjusted | 0.99 | 0.83–1.17 | 1.25 | 1.18–1.33 | 0.79 | 0.65–0.95 | 0.011 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamm, S.R.; Loft, J.A.; Pérez-Alós, L.; Heftdal, L.D.; Hansen, C.B.; Møller, D.L.; Pries-Heje, M.M.; Hasselbalch, R.B.; Fogh, K.; Hald, A.; et al. The Impact of Time between Booster Doses on Humoral Immune Response in Solid Organ Transplant Recipients Vaccinated with BNT162b2 Vaccines. Viruses 2024, 16, 860. https://doi.org/10.3390/v16060860
Hamm SR, Loft JA, Pérez-Alós L, Heftdal LD, Hansen CB, Møller DL, Pries-Heje MM, Hasselbalch RB, Fogh K, Hald A, et al. The Impact of Time between Booster Doses on Humoral Immune Response in Solid Organ Transplant Recipients Vaccinated with BNT162b2 Vaccines. Viruses. 2024; 16(6):860. https://doi.org/10.3390/v16060860
Chicago/Turabian StyleHamm, Sebastian Rask, Josefine Amalie Loft, Laura Pérez-Alós, Line Dam Heftdal, Cecilie Bo Hansen, Dina Leth Møller, Mia Marie Pries-Heje, Rasmus Bo Hasselbalch, Kamille Fogh, Annemette Hald, and et al. 2024. "The Impact of Time between Booster Doses on Humoral Immune Response in Solid Organ Transplant Recipients Vaccinated with BNT162b2 Vaccines" Viruses 16, no. 6: 860. https://doi.org/10.3390/v16060860
APA StyleHamm, S. R., Loft, J. A., Pérez-Alós, L., Heftdal, L. D., Hansen, C. B., Møller, D. L., Pries-Heje, M. M., Hasselbalch, R. B., Fogh, K., Hald, A., Ostrowski, S. R., Frikke-Schmidt, R., Sørensen, E., Hilsted, L., Bundgaard, H., Garred, P., Iversen, K., Perch, M., Sørensen, S. S., ... Nielsen, S. D. (2024). The Impact of Time between Booster Doses on Humoral Immune Response in Solid Organ Transplant Recipients Vaccinated with BNT162b2 Vaccines. Viruses, 16(6), 860. https://doi.org/10.3390/v16060860






