Assessment of Survival Kinetics for Emergent Highly Pathogenic Clade 2.3.4.4 H5Nx Avian Influenza Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Safety Statement
2.2. Cell Lines
2.3. Preparation of Replicate Samples for Infectivity Assessment at Different Temperatures
2.4. Virus Quantification after Incubation at Different Temperatures
2.5. Statistical Analyses
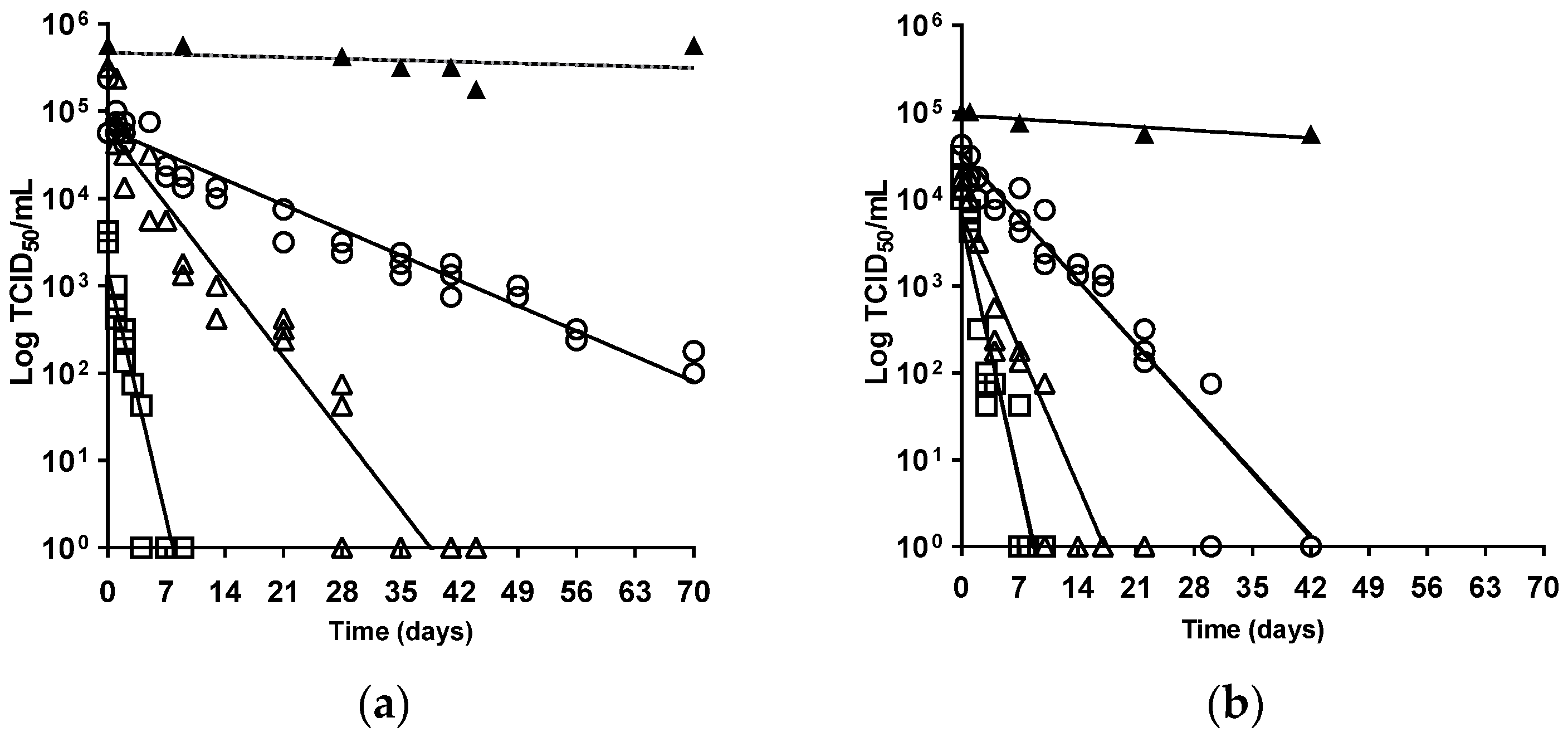
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, D.J. An overview of the epidemiology of avian influenza. Vaccine 2007, 25, 5637–5644. [Google Scholar] [CrossRef] [PubMed]
- WOAH. Infection with high pathogenicity avian influenza viruses. In Terrestrial Animal Health Code; Chapter 10.4; World Organisation for Animal Health: Paris, France, 2023; Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/ (accessed on 23 May 2024).
- Alexander, D.J.; Brown, I.H. History of highly pathogenic avian influenza. OIE Rev. Sci. Tech. 2009, 28, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, J.G.B.; Hoyle, B.J.; Verhagen, J.H.; Nolet, B.A.; Fouchier, R.A.M.; Klaassen, M. Juveniles and migrants as drivers for seasonal epizootics of avian influenza virus. J. Anim. Ecol. 2014, 83, 266–275. [Google Scholar] [CrossRef] [PubMed]
- WHO. Evolution of H5N1 avian influenza viruses in Asia. Emerg. Infect. Dis. 2005, 11, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.H.; Banks, J.; Manvell, R.; Essen, S.C.; Shell, W.; Slomka, M.; Löndt, B.; Alexander, D.J. Recent epidemiology and ecology of influenza A viruses in avian species in Europe and the Middle East. Dev. Biol. 2006, 124, 45–50. [Google Scholar]
- Antigua, K.J.C.; Choi, W.S.; Baek, Y.H.; Song, M.S. The Emergence and Decennary Distribution of Clade 2.3.4.4 HPAI H5Nx. Microorganisms 2019, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Nunez, I.A.; Ross, T.M. A review of H5Nx avian influenza viruses. Ther. Adv. Vaccines Immunother. 2019, 7, 2515135518821625. [Google Scholar] [PubMed]
- Global Consortium for H5N8 and Related Influenza Viruses. Role for migratory wild birds in the global spread of avian influenza H5N8. Science 2016, 354, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, V.; Lewis, N.S.; Pohlmann, A.; Baillie, S.R.; Banyard, A.C.; Beer, M.; Brown, I.H.; Fouchier, R.A.M.; Hansen, R.D.E.; Lameris, T.K.; et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. Sci. Rep. 2022, 12, 11729. [Google Scholar] [CrossRef]
- WOAH. Avian Influenza—Sitaution Reports; WOAH–World Organisation for Animal Health: Paris, France, 2024; Available online: https://www.woah.org/app/uploads/2024/01/hpai-situation-report-20240109.pdf (accessed on 23 May 2024).
- Alarcon, P.; Brouwer, A.; Venkatesh, D.; Duncan, D.; Dovas, C.I.; Georgiades, G.; Monne, I.; Fusaro, A.; Dan, A.; Śmietanka, K.; et al. Comparison of 2016–2017 and previous epizootics of highly pathogenic avian influenza H5 Guangdong lineage in Europe. Emerg. Infect. Dis. 2018, 24, 2270–2283. [Google Scholar] [CrossRef]
- Pohlmann, A.; King, J.; Fusaro, A.; Zecchin, B.; Banyard, A.C.; Brown, I.H.; Byrne, A.M.P.; Beerens, N.; Liang, Y.; Heutink, R.; et al. Has Epizootic Become Enzootic? Evidence for a Fundamental Change in the Infection Dynamics of Highly Pathogenic Avian Influenza in Europe, 2021. MBio 2022, 13, e0060922. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.M.P.; James, J.; Mollett, B.C.; Meyer, S.M.; Lewis, T.; Czepiel, M.; Seekings, A.H.; Mahmood, S.; Thomas, S.S.; Ross, C.R.; et al. Investigating the genetic diversity of H5 avian influenza in the UK 2020–2022. Microbiol. Spectr. 2023, 11, e04776-22. [Google Scholar] [CrossRef] [PubMed]
- Furness, R.W.; Gear, S.C.; Camphuysen, K.; Tyler, G.; de Silva, D.; Warren, C.J.; James, J.; Reid, S.M.; Banyard, A.C. Environmental Samples Test Negative for Avian Influenza Virus H5N1 Four Months after Mass Mortality at A Seabird Colony. Pathogens 2023, 12, 584. [Google Scholar] [CrossRef] [PubMed]
- Defra. Updated Outbreak Assessment #42. Highly pathogenic avian influenza (HPAI) in the UK and Europe, 4 May 2023. In Avian Influenza (Bird Flu) in Europe, Russia and the UK; Department for Environment, Food and Rural Affairs: London, UK, 2023. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1156065/4_May_2023_Highly_pathogenic_avian_influenza__HPAI__in_the_UK_and_Europe.pdf (accessed on 23 May 2024).
- Swayne, D.E. Epidemiology of avian influenza in agricultural and other man-made systems. In Avian Influenza, 1st ed.; Swayne, D.E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; Chapter 4; pp. 59–85. [Google Scholar]
- Hauck, R.; Crossley, B.M.; Rejmanek, D.; Zhou, H.; Gallardo, R.A. Persistence of highly pathogenic and low pathogenic avian influenza viruses in footbaths and poultry manure. Avian Dis. 2017, 61, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Stallknecht, D.E.; Goekjian, V.H.; Wilcox, B.R.; Poulson, R.L.; Brown, J.D. Avian influenza virus in aquatic habitats: What do we need to learn? Avian Dis. 2010, 54, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.P.; Choi, Y.W.; Chappie, D.J.; Rogers, J.V.; Kaye, J.Z. Environmental persistence of a highly pathogenic avian influenza (H5N1) virus. Environ. Sci. Technol. 2010, 44, 7515–7520. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.A.; Buchy, P. Contaminated soil and transmission of influenza virus (H5N1). Emerg. Infect. Dis. 2012, 18, 1530–1532. [Google Scholar] [CrossRef] [PubMed]
- Swayne, D.E.; Beck, J.R. Heat inactivation of avian influenza and Newcastle disease viruses in egg products. Avian Pathol. 2004, 33, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Spackman, E.A. Review of the Stability of Avian Influenza Virus in Materials from Poultry Farms. Avian. Dis. 2023, 67, 229–236. [Google Scholar] [CrossRef]
- Stallknecht, D.E.; Kearney, M.T.; Shane, S.M.; Zwank, P.J. Effects of pH, temperature, and salinity on persistence of avian influenza viruses in water. Avian Dis. 1990, 34, 412–418. [Google Scholar] [CrossRef]
- Nazir, J.; Haumacher, R.; Ike, A.; Stumpf, P.; Boehm, R.; Marschang, R.E. Long-Term Study on Tenacity of Avian Influenza Viruses in Water (Distilled Water, Normal Saline, and Surface Water) at Different Temperatures. Avian Dis. 2010, 54, 720–724. [Google Scholar] [CrossRef]
- Rohani, P.; Breban, R.; Stallknecht, D.E.; Drake, J.M. Environmental transmission of low pathogenicity avian influenza viruses and its implications for pathogen invasion. Proc. Natl. Acad. Sci. USA 2009, 106, 10365–10369. [Google Scholar] [CrossRef]
- Guan, J.; Chan, M.; Grenier, C.; Wilkie, D.C.; Brooks, B.W.; Spencer, J.L. Survival of avian influenza and Newcastle disease viruses in compost and at ambient temperatures based on virus isolation and real-time reverse transcriptase PCR. Avian Dis. 2009, 53, 26–33. [Google Scholar] [CrossRef]
- WOAH. Avian Influenza (including infection with high pathogenicity avian influenza viruses). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 12th ed.; Chapter 3.3.4; World Organisation for Animal Health: Paris, France, 2023; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/A_summry.htm (accessed on 23 May 2024).
- Slomka, M.J.; Seekings, A.H.; Mahmood, S.; Thomas, S.S.; Puranik, A.; Watson, S.; Byrne, A.M.P.; Hicks, D.; Nunez, A.; Brown, I.H.; et al. Unexpected infection outcomes of China-origin H7N9 low pathogenicity avian influenza virus in turkeys. Sci. Rep. 2018, 8, 7322. [Google Scholar] [CrossRef]
- Villegas, P. Titration of Biological Suspensions. In A Laboratory Manual for the Isolation and Identification of Avian Pathogens, 1st ed.; Swayne, D.E., Glisson, J.R., Jackwood, M.W., Pearson, J.E., Reed, W.M., Eds.; The American Association of Avian: Pathologists, PA, USA, 1998; pp. 248–254. [Google Scholar]
- Kamolsiripichaiporn, S.; Subharat, S.; Udon, R.; Thongtha, P.; Nuanualsuwan, S. Thermal inactivation of foot-and-mouth disease viruses in suspension. Appl. Environ. Microbiol. 2007, 73, 7177–7184. [Google Scholar] [CrossRef]
- Leyson, C.; Youk, S.S.; Smith, D.; Dimitrov, K.; Lee, D.H.; Larsen, L.E.; Swayne, D.E.; Pantin-Jackwood, M.J. Pathogenicity and genomic changes of a 2016 European H5N8 highly pathogenic avian influenza virus (clade 2.3.4.4) in experimentally infected mallards and chickens. Virology 2019, 537, 172–185. [Google Scholar] [CrossRef]
- Slomka, M.J.; Puranik, A.; Mahmood, S.; Thomas, S.S.; Seekings, A.H.; Byrne, A.M.P.; Nunez, A.; Bianco, C.; Mollett, B.C.; Watson, S.; et al. Ducks Are Susceptible to Infection with a Range of Doses of H5N8 Highly Pathogenic Avian Influenza Virus (2016, Clade 2.3.4.4b) and Are Largely Resistant to Virus-Specific Mortality, but Efficiently Transmit Infection to Contact Turkeys. Avian Dis. 2019, 63, 172–180. [Google Scholar] [CrossRef]
- Puranik, A.; Slomka, M.J.; Warren, C.J.; Thomas, S.S.; Mahmood, S.; Byrne, A.M.P.; Ramsay, A.M.; Skinner, P.; Watson, S.; Everett, H.E.; et al. Transmission dynamics between infected waterfowl and terrestrial poultry: Differences between the transmission and tropism of H5N8 highly pathogenic avian influenza virus (clade 2.3.4.4a) among ducks, chickens and turkeys. Virology 2020, 541, 113–123. [Google Scholar] [CrossRef]
- Seekings, A.H.; Warren, C.J.; Thomas, S.S.; Mahmood, S.; James, J.; Byrne, A.M.P.; Watson, S.; Bianco, C.; Nunez, A.; Brown, I.H.; et al. Highly pathogenic avian influenza virus H5N6 (clade 2.3.4.4b) has a preferable host tropism for waterfowl reflected in its inefficient transmission to terrestrial poultry. Virology. 2021, 559, 74–85. [Google Scholar] [CrossRef]
- James, J.; Billington, E.; Warren, C.J.; De Sliva, D.; Di Genova, C.; Airey, M.; Meyer, S.M.; Lewis, T.; Peers-Dent, J.; Thomas, S.S.; et al. Clade 2.3.4.4b H5N1 high pathogenicity avian influenza virus (HPAIV) from the 2021/22 epizootic is highly duck adapted and poorly adapted to chickens. J. Gen. Virol. 2023, 104, 001852. [Google Scholar] [CrossRef]
- Parker, C.D.; Irvine, R.M.; Slomka, M.J.; Pavlidis, T.; Hesterberg, U.; Essen, S.; Cox, B.; Ceeraz, V.; Alexander, D.J.; Manvell, R.; et al. Outbreak of Eurasian lineage H5N1 highly pathogenic avian influenza in turkeys in Great Britain in November 2007. Vet. Rec. 2014, 175, 282. [Google Scholar] [CrossRef]
- James, J.; Warren, C.J.; de Silva, D.; Lewis, T.; Grace, K.; Reid, S.M.; Falchieri, M.; Brown, I.H.; Banyard, A.C. The Role of Airborne Particles in the Epidemiology of Clade 2.3.4.4b H5N1 High Pathogenicity Avian Influenza Virus in Commercial Poultry Production Units. Viruses 2023, 15, 1002. [Google Scholar] [CrossRef]
- Brown, J.D.; Goekjian, G.; Poulson, R.; Valeika, S.; Stallknecht, D.E. Avian influenza virus in water: Infectivity is dependent on pH, salinity and temperature. Vet. Microbiol. 2009, 136, 20–26. [Google Scholar] [CrossRef]
- Poen, M.J.; Venkatesh, D.; Bestebroer, T.M.; Vuong, O.; Scheuer, R.D.; Oude Munnink, B.B.; de Meulder, D.; Richard, M.; Kuiken, T.; Koopmans, M.P.G.; et al. Co-circulation of genetically distinct highly pathogenic avian influenza A clade 2.3.4.4 (H5N6) viruses in wild waterfowl and poultry in Europe and East Asia, 2017–2018. Virus Evol. 2019, 5, vez004. [Google Scholar] [CrossRef]
- Dou, D.R.R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef]
- Lenard, J. Viral Membranes. In Encyclopedia of Virology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 308–314. [Google Scholar]
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. (Eds.) Virion Structure and Composition. In Fenner and White’s Medical Virology, 5th ed.; Elsevier: London, UK, 2017; Chapter 3, pp. 27–37. [Google Scholar]
- Gummersheimer, S.L.; Danthi, P. Reovirus Core Proteins λ1 and σ2 Promote Stability of Disassembly Intermediates and Influence Early Replication Events. J. Virol. 2020, 94, e00491-20. [Google Scholar] [CrossRef]
- Lane, J.V.; Jeglinski, J.W.E.; Avery-Gomm, S.; Ballstaedt, E.; Banyard, A.C.; Barychka, T.; Brown, I.H.; Brugger, B.; Burt, T.V.; Careen, N.; et al. High pathogenicity avian influenza (H5N1) in Northern Gannets (Morus bassanus): Global spread, clinical signs and demographic consequences. IBIS Av. Repro. Spec. Issue 2024, 166, 633–650. [Google Scholar] [CrossRef]
- Vigeveno, R.M.; Poen, M.J.; Parker, E.; Holwerda, M.; de Haan, K.; van Montfort, T.; Lewis, N.S.; Russell, C.A.; Fouchier, R.A.M.; de Jong, M.D.; et al. Outbreak severity of highly pathogenic avian influenza A(H5N8) viruses is inversely correlated to polymerase complex activity and interferon induction. J. Virol. 2020, 94, 11. [Google Scholar] [CrossRef]
- Hill, S.C.; Manvell, R.J.; Schulenburg, B.; Shell, W.; Wikramaratna, P.S.; Perrins, C.; Sheldon, B.C.; Brown, I.H.; Pybus, O.G. Antibody responses to avian influenza viruses in wild birds broaden with age. Proc. Biol. Sci. 2016, 283, 20162159. [Google Scholar] [CrossRef]
- Seekings, A.H.; Warren, C.J.; Thomas, S.S.; Lean, F.Z.X.; Selden, D.; Mollett, B.C.; van Diemen, P.M.; Banyard, A.C.; Slomka, M.J. Different Outcomes of Chicken Infection with UK-Origin H5N1-2020 and H5N8-2020 High-Pathogenicity Avian Influenza Viruses (Clade 2.3.4.4b). Viruses 2023, 15, 1909. [Google Scholar] [CrossRef]
- Seekings, A.; Liang, Y.; Warren, C.J.; Hjulsager, C.K.; Thomas, S.S.; Lean, F.Z.X.; Núñez, A.; Skinner, P.; Selden, D.; Falchieri, M.; et al. Transmission dynamics and pathogenesis differ between pheasants and partridges infected with clade 2.3.4.4b H5N8 and H5N1 high-pathogenicity avian influenza viruses. J. Gen. Virol. 2024, 105, 001946. [Google Scholar] [CrossRef]
- Pan American Health Organisation (PAHO). PAHO Issues Alert on Outbreaks of Avian Influenza in Birds in Ten Countries of the Americas; Pan American Health Organization: Washington, DC, USA, 2023. [Google Scholar]
- Bennison, A.; Byrne, A.M.P.; Reid, S.M.; Lynton-Jenkins, J.G.; Mollett, B.; de Sliva, D.; Peers-Dent, J.; Finlayson, K.; Hall, R.; Blockley, F.; et al. Detection and spread of high pathogenicity avian influenza virus H5N1 in the Antarctic Region. 2024. Available online: https://www.biorxiv.org/content/10.1101/2023.11.23.568045v1 (accessed on 23 May 2024).
- European Council. Council Directive 2005/94/EC of 20 December 2005 on Community measures for the control of avian influenza and repealing Directive 92/40/EEC. Off. J. Eur. Union L. 2005, 10, 16–65. Available online: https://www.legislation.gov.uk/eudr/2005/94/contents (accessed on 23 May 2024).
- Defra. Notifiable Avian Disease Control Strategy for Great Britain. Last updated September 2019. Derogations 46-48. 2019. Available online: https://www.gov.uk/government/publications/notifiable-avian-disease-control-strategy (accessed on 23 May 2024).
- Stephens, C.B.; Spackman, E. Thermal Inactivation of avian influenza virus in poultry litter as a method to decontaminate poultry houses. Prev. Vet. Med. 2017, 145, 73–77. [Google Scholar] [CrossRef]
- APHIS. HPAI Response. In Cleaning and Disinfection Basics (Virus Elimination); USDA Animal and Plant Health Inspection Service: Riverdale, MD, USA, 2022. Available online: https://www.aphis.usda.gov/sites/default/files/cleaning_disinfection.pdf (accessed on 23 May 2024).
| Isolate | Subtype/Clade | Descriptor in Text | Collection Date 1 |
|---|---|---|---|
| A/duck/England/1279/2014 | H5N8 2.3.4.4a | H5N8-2014 | 16 November 2014 |
| A/wigeon/Wales/52833/2016 | H5N8 2.3.4.4b | H5N8-2016 | 14 December 2016 |
| A/swan/England/001986/2017 | H5N6 2.3.4.4b | H5N6-2017 | 31 December 2017 |
| A/chicken/England/030786/2020 | H5N8 2.3.4.4b | H5N8-2020 | 1 November 2020 |
| A/chicken/Wales/053969/2021 | H5N1 2.3.4.4b | H5N1-2021 | 31 October 2021 |
| Temperature | 4 °C | 20 °C | 30 °C | ||||
|---|---|---|---|---|---|---|---|
| Strain | DT | Extinction 1 | DT | Extinction 1 | Strain | DT (days) | Extinction 1 |
| H5N8-2020 | 24.2 | 116.1 | 8.0 | 38.5 | H5N8-2016 | 2.7 | 13.2 |
| H5N8-2016 | 14.0 | 69.8 | 6.1 | 28.8 | H5N1-2021 | 2.3 | 8.6 |
| H5N1-2021 | 9.5 | 43.2 | 4.5 | 17.2 | H5N8-2020 | 2.5 | 7.8 |
| H5N6-2017 | 11.2 | 40.0 | 2.8 | 10.4 | |||
| H5N8-2014 | 7.8 | 22.9 | 3.0 | 8.7 | |||
| HPAIV | Result of Multiple Comparisons of DT Value/Extinction Time at 4 °C | ||||
|---|---|---|---|---|---|
| HPAIV Isolate | H5N8-2014 | H5N8-2016 | H5N6-2017 | H5N8-2020 | H5N1-2021 |
| H5N8-2014 | - | <0.05/<0.001 | <0.001 | <0.001 | 0.33/<0.001 |
| H5N8-2016 | - | - | 0.06/<0.001 | <0.001 | <0.001 |
| H5N6-2017 | - | - | - | <0.001 | 0.22/0.58 |
| H5N8-2020 | - | - | - | - | <0.001 |
| H5N1-2021 | - | - | - | - | - |
| HPAIV | Result of Multiple Comparisons of DT Value/Extinction Time at 20 °C | ||||
|---|---|---|---|---|---|
| HPAIV Isolate | H5N8-2014 | H5N8-2016 | H5N6-2017 | H5N8-2020 | H5N1-2021 |
| H5N8-2014 | - | <0.01/<0.001 | 0.35/0.97 | <0.001 | 0.55/<0.05 |
| H5N8-2016 | - | - | <0.001 | <0.01/<0.001 | <0.001 |
| H5N6-2017 | - | - | - | <0.001 | <0.001/0.13 |
| H5N8-2020 | - | - | - | - | <0.001 |
| H5N1-2021 | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warren, C.J.; Brookes, S.M.; Arnold, M.E.; Irvine, R.M.; Hansen, R.D.E.; Brown, I.H.; Banyard, A.C.; Slomka, M.J. Assessment of Survival Kinetics for Emergent Highly Pathogenic Clade 2.3.4.4 H5Nx Avian Influenza Viruses. Viruses 2024, 16, 889. https://doi.org/10.3390/v16060889
Warren CJ, Brookes SM, Arnold ME, Irvine RM, Hansen RDE, Brown IH, Banyard AC, Slomka MJ. Assessment of Survival Kinetics for Emergent Highly Pathogenic Clade 2.3.4.4 H5Nx Avian Influenza Viruses. Viruses. 2024; 16(6):889. https://doi.org/10.3390/v16060889
Chicago/Turabian StyleWarren, Caroline J., Sharon M. Brookes, Mark E. Arnold, Richard M. Irvine, Rowena D. E. Hansen, Ian H. Brown, Ashley C. Banyard, and Marek J. Slomka. 2024. "Assessment of Survival Kinetics for Emergent Highly Pathogenic Clade 2.3.4.4 H5Nx Avian Influenza Viruses" Viruses 16, no. 6: 889. https://doi.org/10.3390/v16060889
APA StyleWarren, C. J., Brookes, S. M., Arnold, M. E., Irvine, R. M., Hansen, R. D. E., Brown, I. H., Banyard, A. C., & Slomka, M. J. (2024). Assessment of Survival Kinetics for Emergent Highly Pathogenic Clade 2.3.4.4 H5Nx Avian Influenza Viruses. Viruses, 16(6), 889. https://doi.org/10.3390/v16060889






