Abstract
The replication of species A rotaviruses (RVAs) involves the recruitment of and interaction with cellular organelles’ lipid droplets (LDs), both physically and functionally. The inhibition of enzymes involved in the cellular fatty acid biosynthesis pathway or the inhibition of cellular lipases that degrade LDs was found to reduce the functions of ‘viral factories’ (viroplasms for rotaviruses or replication compartments of other RNA viruses) and decrease the production of infectious progeny viruses. While many other RNA viruses utilize cellular lipids for their replication, their detailed analysis is far beyond this review; only a few annotations are made relating to hepatitis C virus (HCV), enteroviruses, SARS-CoV-2, and HIV-1.
1. Introduction
While the replication cycles of many RNA and DNA viruses have been well researched, it has only relatively recently been recognized that various cellular lipids are involved in many of the viral replication steps [1,2,3,4,5,6,7].
Cellular lipids, collectively termed the ‘liposome’, are classified as membrane lipids (phospholipids, sphingolipids, glycolipids), cholesterol, steroids, triacylglycerol (TAG), fatty acids, and eicosanoids [8]. Viruses interact with host cell lipids in various ways, affecting their biosynthesis and metabolism and gaining energy for their own replication [3]. In this study, the significance of cellular lipids for the replication of rotaviruses will be reviewed in some detail. Analogous data have been acquired for many other RNA viruses. The fact that the disturbance of cellular lipid metabolism decreases the yield of infectious viral progeny for many viruses has made the search for lipid-disturbing compounds an attractive aim for the development of broadly active candidate antivirals [1,5,6,7,9].
2. Rotaviruses
Species A rotaviruses (RVAs) are a major cause of severe gastroenteritis in infants and young children worldwide and in the young of many mammalian and avian host species [10]. RVA-associated disease leads to the death of approximately 130,000 children under the age of 5 y annually worldwide, mainly in low-income countries [11]. Two RVA vaccines have been licensed since 2006 and are now used in expanded programs of immunization (EPIs) in over 100 countries [12]. In addition, alternative RV vaccines were licensed in India, Vietnam, and China [12]. RVA EPIs have significantly decreased RVA-associated disease, although at different levels in different parts of the world [13,14]. The molecular biology of RVA replication has been well studied [10]. During replication, ‘viroplasms’ form, which are intracytoplasmic inclusion bodies utilized for early RVA morphogenesis and viral RNA replications to take place; they have been termed ‘viral factories’ and have been recognized as essential [10]. The rotavirus-encoded components of viroplasms, NSP2 and NSP5, are subject to liquid–liquid phase separation [15,16]. In 2010, rotavirus viroplasms (containing VP1, VP2, VP3, VP6 and viral RNA segments) were discovered to be associated with components of cellular organelles’ lipid droplets (LDs) [17] [Figure 1]. LDs of various sizes (0.05–200 µm in diameter) are storage sites of sterol esters and triglycerides, which serve as major cellular energy sources and are in close contact with the mitochondria and the 65 endoplasmic reticulum (ER). The phospholipid monolayer of LDs contains various proteins such as perilipins [PLINs], diacylglycerol acyl transferase 2 [DGAT2], Rab18 [a transport protein], CTP-phosphocholine cytidylyltransferase [CCT], the rate-limiting enzyme of phosphatidylcholine synthesis [18], and various other proteins such as ADRP adipophilin and tip47, often bound by amphipathic helices [18]. LDs also contain acyl-CoA cholesterol acyltransferases 1 and 2 (ACAT1, ACAT2), which catalyze cholesterol esterification. A genome-wide, siRNA- based screen identified 550/18,000 genes of human macrophages (THP-1) as being involved in modulating lipid storage according to number, size, and cellular localization and functions such as proteasome activity, intracellular transport, transcription activity, E3 ligase activity, and lipid-modifying enzymes [19]. In summary, LDs are central to the regulation of cellular lipid homeostasis [19]. Fluorescence resonance energy transfer (FRET) has been shown to occur between perilipin A and NSP2, proving the close spatial proximity of LDs and viroplasms [17].
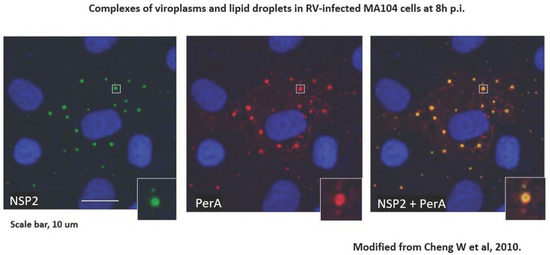
Figure 1.
RV-infected cells grown on coverslips were processed for confocal microscopy (CM) as described [20]. Cells were blocked with 1% BSA–0.1% triton X-100. Primary antibodies against perilipin A (rabbit polyclonal) were from Abcam, and those against NSP2 (mouse monoclonal) were a kind gift of Dr Oscar Burrone, ICGEB, Trieste IT. Secondary antibodies were goat anti-rabbit IgG conjugated with Alexafluor 633 and goat anti-mouse IgG conjugated with Alexafluor 488, both from Invitrogen. Staining with secondary antibodies was carried out in the presence of 2 µg/mL of Hoechst 33,342 (from Sigma). Coverslips were mounted on glass slides with Prolong bold Antifade mounting medium (from Molecular Probes) and observed by CM using a Leica DM Libre TCS SP instrument. An individual viroplasm–LD complex is magnified in the inserts. From [17].
The iodixanol gradient velocity ultracentrifugation of RV-infected cell extracts resulted in the co-sedimentation of viral dsRNA, NSP2, and perilipin A in low-density fractions (1.11–1.15 g/mL). In contrast, rotavirus dual-layered particles (DLPs, with a density of 1.38 g/mL) spiked into mock-infected lysates sedimented to the bottom of the gradient [17]. Iodixanol gradient fractions of low density, containing peaks of the RV dsRNA genome and LD- and viroplasm-associated proteins, were analyzed for 14 different classes of lipids by mass spectrometry and found to contain maximum amounts of lipids as typical components of LDs, confirming the close interaction of LDs with viroplasms [21]. The molecular mechanisms underlying the complex formation of LDs and viroplasms are underexplored [16]. The viral NSP5 protein, an essential component of viroplasms, contains an amphipathic helix [22], which possibly mediates the interaction with LDs, as do other cellular proteins containing amphipathic helices [23].
The complex formation of viroplasms and LDs has functional consequences. LD formation depends critically on the presence of long-chain fatty acids. The following enzymes contribute to their biosynthesis: acetyl-CoA cocarboxylase (ACC-1), fatty acid synthase (FASN), long-chain acyl-CoA synthetase (HCSL), and various glycerol-acyl transferases (GATs), the latter converting long-chain fatty acids into triacyl esters (neutral fats), which are incorporated into the precursors of LDs in the endoplasmic reticulum (ER). Lipid droplets bud from the lipid bilayer of the ER into the cytoplasm and acquire various lipid droplet-associated proteins (PLINs 1–5 and many others) [2].
The key inhibitors of these pathways are TOFA (inhibiting ACC-1), C75 (anti-FASN), triacsin C (anti-ACSL), and A922500 (anti-DGAT-1) [2,24]. The application of these inhibitors to RV-infected cells significantly reduces the size and number of viroplasms formed, the production of viral dsRNA, and the titers of infectious viral progeny. Similarly, the treatment of cells with a combination of [isoproterenol + IBMX], which fragments LDs into smaller microdroplets, also reduces RV progeny production. Representative data for inhibition with TOFA, triacsin C, and [isoproterenol + IBMX] are presented in Table 1 [17,25]. The inhibitory effects of those compounds were reproducibly recorded at non-cytotoxic concentrations; the off-target effects of the compounds cannot be fully excluded at this stage.

Table 1.
Comparison of inhibitory effects of different compounds affecting lipid droplet homeostasis on rotavirus replication.
Furthermore, TOFA-treated and RV-infected cells were analyzed for the production of DLPs and TLPs (purified by CsCl gradient equilibrium ultracentrifugation). In the presence of TOFA, the production of DLPs decreased by a factor of two, but that of TLPs decreased by a factor of 20 compared to the DLP and TLP production in the untreated cells. This suggests that the inhibition of fatty acid biosynthesis affects not only the recruitment of LDs by viroplasms but is also involved in interfering with the later stages of RV maturation [26]. The molecular mechanism is not clear but possibly involves alterations of intracellular membranes, which provide transient envelopes during particle maturation in the cytoplasm [27]. The mechanism of the interaction of viroplasms with LDs was explored further. Following cellular infection with an RVA mutant with delayed viroplasm formation (rRV NSP2 S313D, engineered by a RVA-specific, plasmid only-based reverse genetics system; according to [28]), an early interaction of viroplasm-bound NSP2 with phospho-perilipin 1 (leading to lipolysis [29]) was observed [30], thus exploiting the lipid metabolism.
Recently, it was found that DGAT1, required for triacylglycerol and LD biosynthesis, is degraded in RV-infected MA104 cells and in human intestinal enteroids (HIEs) [31]. In an uninfected cell, DGAT1 synthesizes TAGs from ER-resident DAGs and cytoplasmic acyl-CoAs, and TAGs are deposited in the ER lipid bilayer. Upon RV infection, NSP2 is expressed, which interacts with DGAT1. The viroplasm–DGAT1 complexes are then tagged with ubiquitin and degraded in proteasomes. The loss in DGAT1 induces the budding of TAGs and LD formation through a mechanism that is still not fully understood [31]. The suppression of DGAT1 by rotavirus infection leads to an increase in the number of viroplasms and in the infectivity titer of viral progeny in MA104 cells [31].
3. Other RNA Viruses
Many RNA viruses other than rotaviruses utilize components of the cellular lipidome for their replication. A detailed analysis of the molecular mechanisms involved is beyond the capacity of this review. In the following, a few observations are noted (Table 2) aimed at emphasizing the great significance of this research.

Table 2.
Lipid metabolism involved in the replication of some RNA viruses other than rotaviruses.
3.1. Hepatitis C Virus
The infection of liver cells with the hepatitis C virus (HCV) was shown to induce cytoplasmic double-membrane vesicle (DMV) compartments, also termed vesicle packets, where major steps of viral replication occur [32]. The viral non-structural protein NS5B interacts with the surface of LDs and, together with oter viral core proteins, is involved in viral RNA replication [32,33]. The protein NS5A binds to LDs via an amphipathic helix (similar to viperin), and LD lipolysis is associated with HCV replication, although the molecular mechanism is not clear yet [34]. While the temporal dynamics of cellular lipid species have been explored by mass spectrometry at early and late time points of HCV infection, the mechanisms correlating HCV replication with lipid dynamics remain to be explored [35].
3.2. Enteroviruses and Other Picornaviruses
Phosphatidyl inositole (PI) and its mono- and diphosphate derivatives (PI4P and PI(4.5)P2, respectively) play key roles in the establishment of replication complexes of enteroviruses and other (+)ssRNA viruses [37]. Lipolysis is a major component of enterovirus RNA replication [36,40,41].
3.3. SARS-CoV-2
For coronaviruses, mainly SARS-CoV-2, the causative agent of COVID-19, ceramide plays a central role during viral cell entry. Inhibitors of acid sphingomyelinase, the rate-limiting enzyme of ceramide biosynthesis, suppress viral replication [9].
3.4. HIV-1
Based on previous findings that ceramide is required for capsid maturation in HIV-1, it was recently observed that the inhibition of neutral sphingomyelinase 2, the rate-limiting enzyme of ceramide biosynthesis, prevented the full maturation of HIV-1 capsids and full infectivity in vitro [38] and in vivo [39].
4. Compounds Interfering with Lipidome Homeostasis
The cellular lipidome is beginning to be considered a ‘therapeutic target’ [9] against RNA virus replication. Thus, some compounds interfering with cellular lipidome metabolism are being explored with the aim of reducing viral replication (Table 3).

Table 3.
Compounds disturbing cellular lipidome homeostasis as potential candidate antivirals.
The HCV NS5A protein, bound to the surface of LDs, interacts with cellular cyclophilin A (CypA), and CypA inhibitors were discovered to block viral RNA replication in HCV-infected cells [42].
Enterovirus replication complexes depend on the lipolysis of triglycerides by hormone-dependent lipase (HDL) or adipose triglyceride lipase (ATGL). In the presence of CAY10499, an HDL inhibitor, or atglistatin, an ATGL inhibitor, enterovirus replication decreased [36].
Against SARS-CoV-2 replication, various drugs reducing the cholesterol synthesis and esterification, LD formation, and ceramide biosynthesis of infected cells are under investigation as potential antivirals [7,9,43].
PDDC, an inhibitor of neutral sphingomyelinase 2 that prevents ceramide biosynthesis, was shown to block the maturation and full infectivity of HIV-1 particles [38,39].
Funding
The writing of this review received no external funding.
Acknowledgments
For rotavirus research, the author gratefully acknowledges the collaboration with Oscar Burrone, Winsome Cheung, Serge Chwetzoff, Nathalie Couroussé, Sue E Crawford, Alessandro Esposito, Eleanor Gaunt, Michael Gill, Clemens F Kaminski, Nandita Keshavan, Andrew M L Lever, James E Richards, Germain Trugnan, the late Michael JO Wakelam, and Qifeng Zhang.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Farías, M.A.; Diethelm-Varela, B.; Kalergis, A.M.; González, P.A. Interplay between lipid metabolism, lipid droplets and RNA virus replication. Crit. Rev. Microbiol. 2023, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.E.; Desselberger, U. Lipid droplets form complexes with viroplasms and are crucial for rotavirus replication. Curr. Opin. Virol. 2016, 19, 11–15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heaton, N.S.; Randall, G. Multifaceted roles for lipids in viral infection. Trends Microbiol. 2011, 19, 368–375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herker, E. Lipid Droplets in Virus Replication. FEBS Lett. 2024. [CrossRef] [PubMed]
- Qu, Y.; Wang, W.; Xiao, M.Z.X.; Zheng, Y.; Liang, Q. The interplay between lipid droplets and virus infection. J. Med. Virol. 2023, 95, e28967. [Google Scholar] [CrossRef] [PubMed]
- Roingeard, P.; Eymieux, S.; Burlaud-Gaillard, J.; Hourioux, C.; Patient, R.; Blanchard, E. The double-membrane vesicle (DMV): A virus-induced organelle dedicated to the replication of SARS-CoV-2 and other positive-sense single-stranded RNA viruses. Cell. Mol. Life Sci. 2022, 79, 425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Theken, K.N.; Tang, S.Y.; Sengupta, S.; FitzGerald, G.A. The roles of lipids in SARS-CoV-2 viral replication and the host immune response. J. Lipid Res. 2021, 62, 100129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid classification, structures and tools. Biochim. Biophys. Acta 2011, 1811, 637–647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cesar-Silva, D.; Pereira-Dutra, F.S.; Giannini, A.L.M.; Maya-Monteiro, C.M.; de Almeida, C.J.G. Lipid compartments and lipid metabolism as therapeutic targets against coronavirus. Front. Immunol. 2023, 14, 1268854. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Primers 2017, 3, 17083. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018, 172, 958–965, Erratum in JAMA Pediatr. 2022, 176, 208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bergman, H.; Henschke, N.; Hungerford, D.; Pitan, F.; Ndwandwe, D.; Cunliffe, N.; Soares-Weiser, K. Vaccines for preventing rotavirus diarrhoea: Vaccines in use. Cochrane Database Syst. Rev. 2021, 2021, CD008521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Desselberger, U. Differences of rotavirus vaccine effectiveness by country: Likely causes and contributing factors. Pathogens 2017, 6, 65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parker, E.P.K.; Ramani, S.; Lopman, B.A.; Church, J.A.; Iturriza-Gomara, M.; Prendergast, A.J.; Grassly, N.C. Causes of impaired oral vaccine efficacy in developing countries. Future Microbiol. 2017, 13, 97–118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geiger, F.; Acker, J.; Papa, G.; Wang, X.; Arter, W.E.; Saar, K.L.; Erkamp, N.A.; Qi, R.; Bravo, J.P.; Strauss, S.; et al. Liquid-liquid phase separation underpins the formation of replication factories in rotaviruses. EMBO J. 2021, 40, e107711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papa, G.; Borodavka, A.; Desselberger, U. Viroplasms: Assembly and functions of rotavirus replication factories. Viruses 2021, 13, 1349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheung, W.; Gill, M.; Esposito, A.; Kaminski, C.F.; Courousse, N.; Chwetzoff, S.; Trugnan, G.; Keshavan, N.; Lever, A.; Desselberger, U. Rotaviruses associate with cellular lipid droplet components to replicate in viroplasms, and compounds disrupting or blocking lipid droplets inhibit viroplasm formation and viral replication. J. Virol. 2010, 84, 6782–6798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farese, R.V., Jr.; Walther, T.C. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 2009, 139, 855–860. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mejhert, N.; Kuruvilla, L.; Gabriel, K.R.; Elliott, S.D.; Guie, M.-A.; Wang, H.; Lai, Z.W.; Lane, E.A.; Christiano, R.; Danial, N.N.; et al. Partitioning of MLX-family transcription factors to lipid droplets regulates metabolic gene expression. Mol. Cell 2020, 77, 1251–1264.e9. [Google Scholar] [CrossRef] [PubMed]
- Nejmeddine, M.; Trugnan, G.; Sapin, C.; Kohli, E.; Svensson, L.; Lopez, S.; Cohen, J. Rotavirus spike protein VP4 is present at the plasma membrane and is associated with microtubules in infected cells. J. Virol. 2000, 74, 3313–3320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaunt, E.R.; Zhang, Q.; Cheung, W.; Wakelam, M.J.O.; Lever, A.M.L.; Desselberger, U. Lipidome analysis of rotavirus-infected cells confirms the close interaction of lipid droplets with viroplasms. J. Gen. Virol. 2013, 94 Pt 7, 1576–1586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sen, A.; Sen, N.; Mackow, E.R. The formation of viroplasm-like structures by the rotavirus NSP5 Protein is calcium regulated and directed by a C-terminal helical domain. J. Virol. 2007, 81, 11758–11767. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prévost, C.; Sharp, M.E.; Kory, N.; Lin, Q.; Voth, G.A.; Farese, R.V., Jr.; Walther, T.C. Mechanism and Determinants of Amphipathic Helix-Containing Protein Targeting to Lipid Droplets. Dev. Cell 2018, 44, 73–86.e4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, Y.; George, D.; Prior, A.M.; Prasain, K.; Hao, S.; Le, D.D.; Hua, D.H.; Chang, K.-O. Novel triacsin C analogs as potential antivirals against rotavirus infections. Eur. J. Med. Chem. 2012, 50, 311–318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaunt, E.R.; Cheung, W.; Richards, J.E.; Lever, A.; Desselberger, U. Inhibition of rotavirus replication by downregulation of fatty acid synthesis. J. Gen. Virol. 2013, 94 Pt 6, 1310–1317, Erratum in J. Gen. Virol. 2013, 94 Pt 9, 2140. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.; Gaunt, E.; Lever, A.; Desselberger, U. Rotavirus replication: The role of lipid droplets. In Viral Gastroenteritis; Svensson, L., Desselberger, U., Greenberg, H.B., Estes, M.K., Eds.; Elsevier-Academic Press: Amsterdam, The Netherlands, 2016; pp. 175–187. [Google Scholar]
- Crawford, S.E.; Ding, S.; Greenberg, H.B.; Estes, M.K. Rotaviruses. In Fields Virology, 7th ed.; Volume 3, RNA viruses; Howley, P.M., Knipe, D.M., Damania, B.A., Cohen, J.I., Whelan, S.P.J., Freed, E.O., Enquist, L., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2023; pp. 362–413. [Google Scholar]
- Kanai, Y.; Komoto, S.; Kawagishi, T.; Nouda, R.; Nagasawa, N.; Onishi, M.; Matsuura, Y.; Taniguchi, K.; Kobayashi, T. Entirely plasmid-based reverse genetics system for rotaviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 2349–2354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tansey, J.T.; Huml, A.M.; Vogt, R.; Davis, K.E.; Jones, J.M.; Fraser, K.A.; Brasaemle, D.L.; Kimmel, A.R.; Londos, C. Functional studies on native and mutated forms of perilipins. A role in protein kinase A-mediated lipolysis of triacylglycerols. J. Biol. Chem. 2003, 278, 8401–8406. [Google Scholar] [CrossRef] [PubMed]
- Criglar, J.M.; Estes, M.K.; Crawford, S.E. Rotavirus-induced lipid droplet biogenesis is critical for virus replication. Front. Physiol. 2022, 13, 836870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Z.; Smith, H.; Criglar, J.M.; Valentin, A.J.; Karandikar, U.; Zeng, X.-L.; Estes, M.K.; Crawford, S.E. Rotavirus-mediated DGAT1 degradation: A pathophysiological mechanism of viral-induced malabsorptive diarrhea. Proc. Natl. Acad. Sci. USA 2023, 120, e2302161120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chatel-Chaix, L.; Bartenschlager, R. Dengue virus- and hepatitis C virus-induced replication and assembly compartments: The enemy inside—Caught in the web. J. Virol. 2014, 88, 5907–5911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyanari, Y.; Atsuzawa, K.; Usuda, N.; Watashi, K.; Hishiki, T.; Zayas, M.; Bartenschlager, R.; Wakita, T.; Hijikata, M.; Shimotohno, K. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol 2007, 9, 1089–1097, Erratum in Nat. Cell Biol. 2007, 9, 1216. [Google Scholar] [CrossRef] [PubMed]
- Vieyres, G.; Pietschmann, T. HCV pit stop at the lipid droplet: Refuel lipids and put on a lipoprotein coat before exit. Cells 2019, 8, 233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Islam, K.U.; Anwar, S.; Patel, A.A.; Mirdad, M.T.; Mirdad, M.T.; Azmi, M.I.; Ahmad, T.; Fatima, Z.; Iqbal, J. Global lipidome profiling revealed multifaceted role of lipid species in hepatitis C virus replication, assembly, and host antiviral response. Viruses 2023, 15, 464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laufman, O.; Perrino, J.; Andino, R. Viral generated inter-organelle contacts redirect lipid flux for genome replication. Cell 2019, 178, 275–289.e16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.; He, G.; Filipowicz, N.A.; Randall, G.; Belov, G.A.; Kopek, B.G.; Wang, X. Host Lipids in Positive-Strand RNA Virus Genome Replication. Front. Microbiol. 2019, 10, 286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waheed, A.A.; Zhu, Y.; Agostino, E.; Naing, L.; Hikichi, Y.; Soheilian, F.; Yoo, S.-W.; Song, Y.; Zhang, P.; Slusher, B.S.; et al. Neutral sphingomyelinase 2 is required for HIV-1 maturation. Proc. Natl. Acad. Sci. USA 2023, 120, e2219475120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoo, S.-W.; Waheed, A.A.; Deme, P.; Tohumeken, S.; Rais, R.; Smith, M.D.; DeMarino, C.; Calabresi, P.A.; Kashanchi, F.; Freed, E.O.; et al. Inhibition of neutral sphingomyelinase 2 impairs HIV-1 envelope formation and substantially delays or eliminates viral rebound. Proc. Natl. Acad. Sci. USA 2023, 120, e2219543120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belov, G.A.; van Kuppeveld, F.J.M. Lipid droplets grease enterovirus replication. Cell Host Microbe 2019, 26, 149–151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Viktorova, E.G.; Nchoutmboube, J.A.; Ford-Siltz, L.A.; Iverson, E.; Belov, G.A. Phospholipid synthesis fueled by lipid droplets drives the structural development of poliovirus replication organelles. PLoS Pathog. 2018, 14, e1007280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Madan, V.; Paul, D.; Lohmann, V.; Bartenschlager, R. Inhibition of HCV replication by cyclophilin antagonists is linked to replication fitness and occurs by inhibition of membranous web formation. Gastroenterology 2014, 146, 1361–1372.e9. [Google Scholar] [CrossRef] [PubMed]
- D’Avila, H.; Lima, C.N.R.; Rampinelli, P.G.; Mateus, L.C.O.; Sousa Silva, R.V.d.; Correa, J.R.; Almeida, P.E.d. Lipid metabolism modulation during SARS-CoV-2 infection: A spotlight on extracellular vesicles and therapeutic prospects. Int. J. Mol. Sci. 2024, 25, 640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).