ISKNV Triggers AMPK/mTOR-Mediated Autophagy Signaling through Oxidative Stress, Inducing Antioxidant Enzyme Expression and Enhancing Viral Replication in GF-1 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Virus
2.2. Quantification of Cell Viability
2.3. ROS (Hydrogen Peroxide, H2O2) Generation Assays
2.4. Labeling of Autophagic Vacuoles with Monodansylcadaverine (MDC)
2.5. Transmission Electron Microscopy (TEM)
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
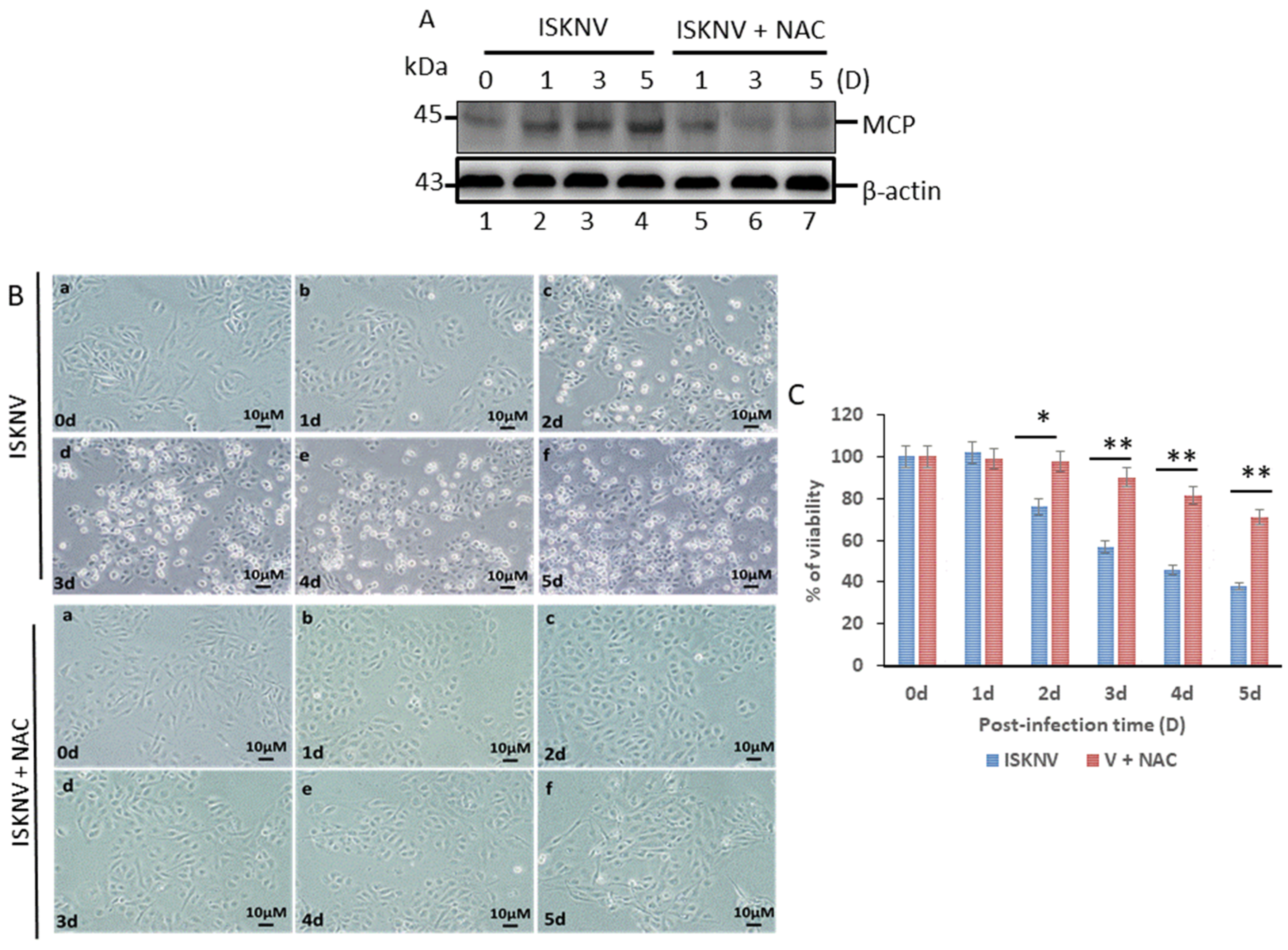
3.1. Blockage of Oxidative Stress Can Enhance Host Cell Survival and Reduce the Viral Titer of ISKNV Infection in GF-1 Cells
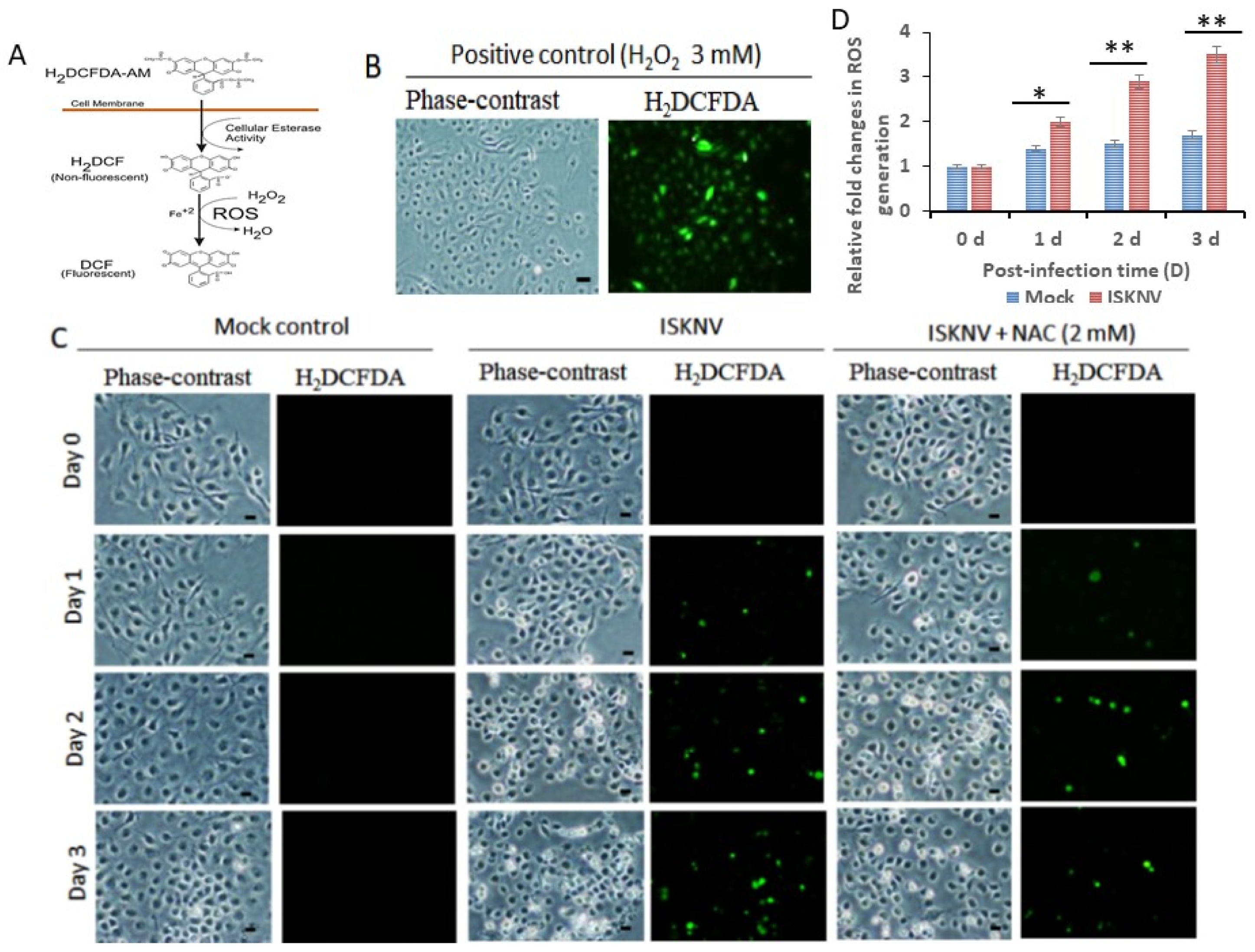
3.2. ISKNV Infection Induces ROS-Mediated Oxidative Stress Signals within Anti-Oxidative Enzyme Expression in GF-1 Cells
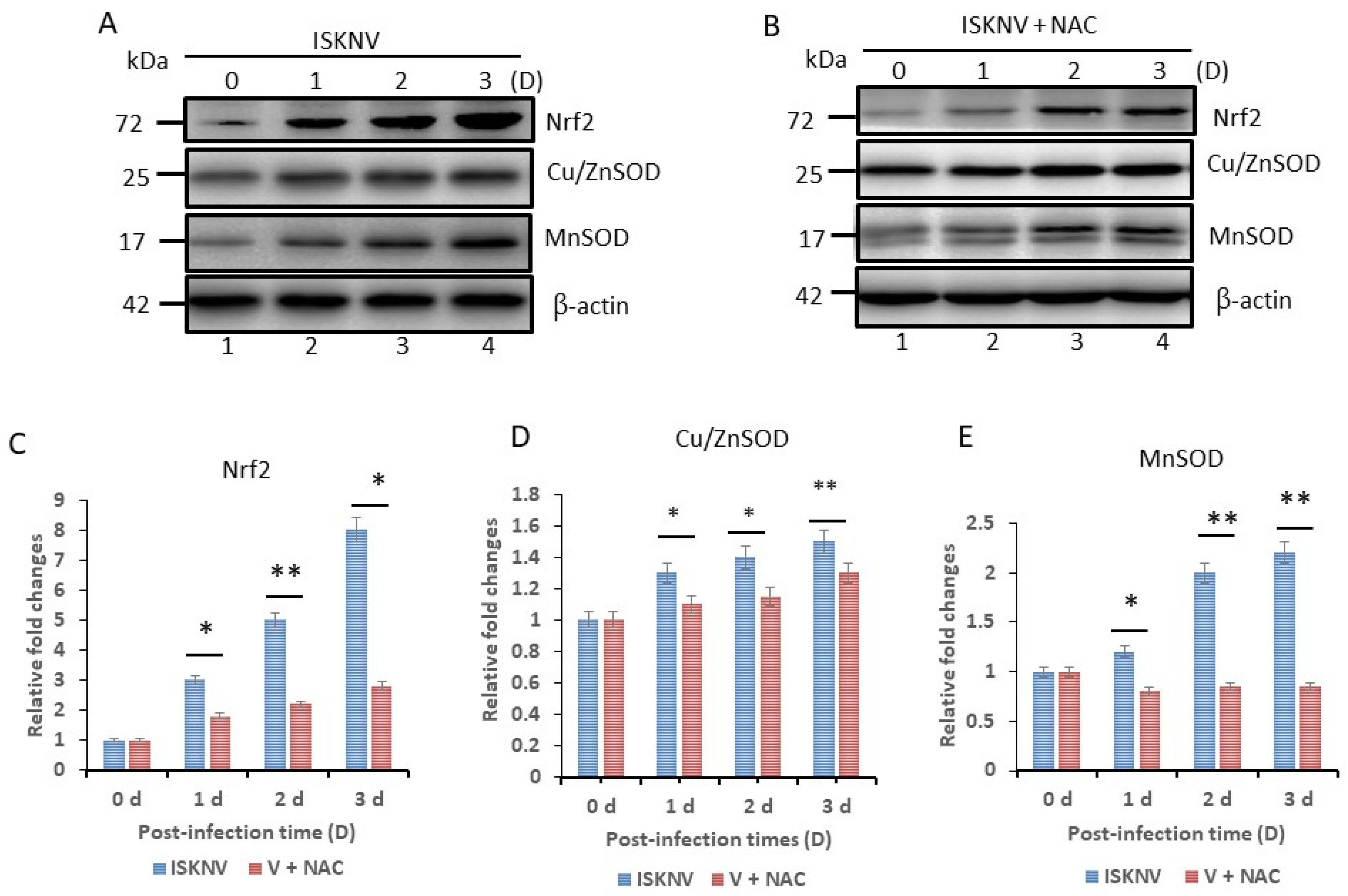
3.3. What Host Antioxidant Responses Does Oxidative Stress Provoke?
3.4. ROS Stress Signaling Is Linked to Autophagy Induction via Puntafomation (MDC)
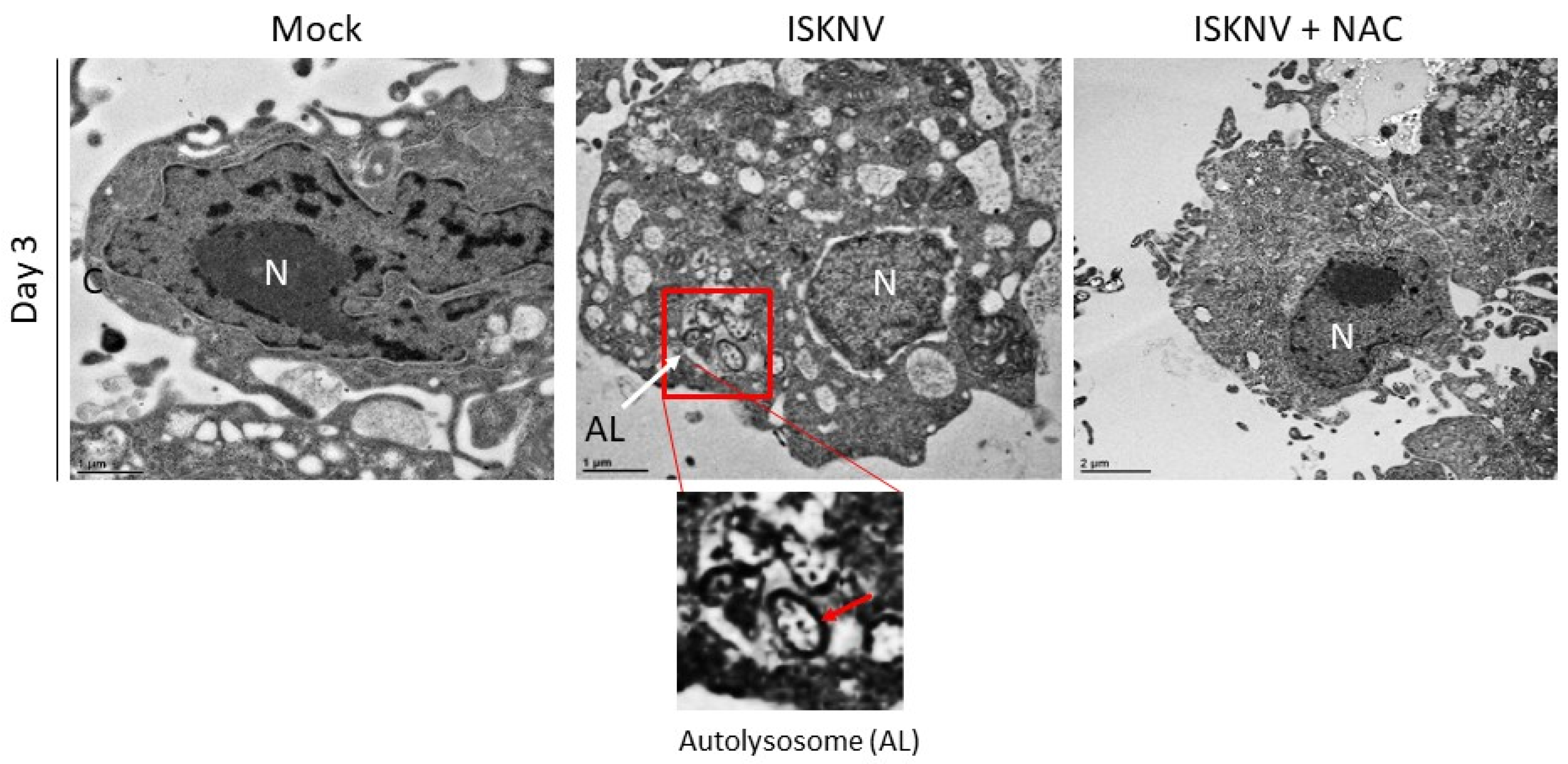
3.5. ROS Stress Signaling Is Linked to Autophagy Induction via Autophagic Markers LC3-II and TEM Criteria
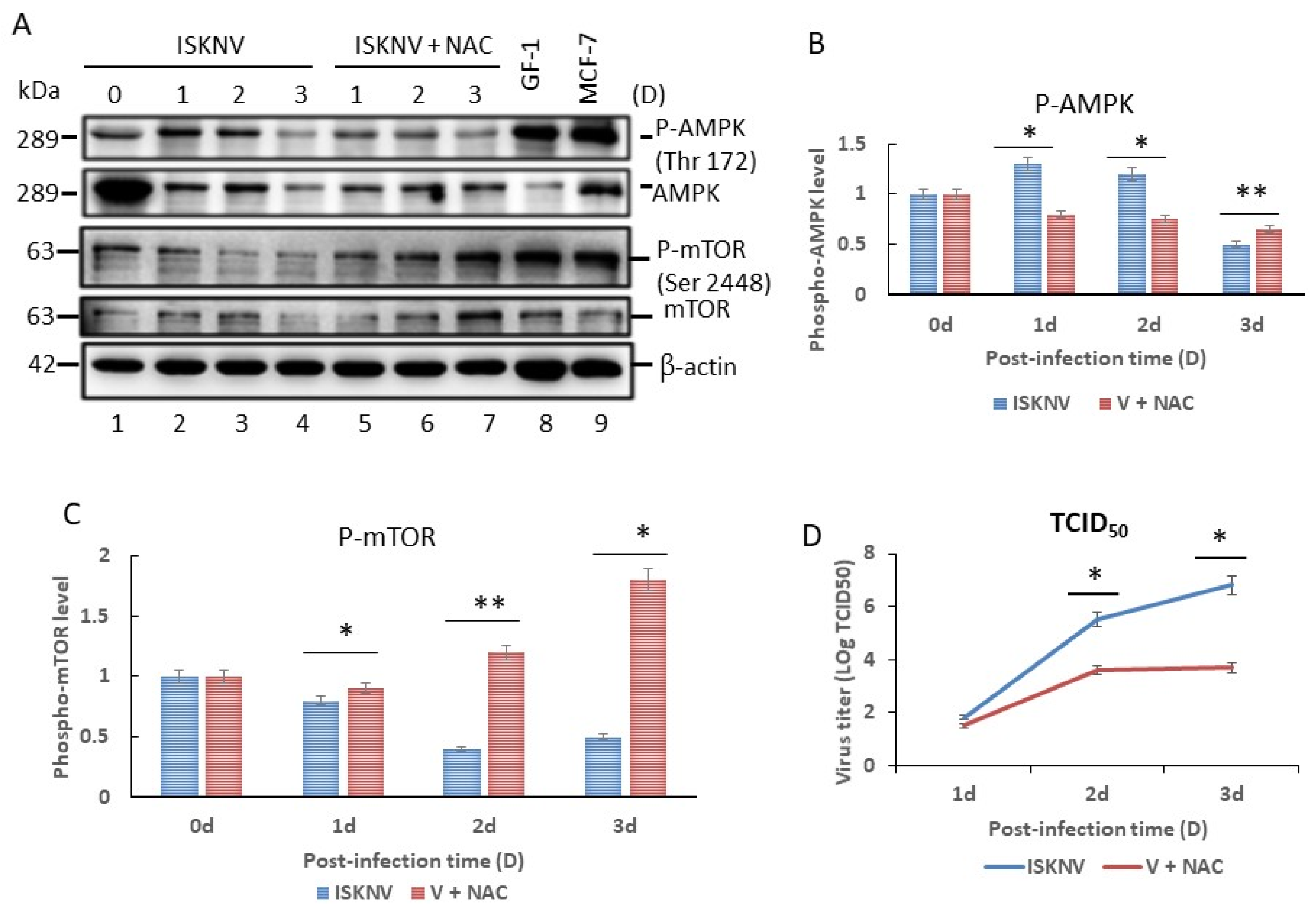
3.6. ROS/Nrf2-Mediated Stress Primes AMPK/mTOR Autophagic Signaling to Regulate Viral Replication in GF-1 Cells
4. Discussion
4.1. Oxidative Stress as a Risk Factor in DNA Viruses
4.2. The Effect of AMPK/mTOR Signaling on Infection with DNA Viruses
4.3. The Effect of Autophagy Flux on DNA Viral Replication
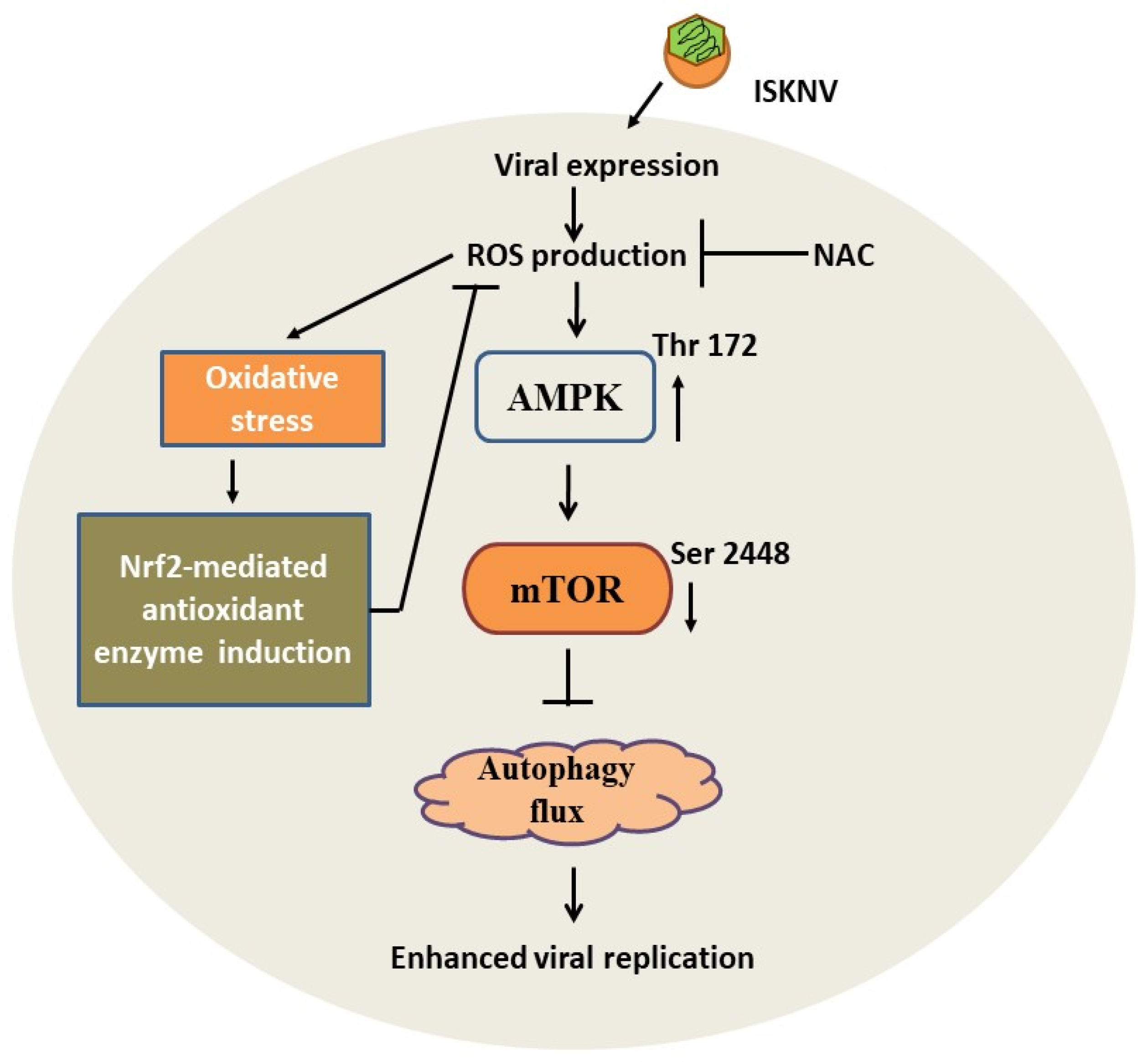
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurita, J.; Nakajima, K. Review: Megalocytivirus. Viruses 2012, 4, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, K.; Shariff, M.; Omar, A.R.; Hair-Bejo, M. Megalocytivirus infection in fish. Rev. Aquac. 2012, 4, 221–233. [Google Scholar] [CrossRef]
- Williams, T.; Barbosa, S.V.; Chinchar, V.G. A decade of advances in iridovirus research. Adv. Virus Res. 2005, 65, 173–248. [Google Scholar]
- Wen, C.M.; Hong, J.R. Complete Genome Sequence of a Giant Sea Perch Iridovirus in Kaohsiung, Taiwan. Genome Announc. 2016, 4, e01759-15. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Y.; Ouyang, Z.; Xu, L.; Yan, Y.; Cui, H.; Han, X.; Qin, Q. Singapore grouper iridovirus, a large DNA virus, induces non apoptotic cell death by a cell type dependent fashion and evokes ERK signaling. Apoptosis 2011, 16, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Krapfenbauer, K.; Engidawork, E.; Cairns, N.; Fountoulakis, M.; Lubee, G. Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res. 2003, 967, 152–160. [Google Scholar] [CrossRef]
- Choongho, L. Therapeutic Modulation of Virus-Induced Oxidative Stress via the Nrf2-Dependent Antioxidative Pathway. Oxid. Med. Cell Longev. 2018, 2018, 6208067. [Google Scholar]
- Chen, P.H.; Hsueh, T.C.; Hong, J.R. ISKNV induces the ROS/Nrf-2-mediated oxidative stress response for the regulation of mitochondrion-mediated Bax/Bak cell death signals in GF-1 cells. Microbiology 2022, 13, 958476. [Google Scholar]
- Desagher, S.; Glowinski, J.; Premont, J. Astrocytes protect neurons from hydrogen peroxide toxicity. J. Neurosci. 1996, 16, 2553–2562. [Google Scholar] [CrossRef]
- Dringen, R.; Hampercht, B. Involvement of glutathione peroxidase and catalase in the disposal of exogenous hydrogen peroxide by cultured astroglial cells. Brain Res. 1997, 759, 67–75. [Google Scholar] [CrossRef]
- Shull, S.; Heintz, N.H.; Periasamy, M.; Manohar, M.; Jansen, Y.M.; Marsh, J.P.; Mossman, B.T. Differential Regulation of Antioxidant Enzymes in Response to Oxidants. J. Biol. Chem. 1991, 266, 24398–24403. [Google Scholar] [CrossRef]
- McClung, C.R. Regulation of catalase in Arabidopsis. Free Radic. Biol. Med. 1997, 23, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Howell, J.M.; Libbey, J.E.; Tainer, J.A.; Fujinami, R.S. Manganese superoxide dismutase induction during measles virus infection. J. Med. Virol. 2003, 70, 470–474. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Glorieux, C.; Juan, M.Z.; Sandoval, M.; Verrax, J.; Calderon, P.B. Regulation of catalase expression in healthy and cancerous cells. Free. Radic. Biol. Med. 2015, 87, 84–97. [Google Scholar] [CrossRef]
- Richetta, C.; Faure, M. Autophagy in antiviral innate immunity. Cell Microbiol. 2012, 15, 368–376. [Google Scholar] [CrossRef]
- English, L.; Chemali, M.; Duron, J.; Rondeau, C.; Laplante, A.; Gingras, D.; Alexander, D.; Leib, D.; Norbury, C.; Lippé, R.; et al. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat. Immunol. 2009, 10, 480–487. [Google Scholar] [CrossRef]
- Paludan, C.; Schmid, D.; Landthaler, M.; Vockerodt, M.; Kube, D.; Tuschl, T.; Münz, C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 2005, 307, 593–596. [Google Scholar] [CrossRef]
- Schmid, D.; Pypaert, M.; Munz, C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity 2007, 26, 79–92. [Google Scholar] [CrossRef]
- Neufeld, T.P. TOR-dependent control of autophagy: Biting the hand that feeds. Curr. Opin. Cell Biol. 2010, 22, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Wang, X. AMPK and mTOR coordinate the regulation of Ulk1 and mammalian autophagy initiation. Autophagy 2011, 7, 924–926. [Google Scholar] [CrossRef]
- Espert, L.; Codogno, P.; Biard-Piechaczyk, M. Involvement of autophagy in viral infections: Antiviral function and subversion by viruses. J. Mol. Med. 2007, 85, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.I.; Nakai, T.; Muroga, K.; Arimoto, M.; Mushiake, K.; Furusawa, I. Properties of a new virus belonging to nodaviridae found in larval striped jack (Pseudocaranx dentex) with nervous necrosis. Virology 1992, 187, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Dobos, P.; Hill, B.; Hallett, R.; Kells, D.; Becht, H.; Teninges, D. Biophysica and biochemical characterization of five animal viruses with bi-segmented double stranded RNA genomes. J. Virol. 1979, 32, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Mullen, P.D.; Brand, R.J.; Parlette, G.N. Evaluation of dye exclusion and colony inhibition techniques for detection of polyomaspecific, cell-mediated immunity. J. Natl. Cancer Inst. 1975, 54, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Chiu, H.W.; Hung, J.C.; Hong, J.R. Beta-nodavirus B2 protein induces hydrogen peroxide production, leading to Drp1-recruited mitochondrial fragmentation and cell death via mitochondrial targeting. Apoptosis 2014, 19, 1457–1470. [Google Scholar] [CrossRef] [PubMed]
- Biederbick, A.; Kern, H.F.; Elsasser, H.P. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur. J. Cell Biol. 1995, 66, 3–14. [Google Scholar]
- Hong, J.R.; Lin, T.L.; Hsu, Y.L.; Wu, J.R. Aproptosis procedes necrosis of fish cell line by infectious pancreatic necrosis virus. Virology 1998, 250, 76–84. [Google Scholar] [CrossRef]
- Li, L.; Oropeza, C.E.; Kaestner, K.H.; McLachlan, A. Limited effects of fasting on hepatitis B virus (HBV) biosynthesis in HBV transgenic mice. J. Virol. 2009, 83, 1682–1688. [Google Scholar] [CrossRef]
- Kain, S.; Mai, K.; Sinai, P. Human multiple tissue western blots: A new immunological tool for the analysis of tissue-specific protein expression. BioTechniques 1994, 17, 982–987. [Google Scholar]
- Chen, M.; Hong, M.J.; Sun, H.; Wang, L.; Shi, X.; Gilbert, B.E.; Corry, D.B.; Kheradmand, F.; Wang, J. Essential role for autophagy in the maintenance of immunological memory against influenza infection. Nat. Med. 2014, 20, 503–510. [Google Scholar] [CrossRef]
- Gabriele, P.; Natasha, I.; Mariapaola, C.; Giovanni, P.; Federica, M.; Vincenzo, A.; Francesco, S.; Domenica, A.; Alessandra, B. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar]
- Jellinger, K.A. General aspects of neurodegeneration. J. Neural Transm. Suppl. 2002, 65, 101–144. [Google Scholar]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Peterhans, E.; Grob, M.; Burge, T.; Zanoni, R. Virus-induced formation of reactive oxygen intermediates in phagocytic cells. Free Radic. Res. Commun. 1987, 3, 39–46. [Google Scholar] [CrossRef]
- Duca, L.; Ottolenghi, S.; Coppola, S.; Rinaldo, R.; Dei Cas, M.; Rubino, F.M.; Paroni, R.; Samaja, M.; Chiumello, D.A.; Motta, I. Differential Redox State and Iron Regulation in Chronic Obstructive Pulmonary Disease, Acute Respiratory Distress Syndrome and Coronavirus Disease 2019. Antioxidants 2021, 10, 1460. [Google Scholar] [CrossRef]
- Veenith, T.; Martin, H.; Breuilly, M.L.; Whitehouse, T.; Smith, F.G.; Duggal, N.; Lord, J.M.; Mian, R.; Sarphie, D.; Moss, P. High generation of reactive oxygen species from neutrophils in patients with severe COVID-19. Sci. Rep. 2022, 12, 10484. [Google Scholar] [CrossRef]
- Vierucci, A.; DeMartino, M.; Graziani, E.; Rossi, M.E.; London, W.T.; Blumberg, B.S. A mechanism for liver cell injury in viral hepatitis: Effects of hepatitis B virus on neutrophil function in vitro and in children with chrinic active hepatitis. Pediatr. Res. 1983, 10, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Muller, F. Reactive oxygen intermediates and human immunodeficiency virus (HIV) infection. Free Radic. Bio Med. 1992, 13, 651–657. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.C.; Levine, B. Autophagy in cellular growth control. FEBS Lett. 2010, 584, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Takamura, A.; Kishi, C.; Iemura, S.-I.; Natsume, T.; Guan, J.-L.; Mizushima, N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J. Cell Biol. 2008, 181, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H. Macroautophagy and its role in nutrient homeostasis. Nutr. Rev. 2009, 67, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Klionsky, D.J. Autophagy and human disease. Cell Cycle. 2007, 6, 1837–1849. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Jung, J.U. Autophagy genes as tumor suppressors. Curr. Opin. Cell Biol. 2010, 22, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Rubinsztein, D.C. Huntington’s disease: Degradation of mutant huntingtin by autophagy. FEBS J. 2008, 275, 4263–4270. [Google Scholar] [CrossRef] [PubMed]
- Cadwell, K.; Stappenbeck, T.S.; Virgin, H.W. Role of autophagy and autophagy genes in inflammatory bowel disease. Curr. Top. Microbiol. Immunol. 2009, 335, 141–167. [Google Scholar] [PubMed]
- Lerena, M.C.; Vazquez, C.L.; Colombo, M.I. Bacterial pathogens and the autophagic response. Cell Microbiol. 2010, 12, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Tal, M.C.; Iwasaki, A. Autophagy and innate recognition systems. Curr. Top. Microbiol. Immunol. 2009, 335, 107–121. [Google Scholar]
- Dan, H.C.; Ebbs, A.; Pasparakis, M.; Van, D.T.; Basseres, D.S.; Baldwin, A.S. Akt-dependent activation of mTORC1 complex involves phosphorylation of mTOR (mammalian target of rapamycin) by IkappaB kinase alpha (IKKalpha). J. Biol. Chem. 2014, 289, 25227–25240. [Google Scholar] [CrossRef] [PubMed]
- Zalckvar, E.; Berissi, H.; Mizrachy, L.; Idelchuk, Y.; Koren, I.; Eisenstein, M.; Sabanay, H.; Pinkas-Kramarski, R.; Kimchi, A. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. Embo. Rep. 2009, 10, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and estoration of Metabolic Balance. Mol. Cell. 2017, 66, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Sanders, M.J.; Underwood, E.; Heath, R.; Mayer, F.V.; Carmena, D.; Jing, C.; Walker, P.A.; Eccleston, J.F.; Haire, L.F.; et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011, 472, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhao, L.; Li, S.; Xu, L.; Wang, Y.; Chen, H. Impaired Cellular Energy Metabolism Contributes to Duck-Enteritis-Virus-Induced Autophagy via the AMPK-TSC2-MTOR Signaling Pathway. Front. Cell. Infect. Microbiol. 2017, 7, 423. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.C.; Shao, S.L.; Jiang, X.J.; Xie, P.Y.; Sun, W.S.; Yu, T.F. Interactions between Autophagy and DNA Viruses. Viruses 2019, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.; Santarelli, R.; Farina, A.; Gonnella, R.; Lotti, L.V.; Faggioni, A.; Cirone, M. Epstein-barr virus blocks the autophagic flux and appropriates the autophagic machinery to enhance viral replication. J. Virol. 2014, 88, 12715–12726. [Google Scholar] [CrossRef]
- Zhai, N.; Liu, K.; Li, H.; Liu, Z.; Wang, H.; Korolchuk, V.I.; Carroll, B.; Pan, C.; Gan, F.; Huang, K.; et al. PCV2 replication promoted by oxidative stress is dependent on the regulation of autophagy on apoptosis. Vet. Res. 2019, 50, 19. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsueh, T.-C.; Chen, P.-H.; Hong, J.-R. ISKNV Triggers AMPK/mTOR-Mediated Autophagy Signaling through Oxidative Stress, Inducing Antioxidant Enzyme Expression and Enhancing Viral Replication in GF-1 Cells. Viruses 2024, 16, 914. https://doi.org/10.3390/v16060914
Hsueh T-C, Chen P-H, Hong J-R. ISKNV Triggers AMPK/mTOR-Mediated Autophagy Signaling through Oxidative Stress, Inducing Antioxidant Enzyme Expression and Enhancing Viral Replication in GF-1 Cells. Viruses. 2024; 16(6):914. https://doi.org/10.3390/v16060914
Chicago/Turabian StyleHsueh, Tsai-Ching, Pin-Han Chen, and Jiann-Ruey Hong. 2024. "ISKNV Triggers AMPK/mTOR-Mediated Autophagy Signaling through Oxidative Stress, Inducing Antioxidant Enzyme Expression and Enhancing Viral Replication in GF-1 Cells" Viruses 16, no. 6: 914. https://doi.org/10.3390/v16060914




