Respiratory Syncytial Virus in Adult Patients at a Tertiary Care Hospital in Germany: Clinical Features and Molecular Epidemiology of the Fusion Protein in the Severe Respiratory Season of 2022/2023
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Clinical Data
2.2. Saisonality, Detection Intervals, Peak Detection
2.3. Meteorological Data
2.4. Nucleic Acid (NA) Extraction and RSV Detection
2.5. Phylogenetic Analysis of the RSV F Gene Sequence
2.6. Amino Acid Analysis
2.7. Statistical Analysis
3. Results
3.1. RSV Detection and Seasonality
3.2. Study Population and Clinical Features
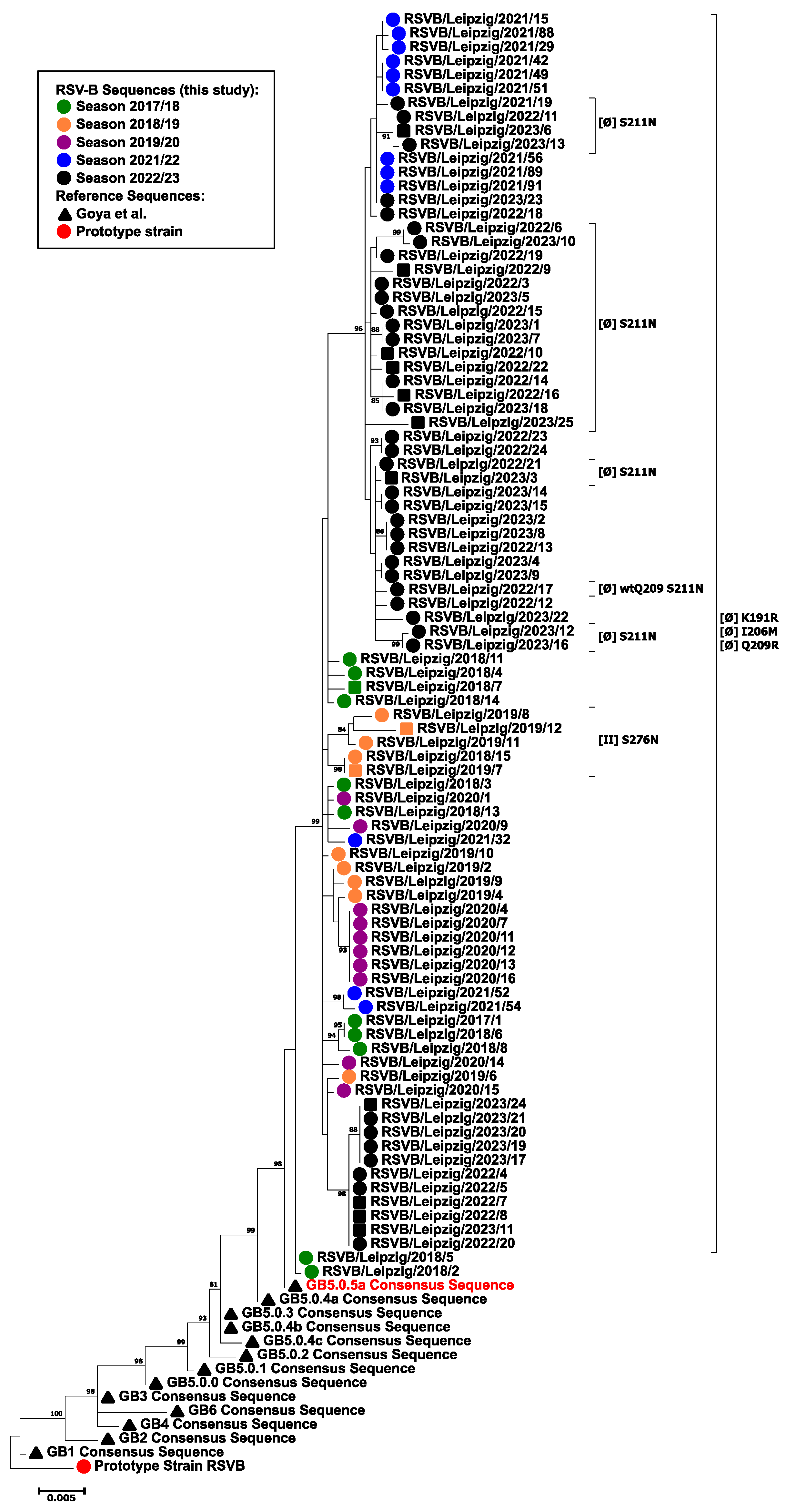
3.3. Phylogenetic Analysis
3.4. Amino Acid Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory syncytial virus—A comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef] [PubMed]
- Krause, C.I. The ABCs of RSV. Nurse Pract. 2018, 43, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Long, C.E.; Schnabel, K.C. Respiratory syncytial virus infections in previously healthy working adults. Clin. Infect. Dis. 2001, 33, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Hönemann, M.; Thiem, S.; Bergs, S.; Berthold, T.; Propach, C.; Siekmeyer, M.; Frille, A.; Wallborn, T.; Maier, M.; Pietsch, C. In-Depth Analysis of the Re-Emergence of Respiratory Syncytial Virus at a Tertiary Care Hospital in Germany in the Summer of 2021 after the Alleviation of Non-Pharmaceutical Interventions Due to the SARS-CoV-2 Pandemic. Viruses 2023, 15, 877. [Google Scholar] [CrossRef] [PubMed]
- Matias, G.; Taylor, R.; Haguinet, F.; Schuck-Paim, C.; Lustig, R.; Shinde, V. Estimates of hospitalization attributable to influenza and RSV in the US during 1997-2009, by age and risk status. BMC Public Health 2017, 17, 271. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, F.; Chemaly, R.F. Respiratory syncytial virus in hematopoietic cell transplant recipients and patients with hematologic malignancies. Haematologica 2019, 104, 1322–1331. [Google Scholar] [CrossRef]
- Vakil, E.; Sheshadri, A.; Faiz, S.A.; Shah, D.P.; Zhu, Y.; Li, L.; Kmeid, J.; Azzi, J.; Balagani, A.; Bashoura, L.; et al. Risk factors for mortality after respiratory syncytial virus lower respiratory tract infection in adults with hematologic malignancies. Transpl. Infect. Dis. 2018, 20, e12994. [Google Scholar] [CrossRef]
- Khalifah, A.P.; Hachem, R.R.; Chakinala, M.M.; Schechtman, K.B.; Patterson, G.A.; Schuster, D.P.; Mohanakumar, T.; Trulock, E.P.; Walter, M.J. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am. J. Respir. Crit. Care Med. 2004, 170, 181–187. [Google Scholar] [CrossRef]
- Falsey, A.R.; McElhaney, J.E.; Beran, J.; van Essen, G.A.; Duval, X.; Esen, M.; Galtier, F.; Gervais, P.; Hwang, S.-J.; Kremsner, P.; et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J. Infect. Dis. 2014, 209, 1873–1881. [Google Scholar] [CrossRef]
- Geevarghese, B.; Weinberg, A. Cell-mediated immune responses to respiratory syncytial virus infection: Magnitude, kinetics, and correlates with morbidity and age. Hum. Vaccin. Immunother. 2014, 10, 1047–1056. [Google Scholar] [CrossRef]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef]
- Hornung, F.; Rogal, J.; Loskill, P.; Löffler, B.; Deinhardt-Emmer, S. The Inflammatory Profile of Obesity and the Role on Pulmonary Bacterial and Viral Infections. Int. J. Mol. Sci. 2021, 22, 3456. [Google Scholar] [CrossRef]
- Frydrych, L.M.; Bian, G.; O’Lone, D.E.; Ward, P.A.; Delano, M.J. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J. Leukoc. Biol. 2018, 104, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Tabor, D.E.; Fernandes, F.; Langedijk, A.C.; Wilkins, D.; Lebbink, R.J.; Tovchigrechko, A.; Ruzin, A.; Kragten-Tabatabaie, L.; Jin, H.; Esser, M.T.; et al. Global Molecular Epidemiology of Respiratory Syncytial Virus from the 2017-2018 INFORM-RSV Study. J. Clin. Microbiol. 2020, 59, e01828-20. [Google Scholar] [CrossRef] [PubMed]
- Trovão, N.S.; Khuri-Bulos, N.; Tan, Y.; Puri, V.; Shilts, M.H.; Halpin, R.A.; Fedorova, N.B.; Nelson, M.I.; Halasa, N.; Das, S.R. Molecular characterization of respiratory syncytial viruses circulating in a paediatric cohort in Amman, Jordan. Microb. Genom. 2021, 7, 000292. [Google Scholar] [CrossRef] [PubMed]
- Goya, S.; Galiano, M.; Nauwelaers, I.; Trento, A.; Openshaw, P.J.; Mistchenko, A.S.; Zambon, M.; Viegas, M. Toward unified molecular surveillance of RSV: A proposal for genotype definition. Influenza Other Respir. Viruses 2020, 14, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Escalante, J.C.; Comas-García, A.; Bernal-Silva, S.; Noyola, D.E. Respiratory syncytial virus B sequence analysis reveals a novel early genotype. Sci. Rep. 2021, 11, 3452. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Escalante, J.C.; Comas-García, A.; Bernal-Silva, S.; Robles-Espinoza, C.D.; Gómez-Leal, G.; Noyola, D.E. Respiratory syncytial virus A genotype classification based on systematic intergenotypic and intragenotypic sequence analysis. Sci. Rep. 2019, 9, 20097. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, H.; Wan, Z.; Li, S.; Li, Y.; Wang, W.; Jin, X.; Li, Y.; Zhang, C. Evolutionary dynamics of group A and B respiratory syncytial virus in China, 2009-2018. Arch. Virol. 2021, 166, 2407–2418. [Google Scholar] [CrossRef]
- Altman, G.; Ahuja, J.; Monrad, J.T.; Dhaliwal, G.; Rogers-Smith, C.; Leech, G.; Snodin, B.; Sandbrink, J.B.; Finnveden, L.; Norman, A.J.; et al. A dataset of non-pharmaceutical interventions on SARS-CoV-2 in Europe. Sci. Data 2022, 9, 145. [Google Scholar] [CrossRef]
- Eden, J.-S.; Sikazwe, C.; Xie, R.; Deng, Y.-M.; Sullivan, S.G.; Michie, A.; Levy, A.; Cutmore, E.; Blyth, C.C.; Britton, P.N.; et al. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat. Commun. 2022, 13, 2884. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Pérez Marc, G.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Pérez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Mas, V.; Nair, H.; Campbell, H.; Melero, J.A.; Williams, T.C. Antigenic and sequence variability of the human respiratory syncytial virus F glycoprotein compared to related viruses in a comprehensive dataset. Vaccine 2018, 36, 6660–6673. [Google Scholar] [CrossRef]
- McLellan, J.S.; Chen, M.; Leung, S.; Graepel, K.W.; Du, X.; Yang, Y.; Zhou, T.; Baxa, U.; Yasuda, E.; Beaumont, T.; et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 2013, 340, 1113–1117. [Google Scholar] [CrossRef]
- McLellan, J.S.; Yang, Y.; Graham, B.S.; Kwong, P.D. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J. Virol. 2011, 85, 7788–7796. [Google Scholar] [CrossRef]
- Gilman, M.S.A.; Castellanos, C.A.; Chen, M.; Ngwuta, J.O.; Goodwin, E.; Moin, S.M.; Mas, V.; Melero, J.A.; Wright, P.F.; Graham, B.S.; et al. Rapid profiling of RSV antibody repertoires from the memory B cells of naturally infected adult donors. Sci. Immunol. 2016, 1, eaaj1879. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Oliver, C.; Prince, G.A.; Hemming, V.G.; Pfarr, D.S.; Wang, S.C.; Dormitzer, M.; O’Grady, J.; Koenig, S.; Tamura, J.K.; et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 1997, 176, 1215–1224. [Google Scholar] [CrossRef]
- Zhu, Q.; McLellan, J.S.; Kallewaard, N.L.; Ulbrandt, N.D.; Palaszynski, S.; Zhang, J.; Moldt, B.; Khan, A.; Svabek, C.; McAuliffe, J.M.; et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci. Transl. Med. 2017, 9, eaaj1928. [Google Scholar] [CrossRef]
- WHO. International Statistical Classification of Diseases and Related Health Problems (ICD): Version 10. Available online: https://icd.who.int/browse10/2019/en (accessed on 31 March 2024).
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.J.; Dolan, A.; Akter, P.; Addison, C.; Dargan, D.J.; Alcendor, D.J.; McGeoch, D.J.; Hayward, G.S. The human cytomegalovirus genome revisited: Comparison with the chimpanzee cytomegalovirus genome. J. Gen. Virol. 2003, 84, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Golke, P.; Hönemann, M.; Bergs, S.; Liebert, U.G. Human Rhinoviruses in Adult Patients in a Tertiary Care Hospital in Germany: Molecular Epidemiology and Clinical Significance. Viruses 2021, 1, 2027. [Google Scholar] [CrossRef] [PubMed]
- Espy, M.J.; Ross, T.K.; Teo, R.; Svien, K.A.; Wold, A.D.; Uhl, J.R.; Smith, T.F. Evaluation of LightCycler PCR for implementation of laboratory diagnosis of herpes simplex virus infections. J. Clin. Microbiol. 2000, 38, 3116–3118. [Google Scholar] [CrossRef] [PubMed]
- Stöcher, M.; Leb, V.; Bozic, M.; Kessler, H.H.; Halwachs-Baumann, G.; Landt, O.; Stekel, H.; Berg, J. Parallel detection of five human herpes virus DNAs by a set of real-time polymerase chain reactions in a single run. J. Clin. Virol. 2003, 26, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Deutscher Wetterdienst. Mean Monthly Temperature Station 2932. Available online: https://www.dwd.de/EN/climate_environment/cdc/clis/clis_node.html (accessed on 15 December 2023).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Iezadi, S.; Gholipour, K.; Azami-Aghdash, S.; Ghiasi, A.; Rezapour, A.; Pourasghari, H.; Pashazadeh, F. Effectiveness of non-pharmaceutical public health interventions against COVID-19: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0260371. [Google Scholar] [CrossRef] [PubMed]
- Bundesministerium der Justiz. Gesetzestext IfSG [German]. Available online: http://www.gesetze-im-internet.de/ifsg/index.html (accessed on 21 January 2023).
- Bundesministerium für Gesundheit. COVID-19-SchG [German]. Available online: https://www.bundesgesundheitsministerium.de/service/gesetze-und-verordnungen/detail/gesetz-zur-staerkung-des-schutzes-der-bevoelkerung-und-insbesondere-vulnerabler-personengruppen-vor-covid-19.html (accessed on 21 January 2023).
- Robert Koch Institut. Zusammenstellung IfSG [German]. Available online: https://www.rki.de/DE/Content/Infekt/IfSG/Gesetze/gesetze_node.html (accessed on 21 January 2023).
- State Government of Saxony. Regulations Saxony [German]. Available online: https://www.coronavirus.sachsen.de/archiv-der-abgelaufenen-amtlichen-bekanntmachungen-7295.html (accessed on 21 January 2023).
- Jørgensen, F.; Bor, A.; Rasmussen, M.S.; Lindholt, M.F.; Petersen, M.B. Pandemic fatigue fueled political discontent during the COVID-19 pandemic. Proc. Natl. Acad. Sci. USA 2022, 119, e2201266119. [Google Scholar] [CrossRef]
- Robert Koch Institut. Weekly Reports of the AGI [German]. Available online: https://influenza.rki.de/Wochenberichte.aspx (accessed on 6 March 2023).
- Greer, A.L.; Tuite, A.; Fisman, D.N. Age, influenza pandemics and disease dynamics. Epidemiol. Infect. 2010, 138, 1542–1549. [Google Scholar] [CrossRef]
- Reicherz, F.; Xu, R.Y.; Abu-Raya, B.; Majdoubi, A.; Michalski, C.; Golding, L.; Stojic, A.; Vineta, M.; Granoski, M.; Cieslak, Z.; et al. Waning Immunity Against Respiratory Syncytial Virus During the Coronavirus Disease 2019 Pandemic. J. Infect. Dis. 2022, 226, 2064–2068. [Google Scholar] [CrossRef] [PubMed]
- Stensballe, L.G.; Ravn, H.; Kristensen, K.; Meakins, T.; Aaby, P.; Simoes, E.A.F. Seasonal variation of maternally derived respiratory syncytial virus antibodies and association with infant hospitalizations for respiratory syncytial virus. J. Pediatr. 2009, 154, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Barclay, W.S.; al-Nakib, W.; Higgins, P.G.; Tyrrell, D.A. The time course of the humoral immune response to rhinovirus infection. Epidemiol. Infect. 1989, 103, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Pons-Salort, M.; Grassly, N.C. Serotype-specific immunity explains the incidence of diseases caused by human enteroviruses. Science 2018, 361, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Hönemann, M.; Martin, D.; Pietsch, C.; Maier, M.; Bergs, S.; Bieck, E.; Liebert, U.G. Influenza B virus infections in Western Saxony, Germany in three consecutive seasons between 2015 and 2018: Analysis of molecular and clinical features. Vaccine 2019, 37, 6550–6557. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Cameron, A.; Branche, A.R.; Walsh, E.E. Perturbations in Respiratory Syncytial Virus Activity During the SARS-CoV-2 Pandemic. J. Infect. Dis. 2022, 227, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Panatto, D.; Domnich, A.; Lai, P.L.; Ogliastro, M.; Bruzzone, B.; Galli, C.; Stefanelli, F.; Pariani, E.; Orsi, A.; Icardi, G. Epidemiology and molecular characteristics of respiratory syncytial virus (RSV) among italian community-dwelling adults, 2021/22 season. BMC Infect. Dis. 2023, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Buda, S.; Dürrwald, R.; Biere, B.; Reiche, J.; Buchholz, U.; Tolksdorf, K.; Schilling, J.; Goerlitz, L.; Streib, V.; Preuß, U.; et al. ARE-Wochenbericht, 2021.
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.-G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.H.; Ison, M.G. Respiratory syncytial virus infection in adults. BMJ 2019, 366, l5021. [Google Scholar] [CrossRef]
- Falsey, A.R.; Formica, M.A.; Hennessey, P.A.; Criddle, M.M.; Sullender, W.M.; Walsh, E.E. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006, 173, 639–643. [Google Scholar] [CrossRef]
- Kurai, D.; Saraya, T.; Ishii, H.; Takizawa, H. Virus-induced exacerbations in asthma and COPD. Front. Microbiol. 2013, 4, 293. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Walsh, E.E.; Esser, M.T.; Shoemaker, K.; Yu, L.; Griffin, M.P. Respiratory syncytial virus–associated illness in adults with advanced chronic obstructive pulmonary disease and/or congestive heart failure. J. Med. Virol. 2018, 91, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Branche, A.R.; Saiman, L.; Walsh, E.E.; Falsey, A.R.; Sieling, W.D.; Greendyke, W.; Peterson, D.R.; Vargas, C.Y.; Phillips, M.; Finelli, L. Incidence of Respiratory Syncytial Virus Infection Among Hospitalized Adults, 2017–2020. Clin. Infect. Dis. 2022, 74, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Lui, G.C.Y.; Wong, K.T.; Li, T.C.M.; Tse, E.C.M.; Chan, J.Y.C.; Yu, J.; Wong, S.S.M.; Choi, K.W.; Wong, R.Y.K.; et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin. Infect. Dis. 2013, 57, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Hönemann, M.; Liebert, U.G. Dynamics of nosocomial parainfluenza virus type 3 and influenza virus infections at a large German University Hospital between 2012 and 2019. Diagn. Microbiol. Infect. Dis. 2021, 99, 115244. [Google Scholar] [CrossRef]
- Levitt, E.E.; Gohari, M.R.; Syan, S.K.; Belisario, K.; Gillard, J.; DeJesus, J.; Levitt, A.; MacKillop, J. Public health guideline compliance and perceived government effectiveness during the COVID-19 pandemic in Canada: Findings from a longitudinal cohort study. Lancet Reg. Health Am. 2022, 9, 100185. [Google Scholar] [CrossRef]
- ECDC/WHO. Influenza Virus Characterization: Summary Report, Europe, February 2023. Copenhagen and Stockholm: WHO Regional Office for Europe and European Centre for Disease Prevention and Control; 2023 Licence: CC BY 3.0 IGO. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Influenza-characterisation-February-2023.pdf (accessed on 1 April 2024).
- Karron, R.A. RSV Illness in the Young and the Old—The Beginning of the End? N. Engl. J. Med. 2023, 388, 1522–1524. [Google Scholar] [CrossRef]
- Arbiza, J.; Taylor, G.; López, J.A.; Furze, J.; Wyld, S.; Whyte, P.; Stott, E.J.; Wertz, G.; Sullender, W.; Trudel, M. Characterization of two antigenic sites recognized by neutralizing monoclonal antibodies directed against the fusion glycoprotein of human respiratory syncytial virus. J. Gen. Virol. 1992, 73 Pt 9, 2225–2234. [Google Scholar] [CrossRef]
- Rossey, I.; Gilman, M.S.A.; Kabeche, S.C.; Sedeyn, K.; Wrapp, D.; Kanekiyo, M.; Chen, M.; Mas, V.; Spitaels, J.; Melero, J.A.; et al. Potent single-domain antibodies that arrest respiratory syncytial virus fusion protein in its prefusion state. Nat. Commun. 2017, 8, 14158. [Google Scholar] [CrossRef]
- Wu, S.-J.; Schmidt, A.; Beil, E.J.; Day, N.D.; Branigan, P.J.; Liu, C.; Gutshall, L.L.; Palomo, C.; Furze, J.; Taylor, G.; et al. Characterization of the epitope for anti-human respiratory syncytial virus F protein monoclonal antibody 101F using synthetic peptides and genetic approaches. J. Gen. Virol. 2007, 88, 2719–2723. [Google Scholar] [CrossRef]
- Zhu, Q.; Lu, B.; McTamney, P.; Palaszynski, S.; Diallo, S.; Ren, K.; Ulbrandt, N.D.; Kallewaard, N.; Wang, W.; Fernandes, F.; et al. Prevalence and Significance of Substitutions in the Fusion Protein of Respiratory Syncytial Virus Resulting in Neutralization Escape from Antibody MEDI8897. J. Infect. Dis. 2018, 218, 572–580. [Google Scholar] [CrossRef] [PubMed]
- López, J.A.; Bustos, R.; Orvell, C.; Berois, M.; Arbiza, J.; García-Barreno, B.; Melero, J.A. Antigenic structure of human respiratory syncytial virus fusion glycoprotein. J. Virol. 1998, 72, 6922–6928. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, F.-P.; Sullender, W.M. Respiratory syncytial virus escape mutant derived in vitro resists palivizumab prophylaxis in cotton rats. Virology 2004, 318, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, F.-P.; Megaw, A.G.; Sullender, W.M. Variable resistance to palivizumab in cotton rats by respiratory syncytial virus mutants. J. Infect. Dis. 2004, 190, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; McAuliffe, J.M.; Patel, N.K.; Palmer-Hill, F.J.; Yang, C.; Liang, B.; Su, L.; Zhu, W.; Wachter, L.; Wilson, S.; et al. Analysis of Respiratory Syncytial Virus Preclinical and Clinical Variants Resistant to Neutralization by Monoclonal Antibodies Palivizumab and/or Motavizumab. J. Infect. Dis. 2011, 203, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Papenburg, J.; Carbonneau, J.; Hamelin, M.-È.; Isabel, S.; Bouhy, X.; Ohoumanne, N.; Déry, P.; Paes, B.A.; Corbeil, J.; Bergeron, M.G.; et al. Molecular Evolution of Respiratory Syncytial Virus Fusion Gene, Canada, 2006–2010. Emerg. Infect. Dis. 2012, 18, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Oraby, A.K.; Stojic, A.; Elawar, F.; Bilawchuk, L.; McClelland, R.; Erwin, K.; Granoski, M.; Griffiths, C.; Arutyunova, E.; Lemieux, M.J.; et al. A Single Amino Acid Mutation Alters the Neutralization Epitopes in the Respiratory Syncytial Virus Fusion Glycoprotein. 2024, Resarch Square preprint.
- Hause, A.M.; Henke, D.M.; Avadhanula, V.; Shaw, C.A.; Tapia, L.I.; Piedra, P.A. Sequence variability of the respiratory syncytial virus (RSV) fusion gene among contemporary and historical genotypes of RSV/A and RSV/B. PLoS ONE 2017, 12, e0175792. [Google Scholar] [CrossRef] [PubMed]
- Schaerlaekens, S.; Jacobs, L.; Stobbelaar, K.; Cos, P.; Delputte, P. All Eyes on the Prefusion-Stabilized F Construct, but Are We Missing the Potential of Alternative Targets for Respiratory Syncytial Virus Vaccine Design? Vaccines 2024, 12, 97. [Google Scholar] [CrossRef]
- Phuah, J.Y.; Maas, B.M.; Tang, A.; Zhang, Y.; Caro, L.; Railkar, R.A.; Swanson, M.D.; Cao, Y.; Li, H.; Roadcap, B.; et al. Quantification of clesrovimab, an investigational, half-life extended, anti-respiratory syncytial virus protein F human monoclonal antibody in the nasal epithelial lining fluid of healthy adults. Biomed. Pharmacother. 2023, 169, 115851. [Google Scholar] [CrossRef]
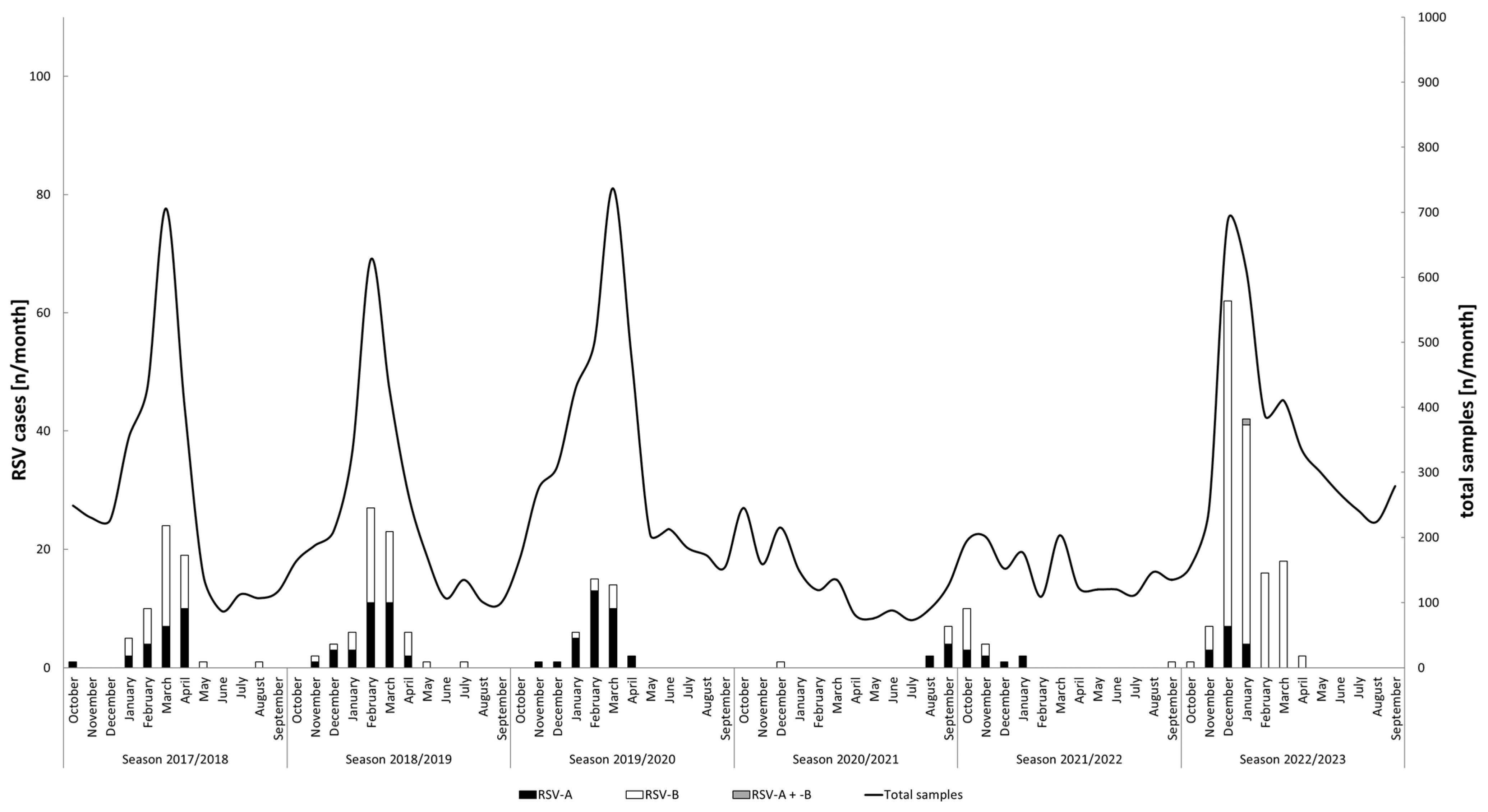
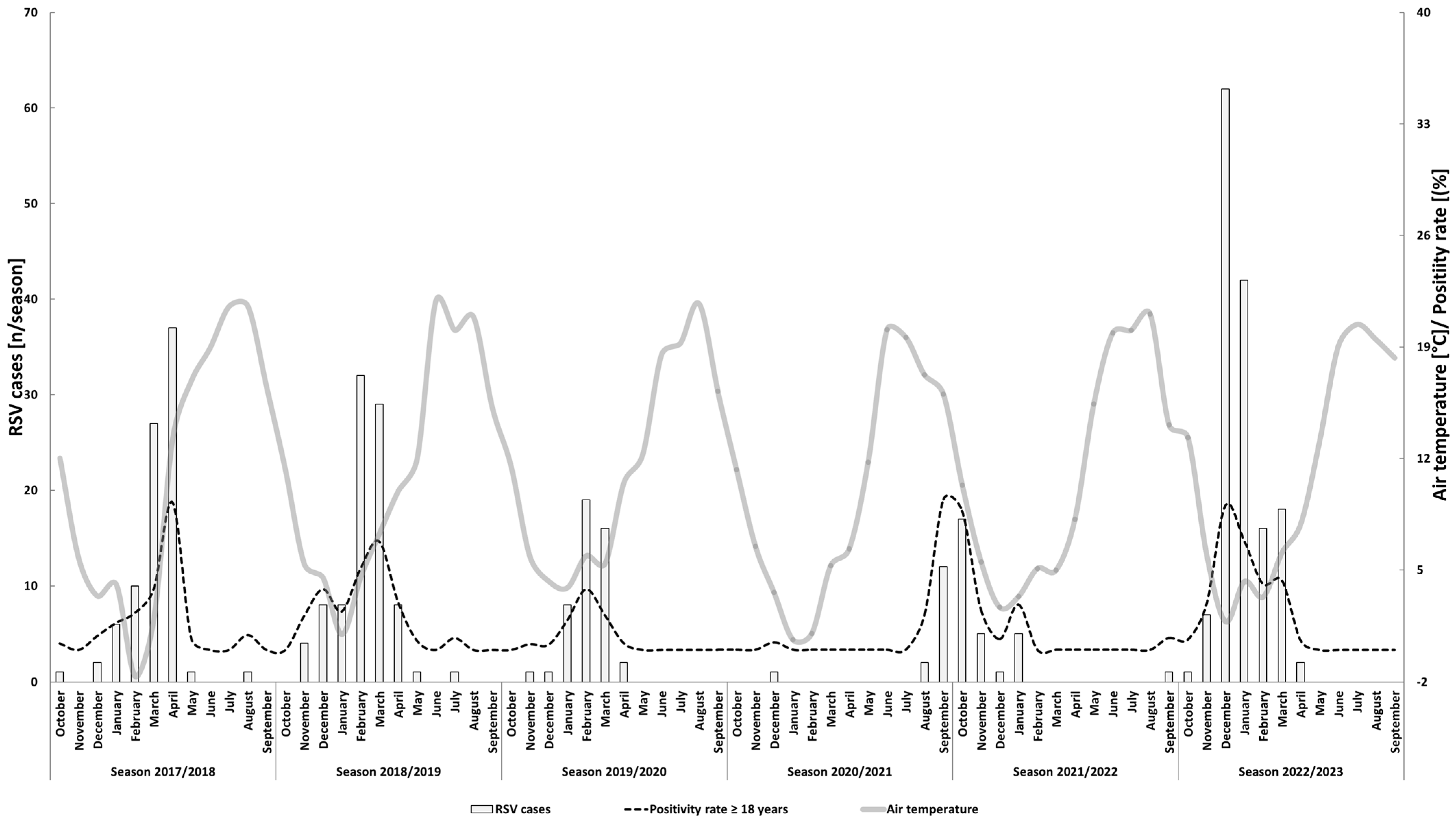
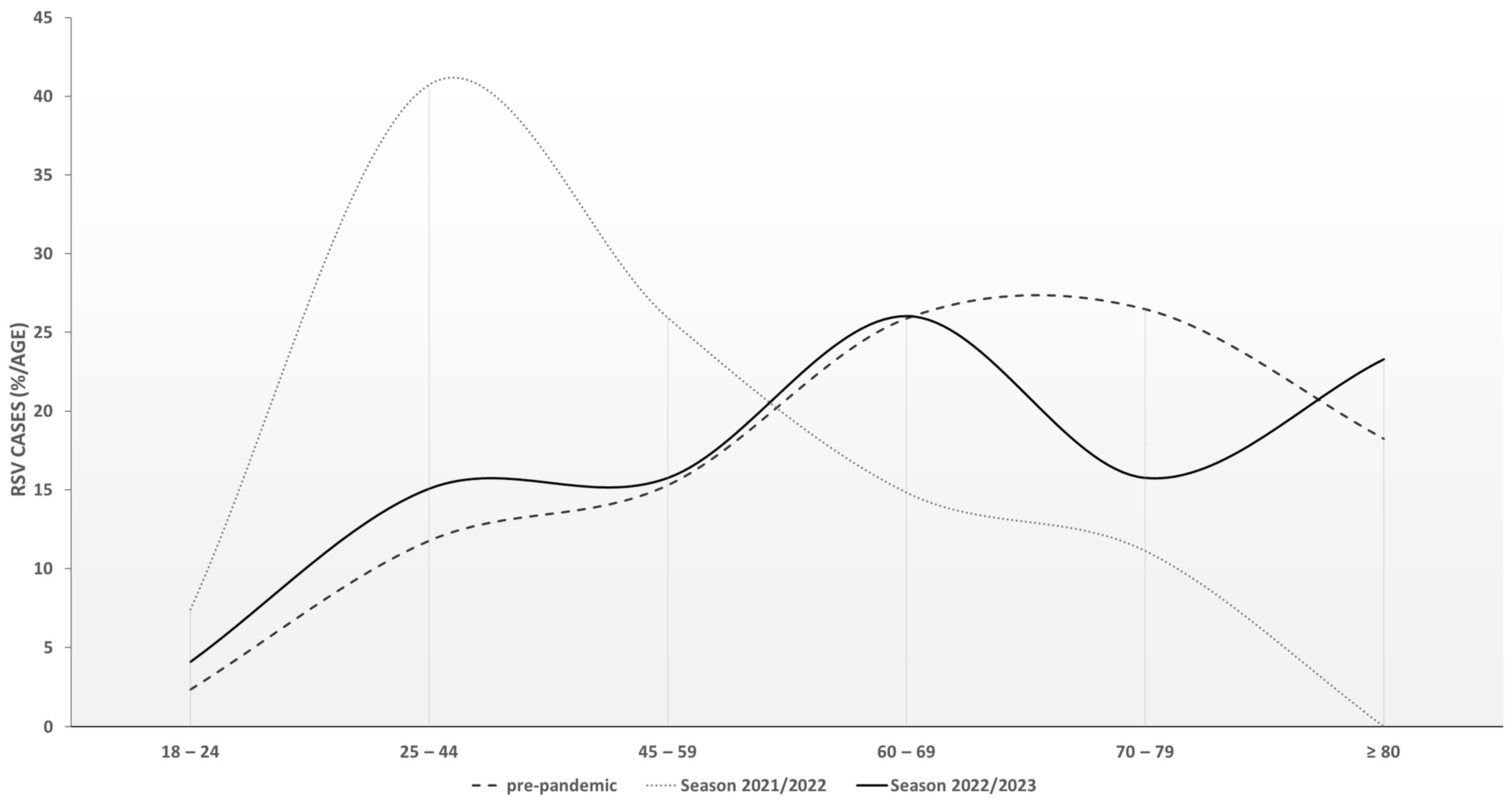
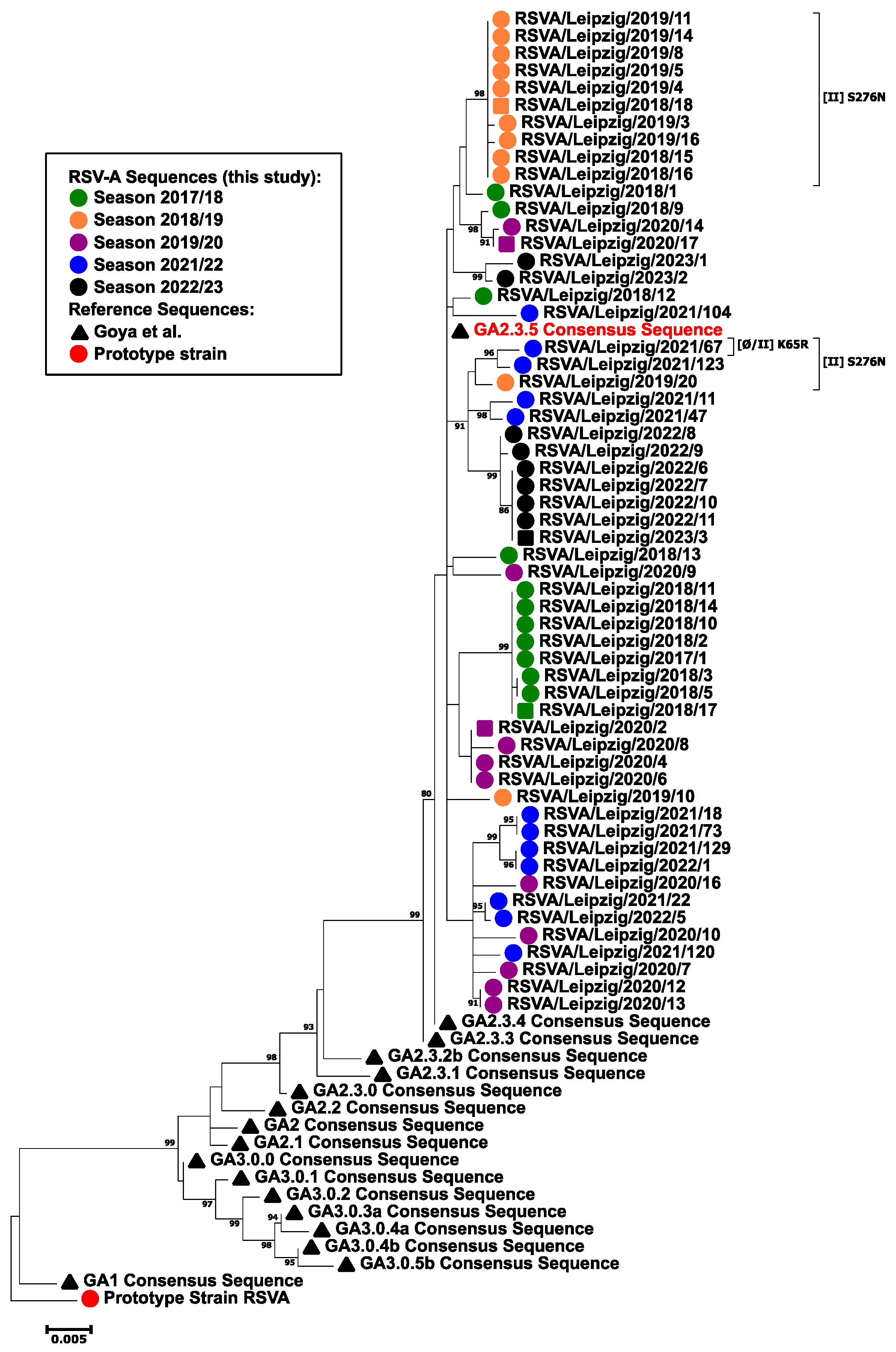
| Seasons | 2017/2018 | 2018/2019 | 2019/2020 | 2021/2022 | 2022/2023 | Total | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Age group | ||||||||
| <60 | [% (n/total)] | 34.4 (21/61) | 21.4 (15/70) | 35.9 (14/39) | 73.1 (19/26) | 34.9 (51/146) | 35.3 (121/343) | <0.001 |
| ≥60 | [% (n/total)] | 65. 6 (40/61) | 78.6 (55/70) | 64.1 (25/39) | 26.9 (7/26) | 65.1 (95/146) | 64.7 (222/343) | |
| Sex | ||||||||
| male | [% (n/total)] | 67.2 (41/61) | 48.6 (34/70) | 56.4 (22/39) | 57.7 (15/26) | 52.1 (76/146) | 55.1 (189/343) | n.s. |
| female | [% (n/total)] | 32.8 (20/61) | 51.4 (36/70) | 43.6 (17/39) | 42.3 (11/26) | 47.9 (70/146) | 44.9 (154/343) | |
| RSV species | ||||||||
| RSV-A | [% (n/total)] | 39.3 (24/61) | 44.3 (31/70) | 82.1 (32/39) | 53.8 (14/26) | 8.2 (12/146) | 32.9 (113/343) | <0.001 |
| RSV-B | [% (n/total)] | 60.7 (37/61) | 55.7 (39/70) | 17.9 (7/39) | 46.2 (12/26) | 91.1 (133/146) | 66.8 (229/343) | |
| mixed | [% (n/total)] | - | - | - | - | 0.7 (1/146) | 0.3 (1/343) |
| Pre-Pandemic | 2022/2023 | Total | p-Value | ||
|---|---|---|---|---|---|
| Study population | |||||
| Female | [% (n/total)] | 42.9 (73/170) | 47.9 (70/146) | 45.3 (143/316) | n.s. |
| Male | [% (n/total)] | 57.1 (97/170) | 52.1 (76/146) | 54.7 (173/316) | |
| Age [years] | [median (IQR)] SD] | 66.5 (56–77) | 65 (49.75–78) | 66 (55–78) | n.s. |
| Inpatients | [% (n/total)] | 79.4 (135/170) | 82.8 (120/145) | 81 (255/315) | n.s. |
| Outpatients | [% (n/total)] | 20.6 (35/170) | 17.2 (25/145) | 19 (60/315) | |
| Length of hospital stay [days] | [median (IQR)] | 13 (7–24.5) | 10 (4–20) | 12 (6–22) | n.s. |
| Comorbidities and risk factors | |||||
| Obstructive lung disease [OLD] | [% (n/total)] | 22.6 (38/168) | 31.2 (44/141) | 26.5 (82/309) | n.s. |
| Lung transplant | [% (n/total)] | 4.7 (8/170) | 0 (0/142) | 2.6 (8/309) | 0.009 |
| Chronic kidney failure | [% (n/total)] | 39.9 (67/159) | 26.4 (37/140) | 33.8 (104/308) | 0.013 |
| Heart failure | [% (n/total)] | 19.0 (32/168) | 17.0 (24/141) | 18.1 (56/309) | n.s. |
| Arterial hypertension | [% (n/total)] | 58.9 (99/168) | 57 (81/142) | 58.1 (180/310) | n.s. |
| Coronary heart disease | [% (n/total)] | 16.7 (28/168) | 14.9 (21/141) | 15.9 (49/309) | n.s. |
| Diabetes | [% (n/total)] | 31.5 (53/168) | 24.8 (35/141) | 28.5 (88/309) | n.s. |
| Immunosuppression | [% (n/total)] | 36.3 (61/168) | 25.4 (36/142) | 31.3 (97/310) | 0.038 |
| Malignancy | [% (n/total)] | 35.7 (60/168) | 28.2 (40/142) | 32.3 (100/310) | n.s. |
| Solid | [% (n/total)] | 11.9 (20/168) | 4.9 (7(142) | 8.7 (27/310) | n.s. |
| Hematologic | [% (n/total)] | 23.2 (39/168) | 22.5 (32/142) | 22.9 (71/310) | |
| Solid and hematologic | [% (n/total)] | 0.6 (1/168) | 0.7 (1/142) | 0.6 (2/310) | |
| CCI | [median (IQR)] | 5 (4–7) | 5 (3–7) | 5 (3–7) | n.s. |
| Clinical presentation and features | |||||
| Fever | [% (n/total)] | 24.1 (38/158) | 35.8 (43/120) | 29.1 (81/178) | 0.032 |
| Newly reported dyspnea | [% (n/total)] | 43.5 (67/154) | 48.4 (59/122) | 45.7 (126/276) | n.s. |
| URTI | [% (n/total)] | 35.4 (28/79) | 34.0 (33/97) | 34.7 (61/176) | n.s. |
| LRTI | [% (n/total)] | 52.5 (83/158) | 77.8 (84/108) | 62.8 (167/266) | <0.001 |
| Bronchitis | [% (n/total)] | 7.6 (12/158) | 10.3 (11/107) | 8.7 (23/265) | n.s. |
| Pneumonia | [% (n/total)] | 33.3 (53/159) | 48.1 (52/108) | 39.3 (105/267) | 0.015 |
| Exacerbation of OLD | [% (n/total)] | 13.8 (22/159) | 26.9 (29/108) | 19.1 (51/167) | 0.008 |
| ICU stay | [% (n/total)] | 25.9 (44/170) | 21.1 (30/142) | 23.7 (74/312) | n.s. |
| Length of ICU stay [days] | [median (IQR)] | 3 (1–10) | 5 (2.75–12.5) | 3.5 (2–10) | n.s. |
| Ventilatory support | [% (n/total)] | 15.3 (26/170) | 23.2 (33/142) | 18.9 (59/312) | n.s. |
| None * | [% (n/total)] | 84.7 (144/170) | 76.8 (109/142) | 81.1 (253/312) | n.s. |
| HFNC | [% (n/total)] | 0.6 (1/170) | 1.4 (2/142) | 1.0 (2/312) | |
| Non-invasive | [% (n/total)] | 7.1 (12/170) | 9.2 (13/142) | 8.0 (25/312) | |
| Invasive | [% (n/total)] | 7.6 (13/170) | 12.7 (18/142) | 9.9 (31/312) | |
| Administration of bronchodilators | [% (n/total)] | 16.9 (28/166) | 30.9 (42/136) | 23.2 (70/302) | 0.004 |
| Syst. prednisolone administration | [% (n/total)] | 14.9 (25/168) | 22.0 (29/132) | 18.0 (54/300) | n.s. |
| Co-infections | [% (n/total)] | 21.2 (36/170) | 27.3 (39/143) | 24.0 (75/313) | n.s. |
| Bacterial | [% (n/total)] | 5.9 (10/170) | 11.9 (17/143) | 8.6 (27/313) | n.s. |
| Viral | [% (n/total)] | 11.2 (19/170) | 9.8 (14/143) | 10.5 (33/313) | |
| Fungal | [% (n/total)] | 1.8 (3/170) | 0.7 (1/143) | 1.3 (4/313) | |
| Combined | [% (n/total)] | 2.4 (4/170) | 5.6 (8/143) | 3.8 (12/313) | |
| Mortality | [% (n/total)] | 6.5 (11/170) | 12 (17/142) | 9 (28/312) | n.s. |
| AA position | 9 | 10 | 12 | 13 | 20 | 65 | 103 | 105 | 113 | 114 | 122 | 127 | 276 | 334 | 377 | 384 | 543 | 547 |
| Subunit | F1 | F1 | F1 | F1 | F1 | F1 | F1 | F1 | F2 | F2 | F2 | F2 | F2 | F2 | ||||
| Region | SP | SP | SP | SP | SP | p27 | p27 | p27 | P27 | CT | CT | |||||||
| Antigenic sites | Ø/II | II/III | I/III | I | ||||||||||||||
| GA2.3.5 consensus | N | A | T | T | L | K | A | S | R | F | T | V | S | L | S | I | A | L |
| 16 strains * | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2017/1 | . | . | . | . | . | . | . | N | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2018/2 | . | . | . | . | . | . | . | N | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2018/3 | . | . | . | . | . | . | . | N | . | . | . | . | . | . | . | . | . | I |
| RSVA/Leipzig/2018/5 | . | . | . | . | . | . | . | N | . | . | . | . | . | . | . | . | . | I |
| RSVA/Leipzig/2018/9 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | T | . | . |
| RSVA/Leipzig/2018/10 | . | . | . | . | . | . | . | N | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2018/11 | . | . | . | . | . | . | . | N | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2018/12 | . | . | . | . | . | . | . | . | . | . | . | A | . | . | . | . | . | . |
| RSVA/Leipzig/2018/14 | . | . | . | . | . | . | . | N | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2018/17 | . | . | . | . | . | . | . | N | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2018/15 | . | . | . | . | . | . | . | . | . | . | . | . | N | . | . | . | . | . |
| RSVA/Leipzig/2018/18 | . | . | . | . | . | . | . | . | . | . | . | . | N | . | . | . | . | . |
| RSVA/Leipzig/2018/16 | . | . | . | . | . | . | . | . | . | . | . | . | N | . | . | . | . | . |
| RSVA/Leipzig/2019/3 | . | . | . | . | . | . | . | . | . | . | . | . | N | . | . | . | . | . |
| RSVA/Leipzig/2019/4 | . | . | . | . | . | . | . | . | . | . | . | . | N | . | . | . | . | . |
| RSVA/Leipzig/2019/5 | . | . | . | . | . | . | . | . | . | . | . | . | N | . | . | . | . | . |
| RSVA/Leipzig/2019/8 | . | . | . | . | . | . | . | . | . | . | . | . | N | . | . | . | . | . |
| RSVA/Leipzig/2019/10 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | T | . |
| RSVA/Leipzig/2019/11 | . | . | . | . | . | . | . | . | . | . | . | . | N | . | . | . | . | . |
| RSVA/Leipzig/2019/14 | . | . | . | . | . | . | . | . | . | . | . | . | N | . | . | . | . | . |
| RSVA/Leipzig/2019/20 | . | . | I | . | . | . | . | . | . | . | . | . | N | . | . | . | . | . |
| RSVA/Leipzig/2019/16 | . | . | . | . | . | . | . | . | . | . | . | . | N | . | . | . | . | . |
| RSVA/Leipzig/2020/10 | . | . | . | . | . | . | . | . | . | S | . | . | . | I | . | . | . | . |
| RSVA/Leipzig/2020/16 | D | . | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2021/11 | . | . | I | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2021/18 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | N | . | . | . |
| RSVA/Leipzig/2021/47 | . | . | I | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2021/67 | . | . | I | . | F | R | . | . | . | . | . | . | N | . | . | . | . | . |
| RSVA/Leipzig/2021/73 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | N | . | . | . |
| RSVA/Leipzig/2021/123 | . | . | I | . | F | . | . | . | . | . | . | . | N | . | . | . | . | . |
| RSVA/Leipzig/2021/129 | . | . | . | . | . | . | . | . | I | . | . | . | . | . | N | . | . | . |
| RSVA/Leipzig/2022/1 | . | . | . | . | . | . | . | . | I | . | . | . | . | . | N | . | . | . |
| RSVA/Leipzig/2022/6 | . | V | I | . | F | . | . | . | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2022/7 | . | V | I | . | F | . | . | . | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2022/8 | . | . | I | . | F | . | . | . | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2022/9 | . | . | I | . | F | . | . | . | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2022/10 | . | V | I | . | F | . | . | . | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2022/11 | . | V | I | . | F | . | . | . | . | . | . | . | . | . | . | . | . | . |
| RSVA/Leipzig/2022/1 | . | . | . | . | . | . | T | . | . | . | A | . | . | . | . | . | . | . |
| RSVA/Leipzig/2022/2 | . | . | . | . | . | . | T | . | . | . | A | . | . | . | . | . | . | . |
| RSVA/Leipzig/2022/3 | . | V | I | . | F | . | . | . | . | . | . | . | . | . | . | . | . | . |
| AA position | 9 | 12 | 22 | 42 | 64 | 108 | 113 | 116 | 123 | 157 | 190 | 191 | 206 | 209 | 211 | 273 | 276 | 295 | 389 | 463 | 477 | 507 | 508 | 514 | 527 |
| Subunit | F1 | F1 | F1 | F1 | F1 | F1 | F2 | F2 | F2 | F2 | F2 | F2 | F2 | F2 | F2 | F2 | F2 | F2 | F2 | F2 | F2 | F2 | |||
| Region | SP | SP | SP | p27 | p27 | p27 | A | A | A | B | B | B | CT | ||||||||||||
| Antigenic sites | I | Ø | V | Ø | Ø | Ø | II | I/II | Ø | I | |||||||||||||||
| GB5.0.5a consensus | S | F | L | R | I | R | Q | N | K | V | S | K | I | Q | S | L | S | E | S | E | Y | R | R | H | I |
| 2 strains a | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 25 strains b | . | . | . | . | . | . | . | . | . | . | . | R | M | R | . | . | . | . | . | . | . | . | . | . | . |
| RSVB/Leipzig/2018/3 | . | . | . | . | . | . | . | . | . | . | . | R | M | R | . | . | . | D | . | . | . | . | . | . | . |
| RSVB/Leipzig/2018/13 | . | . | . | . | . | . | . | . | . | . | . | R | M | R | . | . | . | . | . | . | . | . | . | . | V |
| RSVB/Leipzig/2018/15 | . | . | . | . | . | . | . | . | . | . | . | R | M | R | . | . | N | . | . | . | . | . | . | . | . |
| RSVB/Leipzig/2019/7 | . | . | . | . | . | . | . | . | . | . | . | R | M | R | . | . | N | . | . | . | . | . | . | . | . |
| RSVB/Leipzig/2019/8 | . | L | . | . | . | . | . | . | . | . | . | R | M | R | . | . | N | . | . | D | . | . | . | . | . |
| RSVB/Leipzig/2019/11 | . | L | . | . | . | . | . | . | . | . | . | R | M | R | . | . | N | . | . | . | . | . | . | . | . |
| RSVB/Leipzig/2019/12 | . | L | F | . | . | . | . | . | . | . | N | R | M | R | . | . | N | . | . | D | . | . | . | . | . |
| RSVB/Leipzig/2021/19 | . | . | . | . | . | . | . | . | . | A | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| 21 strains c | . | . | . | . | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2022/3 | . | . | . | K | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2022/4 | . | . | . | . | . | . | . | . | R | . | . | R | M | R | . | . | . | . | . | . | . | . | . | . | . |
| RSVB/Leipzig/2022/5 | . | . | . | . | . | . | . | . | R | . | . | R | M | R | . | . | . | . | . | . | . | . | . | . | . |
| RSVB/Leipzig/2022/6 | . | . | . | . | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | H | . | R | . |
| RSVB/Leipzig/2022/7 | . | . | . | . | . | . | . | . | R | . | . | R | M | R | . | . | . | . | . | . | . | . | . | . | . |
| RSVB/Leipzig/2022/8 | . | . | . | . | . | . | . | . | R | . | . | R | M | R | . | . | . | . | . | . | . | . | . | . | . |
| RSVB/Leipzig/2022/9 | . | . | . | K | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2022/10 | . | . | . | K | V | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2022/11 | . | . | . | . | . | . | . | S | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2022/14 | . | . | . | K | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2022/15 | . | . | . | K | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2022/16 | N | . | . | K | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2022/17 | . | . | . | . | . | . | . | . | . | . | N | R | M | . | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2022/18 | . | . | . | . | . | . | R | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2022/19 | . | . | . | K | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2022/20 | . | . | . | . | . | . | . | . | R | . | . | R | M | R | . | . | . | . | . | . | . | . | . | . | . |
| RSVB/Leipzig/2022/21 | . | . | . | . | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2022/22 | . | . | . | K | . | K | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2023/1 | . | . | . | K | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2023/3 | . | . | . | . | . | . | . | . | . | . | N | R | M | R | N | I | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2023/5 | . | . | . | K | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2023/6 | . | . | . | . | . | . | . | S | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2023/7 | . | . | . | K | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2023/10 | . | . | . | . | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | H | . | R | . |
| RSVB/Leipzig/2023/11 | . | . | . | . | . | . | . | . | R | . | . | R | M | R | . | . | . | . | . | . | . | . | . | . | . |
| RSVB/Leipzig/2023/12 | . | . | . | . | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | H | . | . | . | . |
| RSVB/Leipzig/2023/13 | . | . | . | . | . | . | . | S | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2023/16 | . | . | . | . | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | H | . | . | . | . |
| RSVB/Leipzig/2023/17 | . | . | . | . | . | . | . | . | R | . | . | R | M | R | . | . | . | . | . | . | . | . | . | . | . |
| RSVB/Leipzig/2023/18 | . | . | . | K | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | . | . | . |
| RSVB/Leipzig/2023/19 | . | . | . | . | . | . | . | . | R | . | . | R | M | R | . | . | . | . | . | . | . | . | . | . | . |
| RSVB/Leipzig/2023/20 | . | . | . | . | . | . | . | . | R | . | . | R | M | R | . | . | . | . | . | . | . | . | . | . | . |
| RSVB/Leipzig/2023/21 | . | . | . | . | . | . | . | . | R | . | . | R | M | R | . | . | . | . | . | . | . | . | . | . | . |
| RSVB/Leipzig/2023/24 | . | . | . | . | . | . | . | . | R | . | . | R | M | R | . | . | . | . | . | . | . | . | . | . | . |
| RSVB/Leipzig/2023/25 | . | I | . | . | . | . | . | . | . | . | N | R | M | R | N | . | . | . | P | . | . | . | K | . | . |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hönemann, M.; Maier, M.; Frille, A.; Thiem, S.; Bergs, S.; Williams, T.C.; Mas, V.; Lübbert, C.; Pietsch, C. Respiratory Syncytial Virus in Adult Patients at a Tertiary Care Hospital in Germany: Clinical Features and Molecular Epidemiology of the Fusion Protein in the Severe Respiratory Season of 2022/2023. Viruses 2024, 16, 943. https://doi.org/10.3390/v16060943
Hönemann M, Maier M, Frille A, Thiem S, Bergs S, Williams TC, Mas V, Lübbert C, Pietsch C. Respiratory Syncytial Virus in Adult Patients at a Tertiary Care Hospital in Germany: Clinical Features and Molecular Epidemiology of the Fusion Protein in the Severe Respiratory Season of 2022/2023. Viruses. 2024; 16(6):943. https://doi.org/10.3390/v16060943
Chicago/Turabian StyleHönemann, Mario, Melanie Maier, Armin Frille, Stephanie Thiem, Sandra Bergs, Thomas C. Williams, Vicente Mas, Christoph Lübbert, and Corinna Pietsch. 2024. "Respiratory Syncytial Virus in Adult Patients at a Tertiary Care Hospital in Germany: Clinical Features and Molecular Epidemiology of the Fusion Protein in the Severe Respiratory Season of 2022/2023" Viruses 16, no. 6: 943. https://doi.org/10.3390/v16060943






