The MGF300-2R Protein of African Swine Fever Virus Promotes IKKβ Ubiquitination by Recruiting the E3 Ubiquitin Ligase TRIM21
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Antibodies, Plasmids and Reagents
2.3. Transfection
2.4. Immunoprecipitation-Mass Spectrometry (IP-MS) and Proteomics Functional Analysis
2.5. Western Blotting and Co-Immunoprecipitation (Co-IP)
2.6. Laser Confocal Microscopy
2.7. Cell Viability Assay
2.8. Statistical Analysis
3. Results
3.1. The Proteins Interacting with MGF300-2R Were Screened by IP-MS
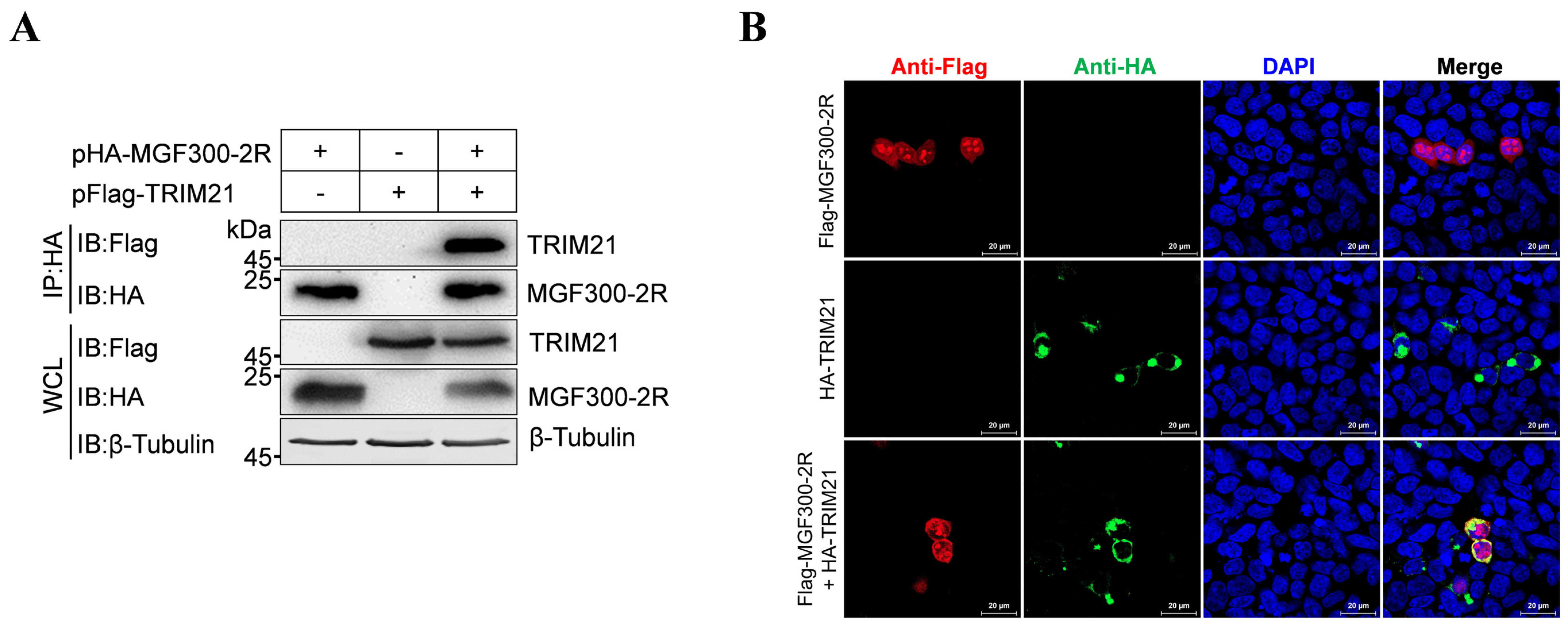
3.2. The E3 Ubiquitin Ligase TRIM21 Interacts with MGF300-2R
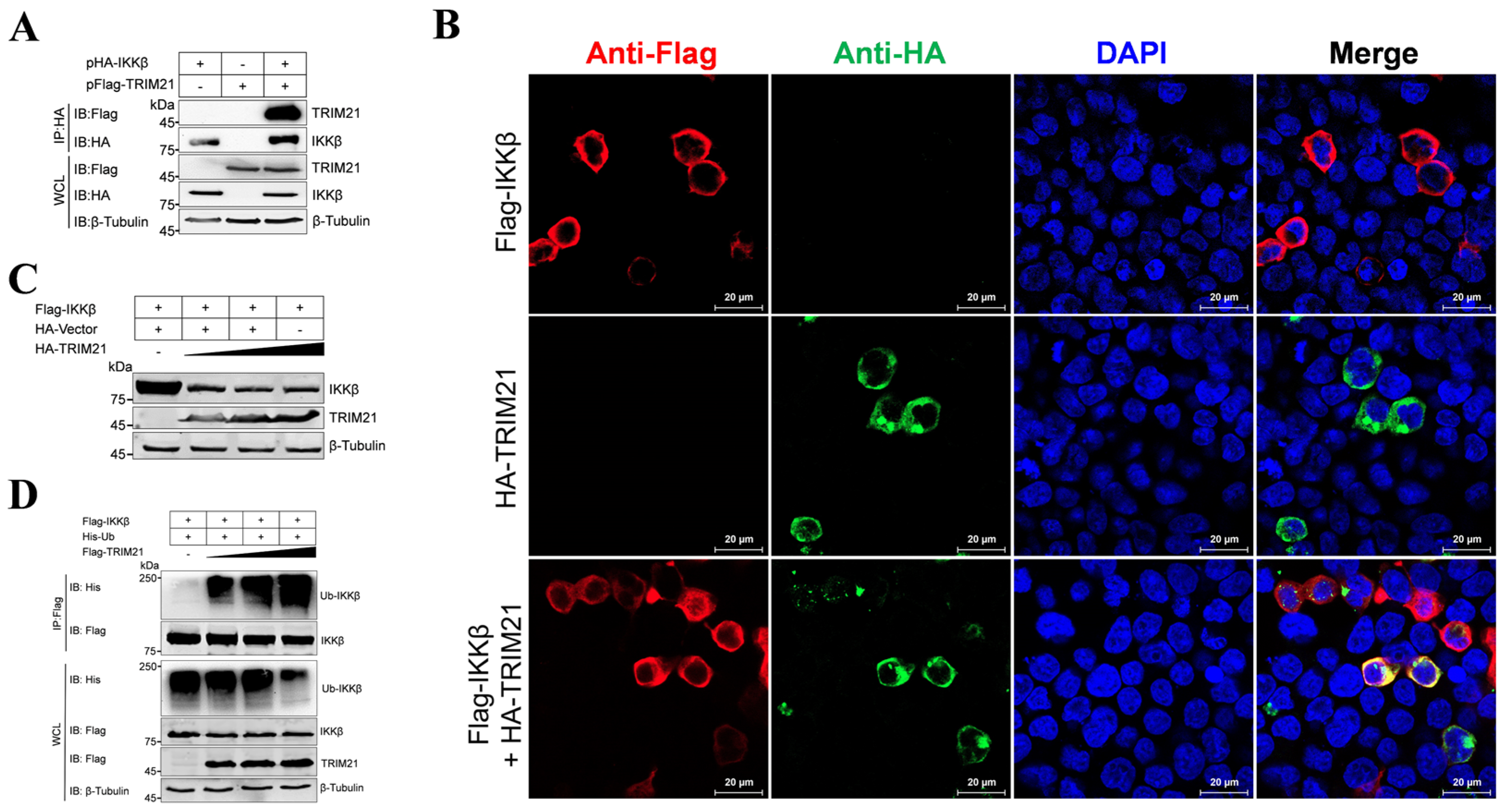
3.3. The E3 Ubiquitin Ligase TRIM21 Promotes the Ubiquitination and Degradation of IKKβ
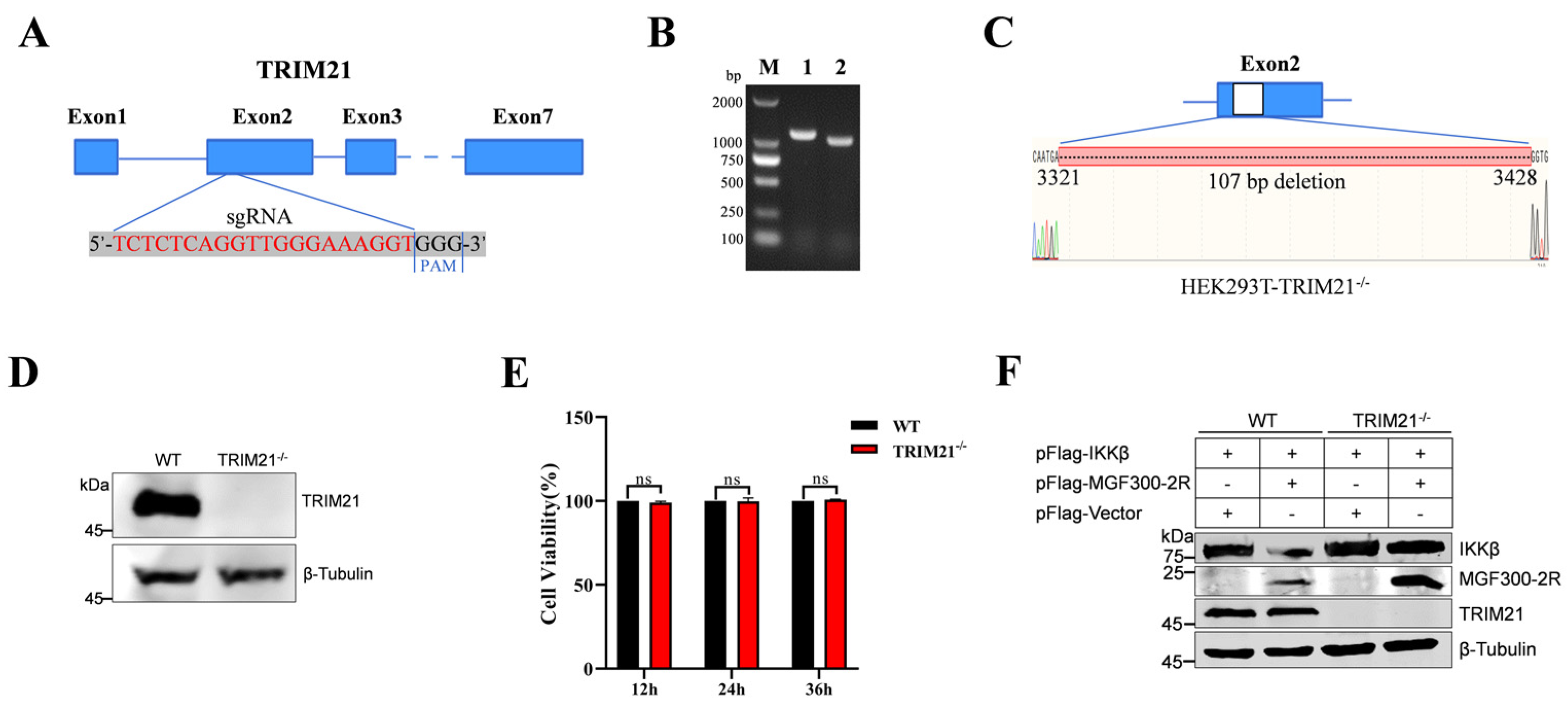
3.4. Degradation of IKKβ by MGF300-2R Depends on TRIM21
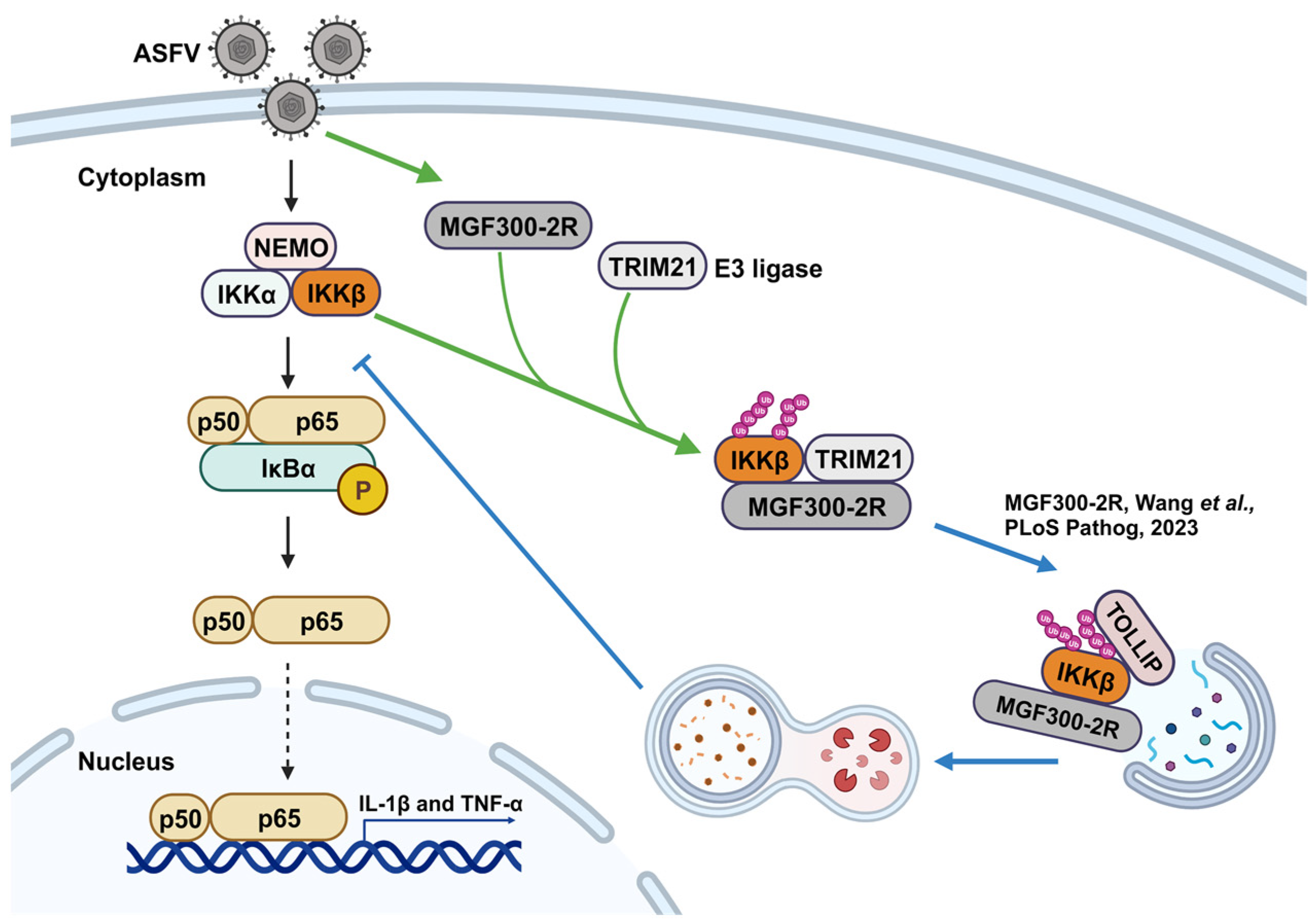
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African swine fever in China, 2018. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African swine fever epidemiology and control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Liu, T.; Zhang, M.; Zhao, X.; Dong, Y.; Wu, B.; Wang, Y.; Li, J.; Wei, X.; Shi, B. African swine fever outbreaks in China led to gross domestic product and economic losses. Nat. Food 2021, 2, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, L.F.; Zhang, K.; Wang, B.; Tang, L.; Li, M.; Wang, T.; Sun, Y.; Li, S.; Qiu, H.J. Deletion of the H240R gene of African swine fever virus decreases infectious progeny virus production due to aberrant virion morphogenesis and enhances inflammatory cytokine expression in porcine macrophages. J. Virol. 2022, 96, e0166721. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yu, S.; Ge, H.; Wang, T.; Li, Y.; Zhou, P.; Pan, L.; Han, Y.; Yang, Y.; Sun, Y.; et al. The A137R protein of African swine fever virus inhibits type I interferon production via the autophagy-mediated lysosomal degradation of TBK1. J. Virol. 2022, 96, e0195721. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Luo, R.; Zhang, J.; Lu, Z.; Li, L.F.; Zheng, Y.H.; Pan, L.; Lan, J.; Zhai, H.; Huang, S.; et al. The MGF300-2R protein of African swine fever virus is associated with viral pathogenicity by promoting the autophagic degradation of IKKα and IKKβ through the recruitment of TOLLIP. PLoS Pathog. 2023, 19, e1011580. [Google Scholar] [CrossRef]
- Wang, T.; Luo, R.; Zhang, J.; Lan, J.; Lu, Z.; Zhai, H.; Li, L.F.; Sun, Y.; Qiu, H.J. The African swine fever virus MGF300-4L protein is associated with viral pathogenicity by promoting the autophagic degradation of IKKβ and increasing the stability of IκBα. Emerg. Microbes Infect. 2024, 13, 2333381. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Luo, R.; Sun, Y.; Qiu, H.J. Current efforts towards safe and effective live attenuated vaccines against African swine fever: Challenges and prospects. Infect. Dis. Poverty 2021, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Behrends, C. Molecular mechanisms of selective autophagy. J. Mol. Biol. 2020, 432, 1–2. [Google Scholar] [CrossRef]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185. [Google Scholar] [CrossRef]
- Kong, Z.; Yin, H.; Wang, F.; Liu, Z.; Luan, X.; Sun, L.; Liu, W.; Shang, Y. Pseudorabies virus tegument protein UL13 recruits RNF5 to inhibit STING-mediated antiviral immunity. PLoS Pathog. 2022, 18, e1010544. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.; Zhao, W.; Liu, J.; Ullah, R.; Fang, P.; Fang, L.; Xiao, S. Porcine reproductive and respiratory syndrome virus degrades DDX10 via SQSTM1/p62-dependent selective autophagy to antagonize its antiviral activity. Autophagy 2023, 19, 2257–2274. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Bai, J.; Jiang, C.; Zhu, H.; Liu, D.; Pan, M.; Wang, X.; Pi, J.; Jiang, P.; Liu, X. Tegument protein UL21 of alpha-herpesvirus inhibits the innate immunity by triggering CGAS degradation through TOLLIP-mediated selective autophagy. Autophagy 2023, 19, 1512–1532. [Google Scholar] [CrossRef] [PubMed]
- de Moura, T.R.; Mozaffari-Jovin, S.; Szabó, C.Z.K.; Schmitzová, J.; Dybkov, O.; Cretu, C.; Kachala, M.; Svergun, D.; Urlaub, H.; Lührmann, R.; et al. Prp19/Pso4 is an autoinhibited ubiquitin ligase activated by stepwise assembly of three splicing factors. Mol. Cell 2018, 69, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, P.; Nassar, J.; Peiffer, I.; Stanojcic, S.; Sterkers, Y.; Delamarre, A.; Bocquet, S.; Méchali, M. The ORC ubiquitin ligase OBI1 promotes DNA replication origin firing. Nat. Commun. 2019, 10, 2426. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Dai, X.; Jiang, W.; Li, Y.; Wei, W. RBR E3 ubiquitin ligases in tumorigenesis. Semin. Cancer Biol. 2020, 67, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Shin, H.C.; Seo, T.; Nawale, L.; Han, G.; Kim, B.Y.; Kim, S.J.; Cha-Molstad, H. Signaling pathways regulated by UBR box-containing E3 ligases. Int. J. Mol. Sci. 2021, 22, 8323. [Google Scholar] [CrossRef]
- Singh, S.; Ng, J.; Sivaraman, J. Exploring the "Other" subfamily of HECT E3-ligases for therapeutic intervention. Pharmacol. Ther. 2021, 224, 107809. [Google Scholar] [CrossRef]
- Xue, B.; Li, H.; Guo, M.; Wang, J.; Xu, Y.; Zou, X.; Deng, R.; Li, G.; Zhu, H. TRIM21 promotes innate immune response to RNA viral infection through Lys27-linked polyubiquitination of MAVS. J. Virol. 2018, 92, e00321-18. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, W.; Yang, W.; Zhang, J.; Li, D.; Zheng, H. Structure of African swine fever virus and associated molecular mechanisms underlying infection and immunosuppression: A review. Front. Immunol. 2021, 12, 715582. [Google Scholar] [CrossRef]
- Mao, S.; Cai, X.; Niu, S.; Wei, J.; Jiang, N.; Deng, H.; Wang, W.; Zhang, J.; Shen, S.; Ma, Y.; et al. TRIM21 promotes ubiquitination of SARS-CoV-2 nucleocapsid protein to regulate innate immunity. J. Med. Virol. 2023, 95, e28719. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, J.; Yang, W.; Li, P.; Ru, Y.; Kang, W.; Li, L.; Ran, Y.; Zheng, H. African swine fever virus protein MGF-505-7R promotes virulence and pathogenesis by inhibiting JAK1- and JAK2-mediated signaling. J. Biol. Chem. 2021, 297, 101190. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Li, D.; Xiong, M.G.; Liu, H.N.; Feng, T.; Shi, Z.W.; Li, Y.H.; Wu, H.N.; Wang, S.Y.; Zheng, H.X.; et al. African swine fever virus I267L acts as an important virulence factor by inhibiting RNA polymerase III-RIG-I-mediated innate immunity. PLoS Pathog. 2022, 18, e1010270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, S.; Xin, T.; Wang, X.; Yu, H.; Chen, S.; Jiang, Y.; Gao, X.; Jiang, Y.; Guo, X.; et al. African swine fever virus MGF360-14L negatively regulates type I interferon signaling by targeting IRF3. Front. Cell. Infect. Microbiol. 2022, 11, 818969. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Peng, J.; Wu, J.; Yi, J.; Wu, P.; Qi, X.; Ren, J.; Peng, G.; Duan, X.; Ru, Y.; et al. African swine fever virus MGF-360-10L is a novel and crucial virulence factor that mediates ubiquitination and degradation of JAK1 by recruiting the E3 ubiquitin ligase HERC5. mBio 2023, 14, e0060623. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Peng, J.L.; Xu, Z.S.; Xiong, M.G.; Wu, H.N.; Wang, S.Y.; Li, D.; Zhu, G.Q.; Ran, Y.; Wang, Y.Y. African swine fever virus cysteine protease pS273R inhibits type I interferon signaling by mediating STAT2 degradation. J. Virol. 2023, 97, e0194222. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lou, H.; Zhao, Y.; Fan, W.; Jiao, P.; Sun, L.; Luo, T.; Liu, W. The I226R protein of African swine fever virus inhibits the cGAS-STING-mediated innate immune response. Sheng Wu Gong Cheng Xue Bao 2023, 39, 4796–4808. [Google Scholar] [PubMed]
- Li, Y.; Huang, L.; Li, H.; Zhu, Y.; Yu, Z.; Zheng, X.; Weng, C.; Feng, W.H. ASFV pA151R negatively regulates type I IFN production via degrading E3 ligase TRAF6. Front. Immunol. 2024, 15, 1339510. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Chen, S.; Zheng, J.; Zhang, H.; Ren, L. Multiple roles of TRIM21 in virus infection. Int. J. Mol. Sci. 2023, 24, 1683. [Google Scholar] [CrossRef]
- Song, Y.; Wu, X.; Xu, Y.; Zhu, J.; Li, J.; Zou, Z.; Chen, L.; Zhang, B.; Hua, C.; Rui, H.; et al. HPV E7 inhibits cell pyroptosis by promoting TRIM21-mediated degradation and ubiquitination of the IFI16 inflammasome. Int. J. Biol. Sci. 2020, 16, 2924–2937. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, D.; Qian, P.; Qian, S.; Wu, M.; Chen, H.; Li, X. Swine TRIM21 restricts FMDV infection via an intracellular neutralization mechanism. Antivir. Res. 2016, 127, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zou, C.; Zeng, S.; Xue, C.; Cao, Y. Characterization of porcine tripartite motif genes as host restriction factors against PRRSV and PEDV infection. Virus Res. 2019, 270, 197647. [Google Scholar] [CrossRef] [PubMed]

| Primers | Sequence (5′-3′) |
|---|---|
| Flag-TRIM21-Forward | CAAGCTTGCGGCCGCGAATTCGATGGCTTCAGCAGCACGC |
| Flag-TRIM21-Reverse | ATCAGATCTATCGATGAATTCTCAATAGTCAGTGGATCCTTGTGATC |
| sgRNA-Forward | TCTCTCAGGTTGGGAAAGGT |
| sgRNA-Reverse | ACCTTTCCCAACCTGAGAGA |
| TRIM21-Forward | GCTTTGGCTATTCTGAGTCT |
| TRIM21-Reverse | TCCCCTTTGAACCTGCATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Luo, R.; Lan, J.; Chen, S.; Qiu, H.-J.; Wang, T.; Sun, Y. The MGF300-2R Protein of African Swine Fever Virus Promotes IKKβ Ubiquitination by Recruiting the E3 Ubiquitin Ligase TRIM21. Viruses 2024, 16, 949. https://doi.org/10.3390/v16060949
Lu Z, Luo R, Lan J, Chen S, Qiu H-J, Wang T, Sun Y. The MGF300-2R Protein of African Swine Fever Virus Promotes IKKβ Ubiquitination by Recruiting the E3 Ubiquitin Ligase TRIM21. Viruses. 2024; 16(6):949. https://doi.org/10.3390/v16060949
Chicago/Turabian StyleLu, Zhanhao, Rui Luo, Jing Lan, Shengmei Chen, Hua-Ji Qiu, Tao Wang, and Yuan Sun. 2024. "The MGF300-2R Protein of African Swine Fever Virus Promotes IKKβ Ubiquitination by Recruiting the E3 Ubiquitin Ligase TRIM21" Viruses 16, no. 6: 949. https://doi.org/10.3390/v16060949





