Abstract
Brazil has earned the moniker “arbovirus hotspot”, providing an ideal breeding ground for a multitude of arboviruses thriving in various zoonotic and urban cycles. As the planet warms and vectors expand their habitat range, a nuanced understanding of lesser-known arboviruses and the factors that could drive their emergence becomes imperative. Among these viruses is the Iguape virus (IGUV), a member of the Orthoflavivirus aroaense species, which was first isolated in 1979 from a sentinel mouse in the municipality of Iguape, within the Vale do Ribeira region of São Paulo State. While evidence suggests that IGUV circulates among birds, wild rodents, marsupials, bats, and domestic birds, there is no information available on its pathogenesis in both humans and animals. The existing literature on IGUV spans decades, is outdated, and is often challenging to access. In this review, we have curated information from the known literature, clarifying its elusive nature and investigating the factors that may influence its emergence. As an orthoflavivirus, IGUV poses a potential threat, which demands our attention and vigilance, considering the serious outbreaks that the Zika virus, another neglected orthoflavivirus, has unleashed in the recent past.
1. Introduction
In recent years, neglected arboviruses have taken center stage as severe outbreaks in various countries [1,2,3,4,5,6,7,8,9,10,11,12,13] have strained healthcare systems and incurred enormous socioeconomic costs [14,15,16]. The global spread of arboviral infections, propelled by expanding mosquito habitats due to heightened trade, uncontrolled urbanization, and climate change, has increased awareness among public health, research, and policy stakeholders [17,18,19,20]. Iguape virus (IGUV), a single-stranded positive-sense RNA virus within the Orthoflavivirus aroaense species (Flaviviridae,/Orthoflavivirus) [21], was initially isolated in 1979 from sentinel mice in São Paulo State, Brazil, following a Rocio virus (ROCV) outbreak [22].
Brazil, renowned for its sprawling ecotypes and biodiversity, has long been considered an “arbovirus hotspot” for fostering ideal conditions for numerous arboviruses in diverse zoonotic and urban transmission cycles [4,12,23,24,25,26,27,28,29,30,31,32,33,34,35]. For IGUV, a potential emergent threat, to date, there is limited information available on its transmission cycles and pathogenicity in both animals and humans. Additionally, the burden of IGUV infections may be significantly underestimated, given the lack of accurate diagnostics and its omission from laboratory screenings. Similar to the trajectories of Zika (ZIKV) and chikungunya (CHIKV) [36] viruses, IGUV infections may evolve into a major health concern. Notably, the existing literature on IGUV spans decades, is outdated, and is often a challenge to access. Amidst the warming planet and expanding vector habitats [19,37,38,39,40], we delve into the known IGUV literature to review the information that is known, identify gaps, and suggest comprehensive studies on the biological aspects, potential vectors, and transmission dynamics that are urgently needed.
2. Discovery, Classification, and Taxonomy
Since 1961, the Section of Arthropod-Transmitted Viruses (S.A.T.V.) at the Adolfo Lutz Institute, a key institution affiliated with the Department of Health of the State of São Paulo, has been conducting ongoing studies in ecology and epidemiology focused on arbovirus infections in the Atlantic rainforest regions of this state. These investigations involved collecting vectors and blood samples from wildlife in the area and were instrumental in the detection and response to the largest recorded encephalitis outbreak in the country in 1975 caused by ROCV (reviewed in [25]). This outbreak affected over 1000 people in the Vale do Ribeira region, with a 10% fatality rate, and 20% were affected with long-term sequelae [25,26]. The response to this outbreak included sustainable surveillance efforts, with the capture of animals and vectors, as well as the use of sentinel animals for the early identification and characterization of new arboviruses.
In January 1979, a new virus, initially designated as SPAn 71686 and later renamed Iguape virus (IGUV), was isolated from sentinel mice in the Atlantic rainforest region within the municipality of Iguape, state of São Paulo. The mice were brought into the laboratory for further observation, where visible signs of infection were noted, including tremors, paralysis, and lethargy. Mice were euthanized, their brains were collected, and filtered brain homogenates were intracerebrally inoculated into suckling mice. The virus was subsequently identified using the complement fixation test [22].
Currently, there is limited available information on the molecular characteristics of IGUV. Ultrastructural observations of mouse brain tissue collected 73 h post-inoculation revealed viral particles predominantly in the cytoplasm of infected cells, as well as in extracellular spaces, with an approximate size of 41 nm [22]. The virus is currently classified as a member of the genus Orthoflavivirus, which encompasses over 70 virus species and is within the Aroa antigenic complex [21,41]. IGUV demonstrated pathogenicity in several laboratory animals where high viral titers were observed in the brains of Swiss mice and suckling hamsters, which developed fatal encephalitis six days post-inoculation via the intracerebral route. However, young adult hamsters (6–8 weeks old) inoculated intraperitoneally developed an encephalitic illness from which they eventually recovered [22].
3. Experimental Studies on Ecology and Transmission Cycles
Following the IGUV discovery, serological surveys were conducted on animals in the region to gain a better understanding of potential viral reservoirs and affected animals in an attempt to trace a possible transmission cycle [22,42]. The studies focused on animals in the Vale do Ribeira and Vale do Rio Iguape regions because of their rich fauna. Serological surveys were conducted on bird samples collected between 1989 and 1990, showing monotypic response to IGUV in 50 birds from the following 16 families: Columbidae, Furnaridae, Formicariidae, Conopophagidae, Piiridae, Tyrannidae, Hirundinidae, Troglodytidae, Turdidae, Motacillidae, Plodeidae, Vireonidae, Icteridae, Parulidae, Thraupidae, and Fringillidae [42] (Table 1). Many of the identified birds were resident–migratory species, notably Myiarchus swainsoni (Tyrannidae/Myiarchus) [42]. This passerine bird, commonly known as Swainson’s flycatcher or Swainson’s Myiarchus, is renowned for its extensive migration patterns, suggesting a possible role in IGUV’s long-distance dispersion. During the breeding season, they migrate from South America to northern regions of the continent, including parts of Central America [43]. Vireo olivaceus (Vireonida,/Vireo), on the other hand, is a small songbird commonly known as the red-eyed vireo, which is primarily found in North and Central America and encompasses a vast geographical range. During the breeding season, they inhabit deciduous forests, but they undergo extensive migrations, moving to wintering grounds in Central and South America [44]. Given that many arboviruses (e.g., West Nile (WNV) [45,46], Ilheus (ILHV) [47], Saint Louis encephalitis (SLEV) [48], ROCV [26,42] and others) infect wild birds and can be amplified at high levels of viremia that make birds infectious to various vector species, it has been suggested that the migratory bird movements could represent a crucial mechanism for the dispersal of these viruses on a local, continental, and intercontinental scale [49,50].

Table 1.
Documented circulation of IGUV among animals/arthropods.
Between 1989 and 1992, Coimbra and colleagues performed serological studies based on the hemagglutination inhibition (HI) test on wild rodents, marsupials, teal, ducks, and chickens, and showed the presence of flavivirus antibodies (Table 1) [22], suggesting their possible role in the transmission cycle. Critically, though, they demonstrated that wild birds had a monotypic response to IGUV, strongly suggesting a key role in the transmission of the virus. Notably, the tested wild bird samples (n = 973) were representative of 33 species belonging to 29 genera and representing 17 families, showing a 9.89% (46/465), 18.90% (40/212) and 19.50% (58/296) positivity rate in 1990–1992, respectively [22]. A similar monotypic response was shown in chickens and ducks, raising a notion that may play a role as bridge hosts of IGUV transmission in urban settings, given their proximity to humans as they are commonly raised in urban and rural environments, often sharing spaces close to human residences.
The highly primatophilic Anopheles (An.) cruzii mosquitoes collected in 1994 from the city of Juquitiba, located in the Vale do Ribeira region about 80 miles from the city of Iguape [51], provide, to date, the only record of IGUV detection and isolation from naturally infected mosquitoes. Notably, An. cruzii mosquitoes are considered the primary vectors of transmission for humans and simian malaria in the Brazilian regions covered by the Atlantic Rainforest [55,56,57]; however, Anopheles spp. are known to be competent vectors of transmission for the o’nyong nyong virus (ONNV), an arbovirus endemic in East Africa [58] and possibly Cacipacore virus (CPCV), a zoonotic arbovirus endemic to Brazil [29,59]. Their high abundance is predominantly in the hills of the Vale do Ribeira, which has intense deforestation and land use changes which may not only have created favorable ecological and microclimate conditions (e.g., natural breeding sites) that may favor the distribution and relative abundance of certain vectors of transmission but also the pathogens they transmit, partly resulting from their opportunistic behavior and feeding habits [56,60,61]. Lastly, additional information based on experimental vector competence is still necessary to determine the role of An. cruzii in the transmission cycle of IGUV.
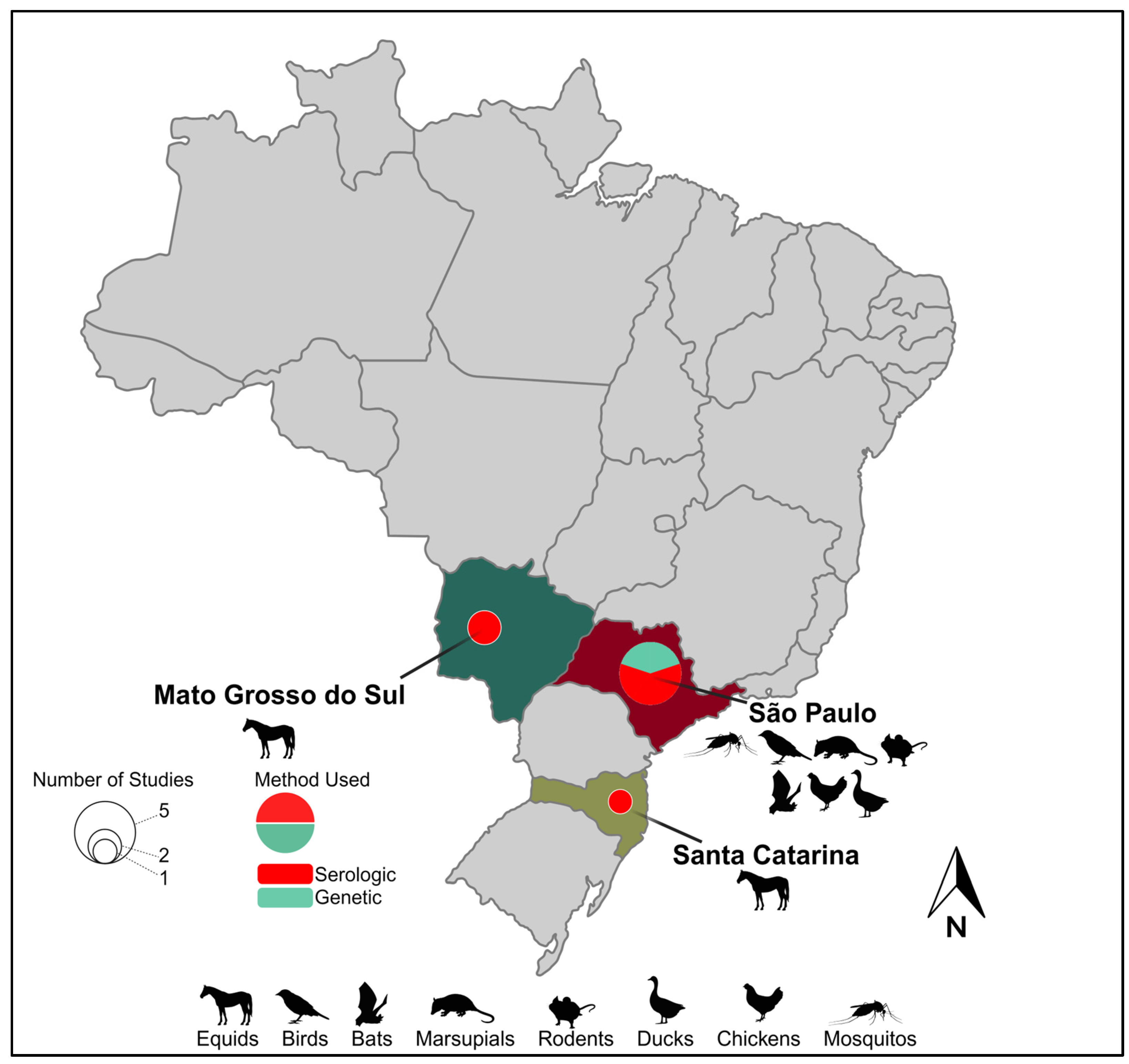
The proximity of horses to human populations is also a concern regarding the dissemination of diseases [62]. Horses are often found in both urban and rural areas bordering forested areas, where they share spaces close to humans for recreational activities, sports, or work. This proximity creates a potential interface for the transmission of arboviruses (e.g., IGUV) between horses and humans through the bites of generalist mosquitoes. Several serosurveys in various regions of Brazil have been performed in the first decade of the 21st century aimed at determining their role in IGUV transmission and have detected their presence in horses sampled at the states of São Paulo State [52,53], Mato Grosso do Sul [53,54], and Santa Catarina [53], demonstrating the circulation of this virus in the central and southern region of Brazil (Figure 1). Beyond the unknown impact of IGUV infection on the health of horses, experimental studies are urgently needed to assess whether horses could serve as reservoirs and/or amplification hosts, thus expanding IGUV’s host range and its potential to seed urban outbreaks.
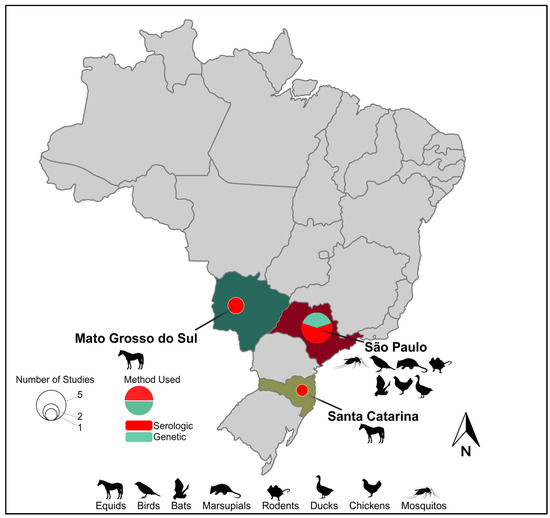
Figure 1.
Geographic range and epidemiological landscape of Iguape virus. Brazilian states with evidence of IGUV circulation are named. Hosts from which IGUV and/or antibodies have been identified within a given Brazilian state are indicated by a representative graphic(s). Pie charts within a given state indicate the number of studies identifying IGUV by size and the method of their identification by color.
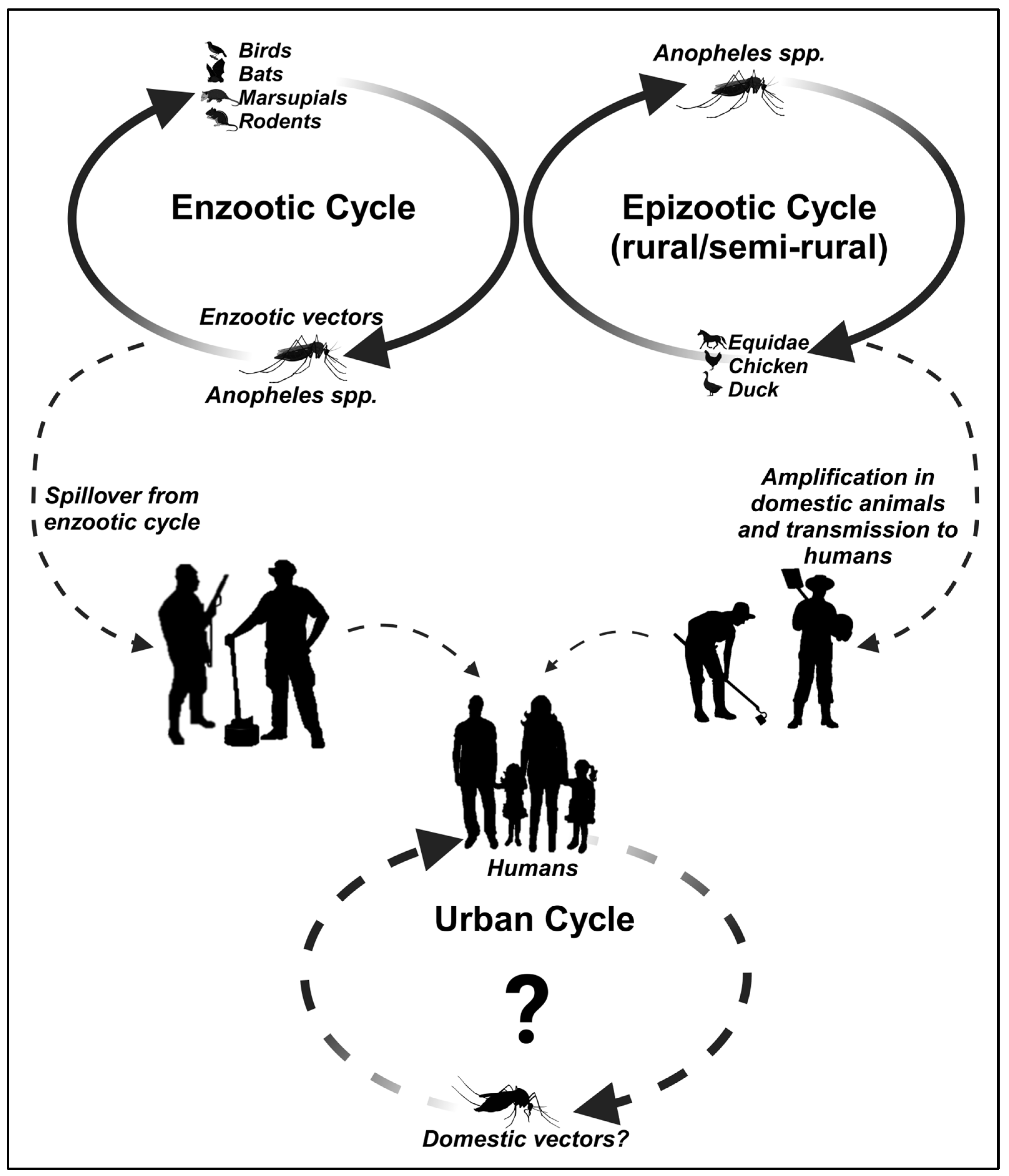
To date, there are no known reports in the literature of IGUV infections in humans nor prevalent data in serological surveys of humans. A possible IGUV transmission cycle is proposed in Figure 2 based on the currently available information in the literature and its potential role in human infections, given that the absence of any evidence is purely speculative. Moreover, it is important to note that despite the detection of monotypic and heterotypic antibodies in serological surveys that identified various vertebrates as potential vertebrate hosts (other than sentinel mice) of IGUV transmission, as reviewed above, no acute infections in animals or humans that could incriminate IGUV as a pathogenic agent have been observed so far.

Figure 2.
The possible transmission cycles of IGUV.
4. Diagnosis, Treatment, and Prevention
As mentioned above, there is no available information on the range of clinical manifestations of IGUV infection in humans. As an exceptionally poorly understood virus, there are no commercial diagnostic tests available as IGUV is not routinely included in any panels of laboratory diagnostic protocols of public health centers in Brazil. Only a few research centers in the country possess the infrastructure and adequate resources needed for its identification, contributing to our lack of understanding of IGUV circulation and, consequently, the actual impact IGUV may have on veterinary and human health across Brazil and beyond. Diagnostic tests mentioned in the literature are in-house research laboratory-developed tests such as the hemagglutination inhibition test (HI) [22,42,52,53,63] and the plaque reduction neutralization test (PRNT) [54,63], used mainly for serological testing. Molecular testing involves RT-qPCR [64] in addition to viral isolation [22,63]. Therefore, due to the lack of infrastructure and limited resources for accurate IGUV identification, an IGUV outbreak could go unnoticed and likely be attributed to other causes, given that Brazil is endemic for various orthoflaviviruses (e.g., SLEV, ROCV, ILHV, CPCV, ZIKV) and other tropical diseases (e.g., malaria) that present with a similar range of symptoms. Therefore, improving diagnostic capabilities is crucial, given the notorious cross-reactivity among orthoflaviviruses [41]. Developing specific serological tests that can accurately distinguish IGUV from other orthoflavivirus infections, while challenging, is essential for the rapid and accurate detection of IGUV in low-resource settings.
There is no licensed vaccine or antiviral treatment for IGUV infections, and given the absence of any documented human infections, the development of an IGUV vaccine candidate may be challenging and unrealistic. The detection of antibodies in animal serological surveys [22,42], while indicating the circulation of the virus in various vertebrate species, has not been accompanied by reports of any veterinary disease manifestations, thus further obfuscating its disease burden in vertebrate animals. On the other hand, IGUV may undergo stochastic mutations over time, leading to vector host range changes, changes in its virulence, or ability to infect humans, leading to its rapid emergence and dissemination across the globe; these are events that have, over the last two decades, been experienced with the emergence from obscurity and global distribution of ZIKV [65,66], CHIKV [67] and SARS-CoV-2 [68,69]. Recent efforts by world bodies (e.g., The World Health Organization (WHO) [70] or the Coalition for Epidemic Preparedness Innovations (CEPI) [71] have ramped up efforts to better predict and respond to sudden attacks by unknown pathogens—also referred to as Disease X—by investing in new methods for the rapid development and deployment of effective countermeasures, such as vaccines or antivirals, as proactive strategies to respond to potential future outbreaks.
All currently available evidence suggests that IGUV may be primarily confined to regions within Brazilian biomes. However, the possibility that IGUV circulates elsewhere in Central and South America and the Caribbean cannot be ruled out. Despite this apparent restriction, occasional spillovers, emergence, and the global spread of the virus cannot be ruled out, as witnessed with WNV [72,73], CHIKV [74,75,76,77,78], and ZIKV [6,79,80,81,82,83,84,85]. Even with these examples, specific antiviral therapies or vaccines are not available to combat most orthoflavivirus infections, with IGUV being no exception. It is noteworthy that among mosquito-borne orthoflaviviruses, only a handful of recently licensed ones are available. These include DENV (tetravalent, live-attenuated dengue vaccine Dengvaxia® manufactured by Sanofi Pasteur [86,87], and tetravalent dengue vaccine TAK-003 manufactured by Takeda Pharmaceuticals [88]), the YFV live-attenuated YF-VAX ® 17D-204 manufactured by Sanofi Pasteur [89], the 17DD manufactured by Bio-Manguinhos/FIOCRUZ [90], and lastly the Japanese Encephalitis Virus (JEV), IXIARO®, a Vero cell-derived inactivated vaccine, manufactured by Valneva [91]. However, despite these notable exceptions, the majority of orthoflaviviruses lack specific antiviral therapies or vaccines, which pose significant challenges in managing their infections. But even though IGUV currently lacks any licensed vaccine or specific antiviral treatment, the fact that the virus appears to have a “low impact/low burden” currently, coupled with the fact that the traditional path of drug discovery is complex, time-consuming, and expensive, with a typical time required to bring a drug from concept to market usually exceeding a decade and costing billions of dollars [92,93], brings to light a probable negative outlook regarding the development of specific therapeutic approaches for IGUV, even though it is necessary and highly encouraged.
Much of the clinical management of patients infected with arboviruses aims only to alleviate the symptoms and complications associated with the infection. Symptomatic treatment focuses on relieving symptoms with analgesic, antipyretic, and non-steroidal anti-inflammatory drugs (NSAIDs), along with counseling the patient to stay adequately hydrated, especially if experiencing vomiting, diarrhea, or fever, as well as recommending proper rest. Additionally, regular medical monitoring is advised to monitor disease progression and potential complications, especially for at-risk groups such as pregnant women and the elderly. The lack of specific antivirals for IGUV represents a significant gap in the ability to deal with a potential IGUV emergence, leaving the medical and scientific community devoid of specific therapeutic options. However, in the face of a potentially devastating outbreak of IGUV, it is crucial to consider alternative treatment strategies. A promising approach would be to explore the potential of drugs that have shown efficacy against other viruses of the Flaviviridae family. An example is niclosamide, originally an FDA-approved anti-helminthic medication. Drug screening studies have found its effectiveness against various orthoflaviviruses in experimental animal models, including the Zika virus (ZIKV) [94,95], by inhibiting viral production and reducing inflammatory response [96]. Additionally, ribavirin, a synthetic nucleoside analog widely used in the treatment of hepatitis B and C, has been shown to suppress ZIKV replication in cells [97,98], although with varying results in animal studies [97,99]. Another possibility is emetine, an FDA-approved compound for the treatment of amoebiasis, which has shown broad-spectrum antiviral activity [100], including ZIKV [101]. These examples underscore the importance of exploring repurposing and existing FDA-approved drugs as potential treatment candidates against IGUV, offering a valuable strategy in situations where specific therapeutic options are limited. Moreover, it is important to highlight that these medications are not currently used for antiviral purposes or orthoflaviviruses infections.
To mitigate the risk of IGUV infection, it is imperative to implement general prevention measures, which are acknowledged as efficacious in averting other arboviruses. Key among these strategies are vector controls and the elimination of mosquito breeding sites, such as stagnant water containers, with the application of insecticides to diminish the adult mosquito populace [102,103]. Maintaining clean environments devoid of waste accumulation is pivotal in thwarting the proliferation of vectors of transmission. The use of insect repellents and clothing that covers most of the body, such as long pants and long-sleeved shirts, can mitigate skin exposure to mosquitoes while employing screens on doors and windows and sleeping under mosquito nets can provide supplementary protection indoors. The larvicide treatment of breeding habitats and aerial and truck spraying may also effectively reduce vector populations [104,105,106], and recently, the controlled release of Aedes aegypti mosquitoes carrying Wolbachia bacterium has been successful in reducing rates of arbovirus transmission [107,108,109,110]. However, determining the feasibility of this strategy for IGUV containment hinges on elucidating the true role of Aedes mosquitoes in the transmission of this disease.
Emphasizing the significance of community outreach and awareness of IGUV is also crucial for promoting preventive practices and reducing virus transmission. Public education can play a pivotal role in disseminating accurate information regarding the risks associated with IGUV, including its modes of transmission, symptoms, and preventive measures. By increasing awareness about IGUV, communities can be empowered to adopt behaviors that mitigate the risk of infection, as outlined previously. Past experiences with other orthoflaviviruses have demonstrated the significant benefits of public education in reducing the transmission of these diseases [111,112,113,114]. Critically, outreach and education can help combat misinformation and disinformation by promoting a simple yet accurate understanding of the disease and its consequences, thereby allowing for the early identification of initial outbreaks and consequently enabling the quicker implementation of control countermeasures. An added benefit of outreach efforts is empowering communities to make informed decisions in adopting behaviors that reduce the risk of infection, ultimately reducing the disease burden on public health systems and protecting public health.
To advance our understanding of IGUV beyond the development of effective countermeasures and diagnostic tools offering robust specificity and sensitivity, sustainable and coordinated efforts are required. These included comprehensive vector and host surveillance studies to identify the primary enzootic vectors and hosts of IGUV transmission. Although Anopheles cruzii has been suggested as a potential vector [51,63], its primatophilic feeding behavior raises questions about its role in IGUV transmission since there is strong evidence to suggest that birds may be the presumptive main enzootic host. Therefore, understanding the ecology and epidemiology of IGUV can contribute to our understanding of IGUV’s transmission dynamics and host range and, importantly, its potential for spillover and emergence into peridomestic and urban settings.
5. Conclusions and Future Perspectives
IGUV remains poorly characterized, with aspects of its transmission, ecology, epidemiology, and genetic diversity still not well understood. Currently, we lack a clear understanding of the actual burden of this virus in affected or at-risk areas. Additionally, there is a lack of rapid, accurate, and sensitive diagnostic tests suitable for implementation in hospitals or for use by clinicians in low-resource settings. Since IGUV circulates in regions of Brazil that are endemic for other arboviruses and febrile illnesses, accurate diagnosis could be challenging due to the similarity of its early symptoms with other illnesses. The enhancement of diagnostic capabilities will not only facilitate the early detection and treatment of IGUV infections but also contribute to a deeper understanding of its epidemiology and its dynamics of co-infection with other pathogens.
Although no documented human IGUV infections have been reported so far, we should not underestimate its potential emergence and impact on veterinary and human health. Urgent comprehensive epidemiological surveillance will require enhanced field and laboratory studies to identify the true breadth and depth of hosts and vectors of transmission, as well as understand the pathogenesis of IGUV infections in order to develop effective prevention and/or therapeutic countermeasures. Critically, there are no prevention methods specific to IGUV; however, already developed and effective protocols for well-known arboviruses can be readily deployed if a need arises. Current treatment is palliative since there is no antiviral therapy available, although the growing database of antivirals against orthoflaviviruses may offer effective repurposing options against IGUV infections. Lastly, the disruption of IGUV spillover and its emergence into peri-urban and urban habitats will likely benefit by leveraging our extensive experience and vast amounts of empirical data acquired when investigating similar pathogens to inform predictive models of emergence that have been successfully employed in recent years [115,116].
Author Contributions
Conceptualization, S.L.R. and N.V.; data curation, M.V.S., S.L.R. and N.V.; formal analysis, M.V.S., M.L.N., S.L.R. and N.V.; funding acquisition, N.V.; investigation M.V.S., M.L.N., S.L.R. and N.V.; methodology M.V.S., M.L.N., S.L.R. and N.V.; project administration, N.V.; resources, M.V.S., M.L.N., S.L.R. and N.V.; writing—original draft, M.V.S., M.L.N., S.L.R. and N.V.; writing—review and editing M.V.S., M.L.N., S.L.R. and N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants 2013/21719-3 and 2022/03645-1 from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), and by the Centers for Research in Emerging Infectious Diseases (CREID), “The Coordinating Research on Emerging Arboviral Threats Encompassing the Neotropics (CREATE-NEO)” grant U01AI151807, which was awarded to N.V. by the National Institutes of Health. S.L.R. is partially supported by U19AI142762. M.L.N. is partially supported by INCT Viral Genomic Surveillance and One Health by grant 4057586/2022-0. M.L.N. is a CNPq research fellow. M.V.S. was supported by a FAPESP PhD Scholarship, numbers 2020/12875-5 and 2023/09590-7.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Possas, C.; Lourenço-de-Oliveira, R.; Tauil, P.L.; de Pinheiro, F.; Pissinatti, A.; da Cunha, R.V.; Freire, M.; Martins, R.M.; Homma, A. Yellow Fever Outbreak in Brazil: The Puzzle of Rapid Viral Spread and Challenges for Immunisation. Mem. Inst. Oswaldo Cruz 2018, 113, e180278. [Google Scholar] [CrossRef] [PubMed]
- Khongwichit, S.; Chuchaona, W.; Vongpunsawad, S.; Poovorawan, Y. Molecular Surveillance of Arboviruses Circulation and Co-Infection during a Large Chikungunya Virus Outbreak in Thailand, October 2018 to February 2020. Sci. Rep. 2022, 12, 22323. [Google Scholar] [CrossRef]
- Diagne, M.M.; Ndione, M.H.D.; Gaye, A.; Barry, M.A.; Diallo, D.; Diallo, A.; Mwakibete, L.L.; Diop, M.; Ndiaye, E.H.; Ahyong, V.; et al. Yellow Fever Outbreak in Eastern Senegal, 2020–2021. Viruses 2021, 13, 1475. [Google Scholar] [CrossRef] [PubMed]
- de Rezende, I.M.; Sacchetto, L.; de Mello, É.M.; Alves, P.A.; de Melo Iani, F.C.; Adelino, T.É.R.; Duarte, M.M.; Cury, A.L.F.; Bernardes, A.F.L.; Santos, T.A.; et al. Persistence of Yellow Fever Virus Outside the Amazon Basin, Causing Epidemics in Southeast Brazil, from 2016 to 2018. PLoS Negl. Trop. Dis. 2018, 12, e0006538. [Google Scholar] [CrossRef] [PubMed]
- Hill, V.; Koch, R.T.; Bialosuknia, S.M.; Ngo, K.; Zink, S.D.; Koetzner, C.A.; Maffei, J.G.; Dupuis, A.P.; Backenson, P.B.; Oliver, J.; et al. Dynamics of Eastern Equine Encephalitis Virus during the 2019 Outbreak in the Northeast United States. Curr. Biol. 2023, 33, 2515–2527.e6. [Google Scholar] [CrossRef] [PubMed]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika Virus Outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef]
- Lednicky, J.; Beau De Rochars, V.M.; El Badry, M.; Loeb, J.; Telisma, T.; Chavannes, S.; Anilis, G.; Cella, E.; Ciccozzi, M.; Rashid, M.; et al. Zika Virus Outbreak in Haiti in 2014: Molecular and Clinical Data. PLoS Negl. Trop. Dis. 2016, 10, e0004687. [Google Scholar] [CrossRef] [PubMed]
- Khongwichit, S.; Chansaenroj, J.; Thongmee, T.; Benjamanukul, S.; Wanlapakorn, N.; Chirathaworn, C.; Poovorawan, Y. Large-Scale Outbreak of Chikungunya Virus Infection in Thailand, 2018–2019. PLoS ONE 2021, 16, e0247314. [Google Scholar] [CrossRef] [PubMed]
- Vairo, F.; Aimè Coussoud-Mavoungou, M.P.; Ntoumi, F.; Castilletti, C.; Kitembo, L.; Haider, N.; Carletti, F.; Colavita, F.; Gruber, C.E.M.; Iannetta, M.; et al. Chikungunya Outbreak in the Republic of the Congo, 2019—Epidemiological, Virological and Entomological Findings of a South-North Multidisciplinary Taskforce Investigation. Viruses 2020, 12, 1020. [Google Scholar] [CrossRef]
- Anwar, S.; Mourosi, J.T.; Khan, M.F.; Ullah, M.O.; Vanakker, O.M.; Hosen, M.J. Chikungunya Outbreak in Bangladesh (2017): Clinical and Hematological Findings. PLoS Negl. Trop. Dis. 2020, 14, e0007466. [Google Scholar] [CrossRef]
- Gaillet, M.; Pichard, C.; Restrepo, J.; Lavergne, A.; Perez, L.; Enfissi, A.; Abboud, P.; Lambert, Y.; Ma, L.; Monot, M.; et al. Outbreak of Oropouche Virus in French Guiana. Emerg. Infect. Dis. 2021, 27, 2711–2714. [Google Scholar] [CrossRef]
- Moreira, H.M.; Sgorlon, G.; Queiroz, J.A.S.; Roca, T.P.; Ribeiro, J.; Teixeira, K.S.; Passos-Silva, A.M.; Araújo, A.; Gasparelo, N.W.F.; Dos Santos, A.d.O.; et al. Outbreak of Oropouche Virus in Frontier Regions in Western Amazon. Microbiol. Spectr. 2024, 12, e01629-23. [Google Scholar] [CrossRef] [PubMed]
- Javelle, E.; de Laval, F.; Durand, G.A.; Dia, A.; Ficko, C.; Bousquet, A.; Delaune, D.; Briolant, S.; Mérens, A.; Brossier, C.; et al. Chikungunya Outbreak in Country with Multiple Vectorborne Diseases, Djibouti, 2019–2020. Emerg. Infect. Dis. 2023, 29, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Ospina, J.A.; Villamil-Gómez, W.E.; Jimenez-Canizales, C.E.; Castañeda-Hernández, D.M.; Rodríguez-Morales, A.J. Estimating the Burden of Disease and the Economic Cost Attributable to Chikungunya, Colombia, 2014. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 793–802. [Google Scholar] [CrossRef] [PubMed]
- LaBeaud, A.; Bashir, F.; King, C.H. Measuring the Burden of Arboviral Diseases: The Spectrum of Morbidity and Mortality from Four Prevalent Infections. Popul. Health Metr. 2011, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Stanaway, J.D. The Global Economic Burden of Dengue: A Systematic Analysis. Lancet Infect. Dis. 2016, 16, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Nelson, C.B.; Picot, V.; Gubler, D.J. Arboviruses: A Global Public Health Threat. Vaccine 2020, 38, 3989–3994. [Google Scholar] [CrossRef] [PubMed]
- Kading, R.C.; Brault, A.C.; Beckham, J.D. Global Perspectives on Arbovirus Outbreaks: A 2020 Snapshot. Trop. Med. Infect. Dis. 2020, 5, 142. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Hay, S.I. The Global Expansion of Dengue: How Aedes Aegypti Mosquitoes Enabled the First Pandemic Arbovirus. Annu. Rev. Entomol. 2020, 65, 191–208. [Google Scholar] [CrossRef]
- Weaver, S.C. Prediction and Prevention of Urban Arbovirus Epidemics: A Challenge for the Global Virology Community. Antiviral Res. 2018, 156, 80–84. [Google Scholar] [CrossRef]
- Genus: Orthoflavivirus|ICTV. Available online: https://ictv.global/report/chapter/flaviviridae/flaviviridae/orthoflavivirus (accessed on 19 March 2024).
- Coimbra, T.L.M.; Nassar, E.S.; Nagamori, A.H.; Ferreira, I.E.; Pereira, L.E.; Rocco, I.M.; Ueda-Ito, M.; Romano, N.S. Iguape: A Newly Recognized Flavivirus from São Paulo State, Brazil. Intervirology 2008, 36, 144–152. [Google Scholar] [CrossRef]
- Silva, N.I.O.; Sacchetto, L.; de Rezende, I.M.; de Trindade, G.S.; LaBeaud, A.D.; de Thoisy, B.; Drumond, B.P. Recent Sylvatic Yellow Fever Virus Transmission in Brazil: The News from an Old Disease. Virol. J. 2020, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, L.T.M. Emergent Arboviruses in Brazil. Rev. Soc. Bras. Med. Trop. 2007, 40, 224–229. [Google Scholar] [CrossRef]
- Saivish, M.V.; Gomes da Costa, V.; de Lima Menezes, G.; Alves da Silva, R.; Dutra da Silva, G.C.; Moreli, M.L.; Sacchetto, L.; Pacca, C.C.; Vasilakis, N.; Nogueira, M.L. Rocio Virus: An Updated View on an Elusive Flavivirus. Viruses 2021, 13, 2293. [Google Scholar] [CrossRef]
- Lopes, O.D.S.; Sacchetta, L.D.A.; Coimbra, T.L.M.; Pinto, G.H.; Glasser, C.M. Emergence of a New Arbovirus Disease in Brazil: II. Epidemiologic Studies on 1975 Epidemic. Am. J. Epidemiol. 1978, 108, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Saivish, M.V.; da Costa, V.G.; Rodrigues, R.L.; Féres, V.C.R.; Montoya-Diaz, E.; Moreli, M.L. Detection of Rocio Virus SPH 34675 during Dengue Epidemics, Brazil, 2011–2013. Emerg. Infect. Dis. 2020, 26, 797–799. [Google Scholar] [CrossRef]
- Azevedo, R.S.S.; Silva, E.V.P.; Carvalho, V.L.; Rodrigues, S.G.; Neto, J.P.N.; Monteiro, H.A.O.; Peixoto, V.S.; Chiang, J.O.; Nunes, M.R.T.; Vasconcelos, P.F.C. Mayaro Fever Virus, Brazilian Amazon. Emerg. Infect. Dis. 2009, 15, 1830–1832. [Google Scholar] [CrossRef]
- Saivish, M.V.; Nogueira, M.L.; Rossi, S.L.; Vasilakis, N. Beyond Borders: Investigating the Mysteries of Cacipacoré, a Lesser-Studied Arbovirus in Brazil. Viruses 2024, 16, 336. [Google Scholar] [CrossRef] [PubMed]
- Batista, W.C.; Tavares, G.D.S.B.; Vieira, D.S.; Honda, E.R.; Pereira, S.S.; Tada, M.S. Notificação do primeiro isolamento do vírus Cacipacoré em um ser humano, no Estado de Rondônia, Brasil. Rev. Soc. Bras. Med. Trop. 2011, 44, 528–530. [Google Scholar] [CrossRef]
- Laemmert, H.W., Jr.; Hughes, T.P. The Virus of Ilhéus Encephalitis*: Isolation, Serological Specificity and Transmission. J. Immunol. 1947, 55, 61–67. [Google Scholar] [CrossRef]
- Milhim, B.H.G.A.; Estofolete, C.F.; da Rocha, L.C.; Liso, E.; Brienze, V.M.S.; Vasilakis, N.; Terzian, A.C.B.; Nogueira, M.L. Fatal Outcome of Ilheus Virus in the Cerebrospinal Fluid of a Patient Diagnosed with Encephalitis. Viruses 2020, 12, 957. [Google Scholar] [CrossRef] [PubMed]
- Gomes, G.; Causey, O.R. Bussuquara, A New Arthropod-Borne Virus. Proc. Soc. Exp. Biol. Med. 1959, 101, 275–279. [Google Scholar] [CrossRef]
- Vieira, M.A.C.S.; Romano, A.P.M.; Borba, A.S.; Silva, E.V.P.; Chiang, J.O.; Eulálio, K.D.; Azevedo, R.S.S.; Rodrigues, S.G.; Almeida-Neto, W.S.; Vasconcelos, P.F.C. West Nile Virus Encephalitis: The First Human Case Recorded in Brazil. Am. J. Trop. Med. Hyg. 2015, 93, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Mondini, A.; Cardeal, I.L.S.; Lázaro, E.; Nunes, S.H.; Moreira, C.C.; Rahal, P.; Maia, I.L.; Franco, C.; Góngora, D.V.N.; Góngora-Rubio, F.; et al. Saint Louis Encephalitis Virus, Brazil. Emerg. Infect. Dis. 2007, 13, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Charlier, C.; Vasilakis, N.; Lecuit, M. Zika, Chikungunya, and Other Emerging Vector-Borne Viral Diseases. Annu. Rev. Med. 2018, 69, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Parida, M.; Dash, P.K. Impact of Transmission Cycles and Vector Competence on Global Expansion and Emergence of Arboviruses. Rev. Med. Virol. 2017, 27, e1941. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and Future Spread of the Arbovirus Vectors Aedes Aegypti and Aedes Albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef] [PubMed]
- de Melo Ximenes, M.F.F.; de Araujo Galvao, J.M.; Inacio, C.L.S.; Macedo e Silva, V.P.; Pereira, R.L.D.N.; Pinheiro, M.P.G.; de Medeiros Silva, M.M.; Gomes, C.E.S. Arbovirus Expansion: New Species of Culicids Infected by the Chikungunya Virus in an Urban Park of Brazil. Acta Trop. 2020, 209, 105538. [Google Scholar] [CrossRef]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global Expansion and Redistribution of Aedes-Borne Virus Transmission Risk with Climate Change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef]
- Calisher, C.H.; Karabatsos, N.; Dalrymple, J.M.; Shope, R.E.; Porterfield, J.S.; Westaway, E.G.; Brandt, W.E. Antigenic Relationships between Flaviviruses as Determined by Cross-Neutralization Tests with Polyclonal Antisera. J. Gen. Virol. 1989, 70, 37–43. [Google Scholar] [CrossRef]
- Ferreira, I.B.; Pereira, L.E.; Rocco, I.M.; Marti, A.T.; de Souza, L.T.M.; Iversson, L.B. Surveillance of Arbovirus Infections in the Atlantic Forest Region, State of São Paulo, Brazil: I. Detection of Hemagglutination-Inhibition Antibodies in Wild Birds between 1978 and 1990. Rev. Inst. Med. Trop. São Paulo 1994, 36, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L. Swainson’s Flycatcher (Myiarchus swainsoni). In Birds of the World; Version 1.0; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020; Available online: https://birdsoftheworld.org/bow/species/swafly1/1.0/introduction (accessed on 19 March 2024).
- Cimprich, D.A.; Moore, F.R.; Guilfoyle, M.P. Red-Eyed Vireo (Vireo olivaceus). In Birds of the World; Version 1.0.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020; Available online: https://birdsoftheworld.org/bow/species/reevir1/cur/introduction (accessed on 19 March 2024).
- Kramer, L.D.; Bernard, K.A. West Nile Virus Infection in Birds and Mammals. Ann. N. Y. Acad. Sci. 2001, 951, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Bernard, K.A.; Maffei, J.G.; Jones, S.A.; Kauffman, E.B.; Ebel, G.; Dupuis, A.P.; Ngo, K.A.; Nicholas, D.C.; Young, D.M.; Shi, P.Y.; et al. West Nile Virus Infection in Birds and Mosquitoes, New York State, 2000. Emerg. Infect. Dis. 2001, 7, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.E.; Suzuki, A.; Coimbra, T.L.M.; Souza, R.P.D.; Chamelet, E.L.B. Arbovírus Ilheus em aves silvestres (Sporophila caerulescens e Molothrus bonariensis). Rev. Saúde Pública 2001, 35, 119–123. [Google Scholar] [CrossRef]
- Gruwell, J.A.; Fogarty, C.L.; Bennett, S.G.; Challet, G.L.; Vanderpool, K.S.; Jozan, M.; Webb, J.P., Jr. Role of peridomestic birds in the transmission of St. Louis encephalitis virus in southern California. J. Wildl. Dis. 2000, 36, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.D.; Meece, J.K.; Henkel, J.S.; Shukla, S.K. Birds, Migration and Emerging Zoonoses: West Nile Virus, Lyme Disease, Influenza A and Enteropathogens. Clin. Med. Res. 2003, 1, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Barrett, A.D.T. Transmission Cycles, Host Range, Evolution and Emergence of Arboviral Disease. Nat. Rev. Microbiol. 2004, 2, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.S.; Luchs, A.; da Costa, A.C.; de Ribeiro, G.O.; dos Santos, F.C.P.; Nogueira, J.S.; Komninakis, S.V.; dos Marinho, R.S.S.; Witkin, S.S.; Villanova, F.; et al. Detection and Characterization of Ilheus and Iguape Virus Genomes in Historical Mosquito Samples from Southern Brazil. Acta Trop. 2020, 205, 105401. [Google Scholar] [CrossRef]
- Cunha, E.M.S.; Villalobos, E.M.C.; Nassar, A.F.C.; Lara, M.C.C.S.H.; Peres, N.F.; Palazzo, J.P.C.; Silva, A.; Stefano, E.D.; Pino, F.A. Prevalência de Anticorpos Contra Agentes Virais em Equídeos No Sul do Estado de São Paulo. Arq. Inst. Biológico 2021, 76, 165–171. [Google Scholar] [CrossRef]
- Araújo, F.A.A. Inquéritos Sorológicos em Equídeos e Aves Silvestres Para Detecção de Anticorpos Anti-Arbovírus de Importância em Saúde Pública No Brasil. Ph.D. Thesis, Universidade Federal de Goiás, Goiânia, Goiás State, Brazil, 2011. [Google Scholar]
- Pauvolid-Corrêa, A.; Campos, Z.; Juliano, R.; Velez, J.; Nogueira, R.M.R.; Komar, N. Serological Evidence of Widespread Circulation of West Nile Virus and Other Flaviviruses in Equines of the Pantanal, Brazil. PLoS Negl. Trop. Dis. 2014, 8, e2706. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Rezende, H.R.; Fux, B.; De Alencar, F.E.C.; Loss, A.C.; Buery, J.C.; De Castro Duarte, A.M.R.; Junior, C.C. Anopheles (Kerteszia) Cruzii Infected by Plasmodium in the Atlantic Forest Indicates That the Malaria Transmission Cycle Is Maintained Even after Howler Monkeys’ Population Decline. Parasitol. Res. 2022, 121, 3627–3634. [Google Scholar] [CrossRef] [PubMed]
- Laporta, G.Z.; Ramos, D.G.; Ribeiro, M.C.; Sallum, M.A.M. Habitat Suitability of Anopheles Vector Species and Association with Human Malaria in the Atlantic Forest in South-Eastern Brazil. Mem. Inst. Oswaldo Cruz 2011, 106, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.; Krüger, R.F.; Cunha, S.K.; Silveira, A.S.; Alves, D.M.C.C.; Rodrigues, G.D.; Peterson, A.T.; Jiménez-García, D. Climate Change Impacts on Anopheles (K.) Cruzii in Urban Areas of Atlantic Forest of Brazil: Challenges for Malaria Diseases. Acta Trop. 2021, 224, 106123. [Google Scholar] [CrossRef] [PubMed]
- Corbet, P.S.; Williams, M.C.; Gillett, J.D. O’nyong-Nyong Fever: An Epidemic Virus Disease in East Africa. Trans. R. Soc. Trop. Med. Hyg. 1961, 55, 463–480. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, M.L.G.; Amarilla, A.A.; de Figueiredo, G.G.; Alfonso, H.L.; Lippi, V.; Maia, F.G.M.; Morais, F.A.; da Costa, C.A.; Henriques, D.A.; Durigon, E.L.; et al. Cacipacore Virus as an Emergent Mosquito-Borne Flavivirus. Rev. Soc. Bras. Med. Trop. 2017, 50, 539–542. [Google Scholar] [CrossRef] [PubMed]
- de Castro, M.C.; Monte-Mór, R.L.; Sawyer, D.O.; Singer, B.H. Malaria Risk on the Amazon Frontier. Proc. Natl. Acad. Sci. USA 2006, 103, 2452–2457. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.H.; Gangnon, R.; Silveira, G.A.; Patz, J.A. Deforestation and Malaria in Mâncio Lima County, Brazil. Emerg. Infect. Dis. 2010, 16, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Lönker, N.S.; Fechner, K.; Abd El Wahed, A. Horses as a Crucial Part of One Health. Vet. Sci. 2020, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Bocato-Chamelet, E.L.; Coimbra, T.L.M.; da Nassar, E.S.; Pereira, L.E.; Ferreira, I.B.; de Souza, L.T.M.; Suzuki, A. Isolamento do flavivírus Iguape a partir de mosquitos Anopheles (Kerteszia) cruzii em Juquitiba—Estado de São Paulo—Brasil. Rev. Inst. Adolfo Lutz 2001, 60, 65–69. [Google Scholar] [CrossRef]
- Cunha, M.S.; Luchs, A.; dos Santos, F.C.P.; Caleiro, G.S.; Nogueira, M.L.; Maiorka, P.C. Applying a Pan-Flavivirus RT-qPCR Assay in Brazilian Public Health Surveillance. Arch. Virol. 2020, 165, 1863–1868. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Du, S.; Shan, C.; Nie, K.; Zhang, R.; Li, X.-F.; Zhang, R.; Wang, T.; Qin, C.-F.; et al. Evolutionary Enhancement of Zika Virus Infectivity in Aedes Aegypti Mosquitoes. Nature 2017, 545, 482–486. [Google Scholar] [CrossRef]
- Yuan, L.; Huang, X.-Y.; Liu, Z.-Y.; Zhang, F.; Zhu, X.-L.; Yu, J.-Y.; Ji, X.; Xu, Y.-P.; Li, G.; Li, C.; et al. A Single Mutation in the prM Protein of Zika Virus Contributes to Fetal Microcephaly. Science 2017, 358, 933–936. [Google Scholar] [CrossRef]
- Tsetsarkin, K.A.; Vanlandingham, D.L.; McGee, C.E.; Higgs, S. A Single Mutation in Chikungunya Virus Affects Vector Specificity and Epidemic Potential. PLOS Pathog. 2007, 3, e201. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A Familial Cluster of Pneumonia Associated with the 2019 Novel Coronavirus Indicating Person-to-Person Transmission: A Study of a Family Cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Research Response to Pathogen X during a Pandemic. Available online: https://www.who.int/news-room/events/detail/2024/01/19/default-calendar/Research-response-to-pathogen-X-during-a-pandemic (accessed on 19 March 2024).
- Preparing for the Next “Disease X”|CEPI. Available online: https://cepi.net (accessed on 19 March 2024).
- Nash, D.; Mostashari, F.; Fine, A.; Miller, J.; O’Leary, D.; Murray, K.; Huang, A.; Rosenberg, A.; Greenberg, A.; Sherman, M.; et al. The Outbreak of West Nile Virus Infection in the New York City Area in 1999. N. Engl. J. Med. 2001, 344, 1807–1814. [Google Scholar] [CrossRef]
- Mostashari, F.; Bunning, M.L.; Kitsutani, P.T.; Singer, D.A.; Nash, D.; Cooper, M.J.; Katz, N.; Liljebjelke, K.A.; Biggerstaff, B.J.; Fine, A.D.; et al. Epidemic West Nile Encephalitis, New York, 1999: Results of a Household-Based Seroepidemiological Survey. Lancet 2001, 358, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Bessaud, M.; Peyrefitte, C.N.; Pastorino, B.A.M.; Tock, F.; Merle, O.; Colpart, J.-J.; Dehecq, J.-S.; Girod, R.; Jaffar-Bandjee, M.-C.; Glass, P.J.; et al. Chikungunya Virus Strains, Reunion Island Outbreak. Emerg. Infect. Dis. 2006, 12, 1604–1605. [Google Scholar] [CrossRef] [PubMed]
- Bortel, W.V.; Dorleans, F.; Rosine, J.; Blateau, A.; Rousset, D.; Matheus, S.; Leparc-Goffart, I.; Flusin, O.; Prat, C.M.; Césaire, R.; et al. Chikungunya Outbreak in the Caribbean Region, December 2013 to March 2014, and the Significance for Europe. Eurosurveillance 2014, 19, 20759. [Google Scholar] [CrossRef]
- Notes from the Field: Chikungunya Virus Spreads in the Americas—Caribbean and South America, 2013–2014. Available online: https://www.cdc.gov/mmWr/preview/mmwrhtml/mm6322a5.htm (accessed on 1 May 2024).
- Nunes, M.R.T.; Faria, N.R.; de Vasconcelos, J.M.; Golding, N.; Kraemer, M.U.; de Oliveira, L.F.; da Silva, A.; da Silva, D.E.A.; da Silva, E.V.P.; da Silva, S.P.; et al. Emergence and Potential for Spread of Chikungunya Virus in Brazil. BMC Med. 2015, 13, 102. [Google Scholar] [CrossRef]
- Faria, N.R.; Lourenço, J.; Marques de Cerqueira, E.; Maia de Lima, M.; Pybus, O.; Alcantara, L.C. Epidemiology of Chikungunya Virus in Bahia, Brazil, 2014–2015. PLoS Curr. 2016, 8, ecurrents.outbreaks.c97507e3e48efb946401755d468c28b2. [Google Scholar] [CrossRef] [PubMed]
- Heang, V.; Yasuda, C.Y.; Sovann, L.; Haddow, A.D.; Travassos da Rosa, A.P.; Tesh, R.B.; Kasper, M.R. Zika Virus Infection, Cambodia, 2010. Emerg. Infect. Dis. 2012, 18, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Alera, M.T.; Hermann, L.; Tac-An, I.A.; Klungthong, C.; Rutvisuttinunt, W.; Manasatienkij, W.; Villa, D.; Thaisomboonsuk, B.; Velasco, J.M.; Chinnawirotpisan, P.; et al. Zika Virus Infection, Philippines, 2012. Emerg. Infect. Dis. 2015, 21, 722–724. [Google Scholar] [CrossRef] [PubMed]
- Wæhre, T.; Maagard, A.; Tappe, D.; Cadar, D.; Schmidt-Chanasit, J. Zika Virus Infection after Travel to Tahiti, December 2013. Emerg. Infect. Dis. 2014, 20, 1412–1414. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.-M.; Roche, C.; Teissier, A.; Robin, E.; Berry, A.-L.; Mallet, H.-P.; Sall, A.A.; Musso, D. Zika Virus, French Polynesia, South Pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Bossin, H.; Mallet, H.P.; Besnard, M.; Broult, J.; Baudouin, L.; Levi, J.E.; Sabino, E.C.; Ghawche, F.; Lanteri, M.C.; et al. Zika Virus in French Polynesia 2013–14: Anatomy of a Completed Outbreak. Lancet Infect. Dis. 2018, 18, e172–e182. [Google Scholar] [CrossRef] [PubMed]
- Zanluca, C.; de Melo, V.C.A.; Mosimann, A.L.P.; dos Santos, G.I.V.; dos Santos, C.N.D.; Luz, K. First Report of Autochthonous Transmission of Zika Virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Styczynski, A.R.; Malta, J.M.A.S.; Krow-Lucal, E.R.; Percio, J.; Nóbrega, M.E.; Vargas, A.; Lanzieri, T.M.; Leite, P.L.; Staples, J.E.; Fischer, M.X.; et al. Increased Rates of Guillain-Barré Syndrome Associated with Zika Virus Outbreak in the Salvador Metropolitan Area, Brazil. PLoS Negl. Trop. Dis. 2017, 11, e0005869. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Barrere, B.; Malinowski, C.; Saville, M.; Teyssou, R.; Lang, J. From Research to Phase III: Preclinical, Industrial and Clinical Development of the Sanofi Pasteur Tetravalent Dengue Vaccine. Vaccine 2011, 29, 7229–7241. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Briand, O.; Lang, J.; Saville, M.; Jackson, N. Development of the Sanofi Pasteur Tetravalent Dengue Vaccine: One More Step Forward. Vaccine 2015, 33, 7100–7111. [Google Scholar] [CrossRef]
- Tricou, V.; Yu, D.; Reynales, H.; Biswal, S.; Saez-Llorens, X.; Sirivichayakul, C.; Lopez, P.; Borja-Tabora, C.; Bravo, L.; Kosalaraksa, P.; et al. Long-Term Efficacy and Safety of a Tetravalent Dengue Vaccine (TAK-003): 4·5-Year Results from a Phase 3, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Glob. Health 2024, 12, e257–e270. [Google Scholar] [CrossRef] [PubMed]
- Freestone, D.S.; Ferris, R.D.; Weinberg, A.L.; Kelly, A. Stabilized 17D Strain Yellow Fever Vaccine: Dose Response Studies, Clinical Reactions and Effects on Hepatic Function. J. Biol. Stand. 1977, 5, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.M.; de Maia, M.L.S.; Farias, R.H.G.; Camacho, L.A.B.; Freire, M.S.; Galler, R.; Yamamura, A.M.Y.; Almeida, L.F.C.; Lima, S.M.B.; Nogueira, R.M.R.; et al. 17DD Yellow Fever Vaccine. Hum. Vaccines Immunother. 2013, 9, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, T. Ixiaro®: A New Vaccine against Japanese Encephalitis. Expert Rev. Vaccines 2009, 8, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Nahle, Z. A Proof-of-Concept Study Poised to Remodel the Drug Development Process. Front. Med. Technol. 2022, 4, 1053588. [Google Scholar] [CrossRef] [PubMed]
- Pammolli, F.; Magazzini, L.; Riccaboni, M. The Productivity Crisis in Pharmaceutical R&D. Nat. Rev. Drug Discov. 2011, 10, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Brecher, M.; Deng, Y.-Q.; Zhang, J.; Sakamuru, S.; Liu, B.; Huang, R.; Koetzner, C.A.; Allen, C.A.; Jones, S.A.; et al. Existing Drugs as Broad-Spectrum and Potent Inhibitors for Zika Virus by Targeting NS2B-NS3 Interaction. Cell Res. 2017, 27, 1046–1064. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.-C.; HuangFu, W.-C.; Tsai, T.-T.; Ho, M.-R.; Jhan, M.-K.; Shen, T.-J.; Tseng, P.-C.; Wang, Y.-T.; Lin, C.-F. The Antiparasitic Drug Niclosamide Inhibits Dengue Virus Infection by Interfering with Endosomal Acidification Independent of mTOR. PLoS Negl. Trop. Dis. 2018, 12, e0006715. [Google Scholar] [CrossRef] [PubMed]
- Cairns, D.M.; Boorgu, D.S.S.K.; Levin, M.; Kaplan, D.L. Niclosamide Rescues Microcephaly in a Humanized In Vivo Model of Zika Infection Using Human Induced Neural Stem Cells. Biol. Open 2018, 7, bio031807. [Google Scholar] [CrossRef]
- Kamiyama, N.; Soma, R.; Hidano, S.; Watanabe, K.; Umekita, H.; Fukuda, C.; Noguchi, K.; Gendo, Y.; Ozaki, T.; Sonoda, A.; et al. Ribavirin Inhibits Zika Virus (ZIKV) Replication in Vitro and Suppresses Viremia in ZIKV-Infected STAT1-Deficient Mice. Antiviral Res. 2017, 146, 1–11. [Google Scholar] [CrossRef]
- Kim, J.-A.; Seong, R.-K.; Kumar, M.; Shin, O.S. Favipiravir and Ribavirin Inhibit Replication of Asian and African Strains of Zika Virus in Different Cell Models. Viruses 2018, 10, 72. [Google Scholar] [CrossRef]
- Chang, J.; Schul, W.; Butters, T.D.; Yip, A.; Liu, B.; Goh, A.; Lakshminarayana, S.B.; Alonzi, D.; Reinkensmeier, G.; Pan, X.; et al. Combination of α-Glucosidase Inhibitor and Ribavirin for the Treatment of Dengue Virus Infection In Vitro and In Vivo. Antivir. Res. 2011, 89, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, N.; Chander, Y.; Rawat, K.D.; Riyesh, T.; Nishanth, C.; Sharma, S.; Jindal, N.; Tripathi, B.N.; Barua, S.; Kumar, N. Emetine Inhibits Replication of RNA and DNA Viruses without Generating Drug-Resistant Virus Variants. Antivir. Res. 2017, 144, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xu, M.; Lee, E.M.; Gorshkov, K.; Shiryaev, S.A.; He, S.; Sun, W.; Cheng, Y.-S.; Hu, X.; Tharappel, A.M.; et al. Emetine Inhibits Zika and Ebola Virus Infections through Two Molecular Mechanisms: Inhibiting Viral Replication and Decreasing Viral Entry. Cell Discov. 2018, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Success in Mosquito Control: An Integrated Approach. Available online: https://www.epa.gov/mosquitocontrol/success-mosquito-control-integrated-approach (accessed on 23 April 2024).
- CDC. What Mosquito Control Programs Do|CDC. Available online: https://www.cdc.gov/mosquitoes/mosquito-control/community/what-mosquito-control-programs-do.html (accessed on 23 April 2024).
- U.S. Environmental Protection Agency. Controlling Mosquitoes at the Larval Stage. Available online: https://www.epa.gov/mosquitocontrol/controlling-mosquitoes-larval-stage (accessed on 23 April 2024).
- CDC. Truck Spraying|CDC. Available online: https://www.cdc.gov/mosquitoes/mosquito-control/community/truck-spraying.html (accessed on 23 April 2024).
- CDC. Aerial Spraying|CDC. Available online: https://www.cdc.gov/mosquitoes/mosquito-control/community/aerial-spraying.html (accessed on 23 April 2024).
- Pereira, T.N.; Rocha, M.N.; Sucupira, P.H.F.; Carvalho, F.D.; Moreira, L.A. Wolbachia Significantly Impacts the Vector Competence of Aedes Aegypti for Mayaro Virus. Sci. Rep. 2018, 8, 6889. [Google Scholar] [CrossRef]
- Aliota, M.T.; Peinado, S.A.; Velez, I.D.; Osorio, J.E. The wMel Strain of Wolbachia Reduces Transmission of Zika Virus by Aedes Aegypti. Sci. Rep. 2016, 6, 28792. [Google Scholar] [CrossRef] [PubMed]
- Anders, K.L.; Indriani, C.; Ahmad, R.A.; Tantowijoyo, W.; Arguni, E.; Andari, B.; Jewell, N.P.; Rances, E.; O’Neill, S.L.; Simmons, C.P.; et al. The AWED Trial (Applying Wolbachia to Eliminate Dengue) to Assess the Efficacy of Wolbachia-Infected Mosquito Deployments to Reduce Dengue Incidence in Yogyakarta, Indonesia: Study Protocol for a Cluster Randomised Controlled Trial. Trials 2018, 19, 302. [Google Scholar] [CrossRef]
- Pinto, S.B.; Riback, T.I.S.; Sylvestre, G.; Costa, G.; Peixoto, J.; Dias, F.B.S.; Tanamas, S.K.; Simmons, C.P.; Dufault, S.M.; Ryan, P.A.; et al. Effectiveness of Wolbachia-Infected Mosquito Deployments in Reducing the Incidence of Dengue and Other Aedes-Borne Diseases in Niterói, Brazil: A Quasi-Experimental Study. PLoS Negl. Trop. Dis. 2021, 15, e0009556. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Smania-Marques, R.; Albino, V.A.; Fernandes, I.D.; Mangueira, F.F.A.; Altafim, R.A.P.; Olinda, R.; Smith, M.; Traxler, J. Prevention and Control of Mosquito-Borne Arboviral Diseases: Lessons Learned from a School-Based Intervention in Brazil (Zikamob). BMC Public Health 2022, 22, 255. [Google Scholar] [CrossRef]
- Paixão, M.M.; Ballouz, T.; Lindahl, J.F. Effect of Education on Improving Knowledge and Behavior for Arboviral Diseases: A Systematic Review and Meta-Analysis. Am. J. Trop. Med. Hyg. 2019, 101, 441–447. [Google Scholar] [CrossRef]
- Nyangau, P.N.; Nzuma, J.M.; Irungu, P.; Junglen, S.; Kassie, M. Health Education Impact on Knowledge and Management of Arboviral Diseases in Kenya: Evidence from Randomised Control Trials. Glob. Public Health 2023, 18, 2274436. [Google Scholar] [CrossRef] [PubMed]
- Abel Mangueira, F.F.; Smania-Marques, R.; Dutra Fernandes, I.; Alves Albino, V.; Olinda, R.; Acácia Santos-Silva, T.; Traxler, J.; Matheson, D.; Santos, S. The Prevention of Arboviral Diseases Using Mobile Devices: A Preliminary Study of the Attitudes and Behaviour Change Produced by Educational Interventions. Trop. Med. Int. Health 2019, 24, 1411–1426. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.V.; Dallas, T.A.; Han, B.A.; Murdock, C.C.; Drake, J.M. Data-Driven Identification of Potential Zika Virus Vectors. eLife 2017, 6, e22053. [Google Scholar] [CrossRef] [PubMed]
- Han, B.A.; O’Regan, S.M.; Paul Schmidt, J.; Drake, J.M. Integrating Data Mining and Transmission Theory in the Ecology of Infectious Diseases. Ecol. Lett. 2020, 23, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).