The Role of Satellites in the Evolution of Begomoviruses
Abstract
:1. Introduction
2. Satellites Associated with Begomoviruses
2.1. Alphasatellites
2.2. Betasatellites
2.3. Deltasatellites
3. Role of Betasatellites in Transmission of Begomoviruses by Bemisia tabaci
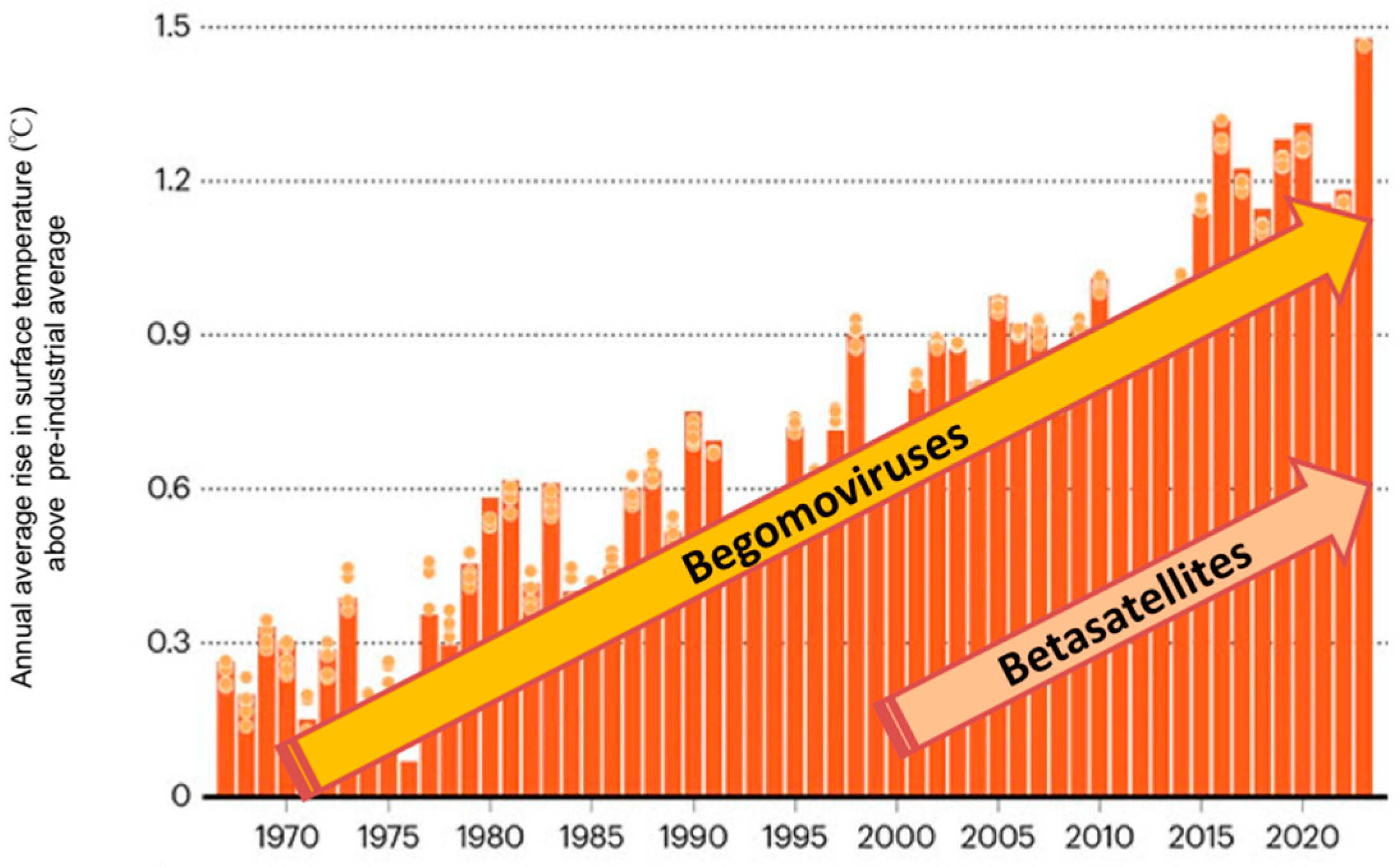
4. Origin and Evolution of Satellites Associated with Begomoviruses
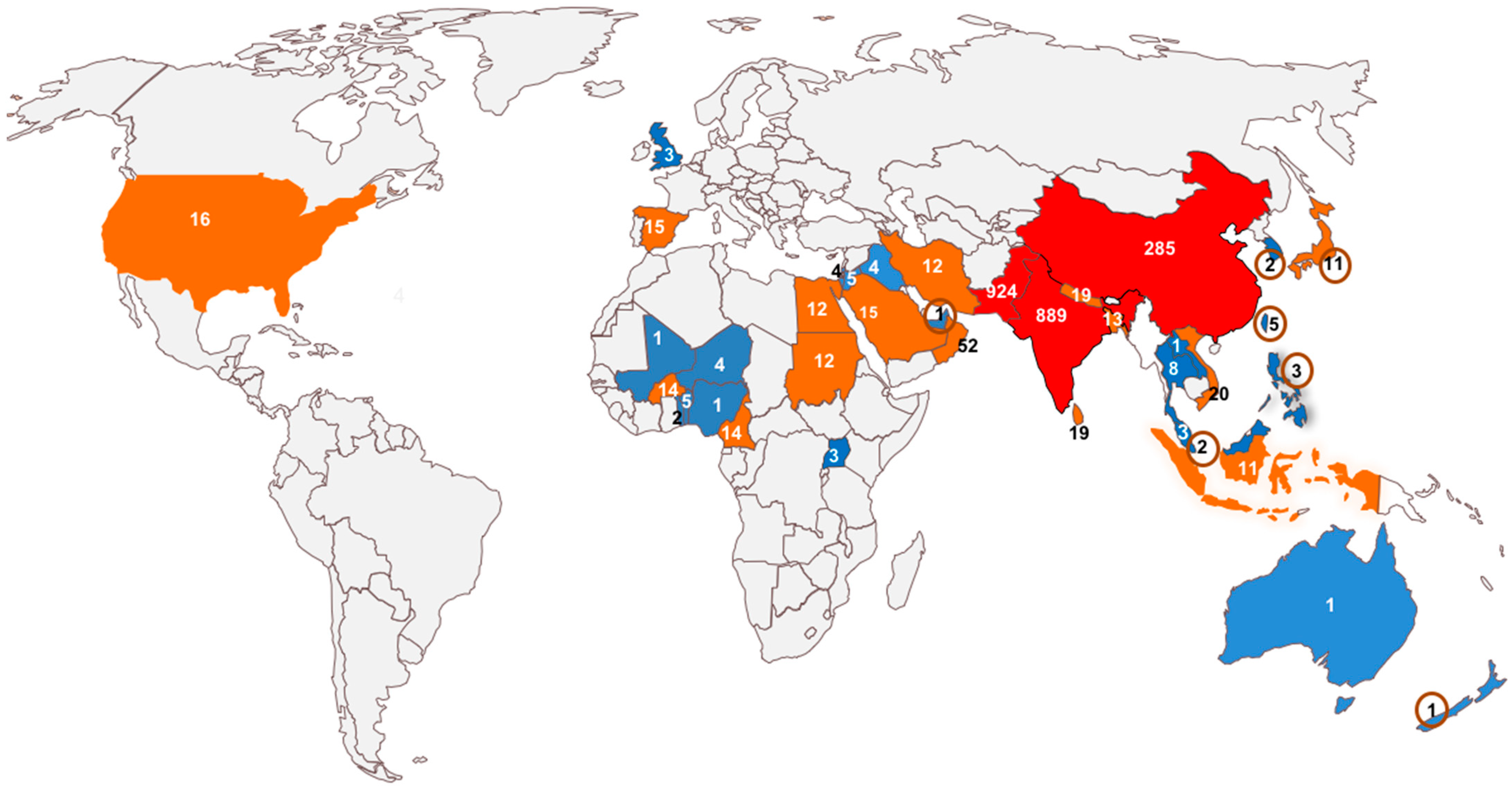
5. Distribution of Betasatellites
6. Discussion
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Varma, A.; Malathi, V.G. Emerging Geminivirus Problems: A Serious Threat to Crop Production. Ann. Appl. Biol. 2003, 142, 145–164. [Google Scholar] [CrossRef]
- Varma, A.; Mandal, B.; Singh, M.K. Global Emergence and Spread of Whitefly (Bemisia Tabaci) Transmitted Geminiviruses. In The Whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) Interaction with Geminivirus-Infected Host Plants; Thompson, W., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 205–292. [Google Scholar] [CrossRef]
- Rojas, M.R.; Macedo, M.A.; Maliano, M.R.; Soto-Aguilar, M.; Souza, J.O.; Briddon, R.W.; Kenyon, L.; Rivera-Bustamante, R.F.; Zerbini, F.M.; Adkins, S.; et al. World Management of Geminiviruses. Annu. Rev. Phytopathol. 2018, 56, 637–677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, M.; Liu, Y.; Ismayil, A. Plant Defense and Viral Counter-Defense during Plant–Geminivirus Interactions. Viruses 2023, 15, 510. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gupta, N.; Chakraborty, S. Geminiviral betasatellites: Critical viral ammunition to conquer plant immunity. Arch. Virol. 2023, 168, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Fiallo-Olivé, E.; Navas-Castillo, J. Begomoviruses: What is the secret(s) of their success? Trends Plant Sci. 2023, 28, 715–727. [Google Scholar] [CrossRef]
- Reddy, M.K.; Nagendran, K.; Kumari, S.; Mohana Pradeep, R.K.; Karthikeyan, G. Global scenario of begomovirus diseases in vegetable crops. Veg. Sci. 2024, 51, 43–53. [Google Scholar] [CrossRef]
- Varma, P.M. Transmission of plant viruses by whiteflies. In Bulletin NISI; National Institute of Science in India: New Delhi, India, 1963; pp. 11–33. [Google Scholar]
- Varma, A.; Singh, M. Cotton. In Viral Diseases of Field and Horticultural Crops, 1st ed.; Awasthi, L.P., Ed.; Academic Press: Cambridge, MA, USA, 2024; pp. 223–232. [Google Scholar] [CrossRef]
- Patil, B.L.; Fauquet, C. Cassava mosaic geminiviruses: Actual knowledge and perspectives. Mol. Plant Pathol. 2009, 10, 685–701. [Google Scholar] [CrossRef]
- Briddon, R.W.; Markham, P.G. Cotton leaf curl virus disease. Virus Res. 2000, 71, 151–159. [Google Scholar] [CrossRef]
- Briddon, R.W.; Mansoor, S.; Bedford, I.; Pinner, M.S.; Saunders, K.; Stanley, J.; Zafar, Y.; Malik, K.A.; Markham, P.G. Identification of DNA components required for induction of cotton leaf curl disease. Virology 2001, 285, 234–243. [Google Scholar] [CrossRef]
- Radhakrishnan, G.; Malathi, V.G.; Varma, A. Detection of DNA A and DNA β Associated with Cotton Leaf Curl and Some Other Plant Diseases Caused by Whitefly Transmitted Geminiviruses. Indian Phytopath. 2004, 57, 53–60. [Google Scholar]
- Farina, A.; Rapisarda, C.; Fiallo-Olivé, E.; Navas-Castillo, J. Tomato Leaf Curl New Delhi Virus Spain Strain Is Not Transmitted by Trialeurodes vaporariorum and Is Inefficiently Transmitted by Bemisia tabaci Mediterranean between Zucchini and the Wild Cucurbit Ecballium elaterium. Insects 2023, 14, 384. [Google Scholar] [CrossRef]
- Czosnek, H.; Hariton-Shalev, A.; Sobol, I.; Gorovits, R.; Ghanim, M. The incredible journey of begomoviruses in their whitefly vector. Viruses 2017, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-Z.; Wang, Y.; Yin, T.-Y.; Fiallo-Olivé, E.; Liu, Y.; Hanley-Bowdoin, L.; Wang, X. A plant DNA virus replicates in the salivary glands of its insect vector via recruitment of host DNA synthesis machinery. Proc. Natl. Acad. Sci. USA 2020, 117, 16928–16937. [Google Scholar] [CrossRef] [PubMed]
- Dry, I.B.; Krake, L.R.; Rigden, J.E.; Rezaian, M.A. A Novel Subviral Agent Associated with a Geminivirus: The First Report of a DNA Satellite. Proc. Natl. Acad. Sci. USA 1997, 94, 7088–7093. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, P.N.; Malathi, V.G.; Varma, A. Molecular Diversity of the DNA-β Satellites Associated with Tomato Leaf Curl Disease in India. Arch. Virol. 2010, 155, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.; Bedford, I.D.; Briddon, R.W.; Markham, P.; Wong, S.-M.; Stanley, J. A Unique Virus Complex Causes Ageratum Yellow Vein Disease. Proc. Natl. Acad. Sci. USA 2000, 97, 6890–6895. [Google Scholar] [CrossRef] [PubMed]
- Villegas, C.; Ramos-Sobrinho, R.; Jifon, J.L.; Keith, C.V.; Al Rwahnih, M.; Sétamou, M.; Brown, J.M.; Alabi, O.J. First Report of Cotton Leaf Curl Gezira Virus and Its Associated Alphasatellite and Betasatellite from Disease Affected Okra Plants in the United States. Plant Dis. 2019, 103, 3291. [Google Scholar] [CrossRef]
- Sivalingam, P.N.; Varma, A. Role of betasatellites in the pathogenesis of a bipartite begomovirus affecting tomato in India. Arch. Virol. 2012, 157, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, P.; Singh, A.K.; Kumar, R.V.; Basu, S.; Chakraborty, S. Host-specific adaptation of diverse betasatellites associated with distinct India tomato-infecting begomoviruses. Virus Genes 2014, 48, 334–342. [Google Scholar] [CrossRef]
- Torkpo, S.K.; Amponsah, E.K.; Arhin, C.D.; Offei, S.K. Occurrence of cassava mosaic begomovirus-associated satellites on cassava in Ghana. Cogent Food Agric. 2021, 7, 1963929. [Google Scholar] [CrossRef]
- Harrison, B.D.; Robinson, D. Natural genomic and antigenic variation in whitefly-transmitted geminiviruses (begomoviruses). Annu. Rev. Phytopathol. 1999, 37, 369–398. [Google Scholar] [CrossRef] [PubMed]
- Fauquet, C.M.; Bisaro, D.M.; Briddon, R.W.; Brown, J.K.; Harrison, B.D.; Rybicki, E.P.; Stenger, D.C. Stanley (Study Group Chair). Virology Division News: Revision of Taxonomic Criteria for Species Demarcation in the Family Geminiviridae, and an Updated List of Begomovirus Species. Arch Virol. 2003, 148, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Taliansky, M.E.; Palukaitis, P. Satellite RNAs and satellite viruses. In Encyclopedia of Virology; Granoff, A., Webster, R.G., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 1607–1615. [Google Scholar]
- Iqbal, Z.; Shafiq, M.; Ali, S.; Mahmood, M.A.; Siddiqui, H.A.; Amin, I.; Briddon, R.W. qPCR Assay as a Tool for Examining Cotton Resistance to the Virus Complex Causing CLCuD: Yield Loss Inversely Correlates with Betasatellite, Not Virus, DNA Titer. Plants 2023, 12, 2645. [Google Scholar] [CrossRef] [PubMed]
- Rosario, K.; Marr, C.; Varsani, A.; Kraberger, S.; Stainton, D.; Moriones, E.; Polston, J.; Breitbart, M. Begomovirus-Associated Satellite DNA Diversity Captured through Vector-Enabled Metagenomic (VEM) Surveys Using Whiteflies (Aleyrodidae). Viruses 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Briddon, R.W.; Martin, D.P.; Roumagnac, P.; Navas-Castillo, J.; Fiallo-Olivé, E.; Moriones, E.; Lett, J.M.; Zerbini, F.M.; Varsani, A. Alphasatellitidae: A new family with two subfamilies for the classification of geminivirus-and nanovirus-associated alphasatellites. Arch Virol. 2018, 163, 2587–2600. [Google Scholar] [CrossRef] [PubMed]
- Venkataravanappa, V.; Reddy, C.L.; Shankarappa, K.; Reddy, M.K. Association of Tomato Leaf Curl New Delhi Virus, Betasatellite, and Alphasatellite with Mosaic Disease of Spine Gourd (Momordica dioica Roxb. Willd) in India. Iran. J. Biotechnol. 2019, 17, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.M.; Shahid, M.S.; Briddon, R.W.; Khan, A.J.; Zhu, J.K.; Brown, J.K. An unusual alphasatellite associated with monopartite begomoviruses attenuates symptoms and reduces betasatellite accumulation. J. Gen. Virol. 2010, 92, 706–717. [Google Scholar] [CrossRef]
- Mar, T.B.; Mendes, I.R.; Lau, D.; Fiallo-Olivé, E.; Navas-Castillo, J.; Alves, M.S.; Murilo Zerbini, F. Interaction between the New World begomovirus Euphorbia yellow mosaic virus and its associated alphasatellite: Effects on infection and transmission by the whitefly Bemisia tabaci. J. Gen. Virol. 2017, 98, 1552–1562. [Google Scholar] [CrossRef]
- Iqbal, Z.; Shafiq, M.; Briddon, R.W. Cotton Leaf Curl Multan Betasatellite Impaired ToLCNDV Ability to Maintain Cotton Leaf Curl Multan Alphasatellite. Braz. J. Biol. 2024, 84, e260922. [Google Scholar] [CrossRef]
- Xu, X.; Qian, Y.; Wang, Y.; Li, Z.; Zhou, X. Iterons Homologous to Helper Geminiviruses Are Essential for Efficient Replication of Betasatellites. J. Virol. 2019, 93, 1128. [Google Scholar] [CrossRef]
- Vo, T.T.B.; Wira Sanjaya, I.G.N.P.; Kil, E.-J.; Lal, A.; Ho, P.T.; Nattanong, B.; Tabassum, M.; Qureshi, M.A.; Lee, T.-K.; Lee, S. Transreplication Preference of the Tomato Leaf Curl Joydebpur Virus for a Noncognate Betasatellite through Iteron Resemblance on Nicotiana bethamiana. Microorganisms 2023, 11, 2907. [Google Scholar] [CrossRef] [PubMed]
- Gelbart, D.; Chen, L.; Alon, T.; Dobrinin, S.; Levin, I.; Lapidot, M. The recent association of a DNA betasatellite with Tomato yellow leaf curl virus in Israel–A new threat to tomato production. Crop Prot. 2020, 128, 104995. [Google Scholar] [CrossRef]
- Gnanasekaran, P.; KishoreKumar, R.; Bhattacharyya, D.; Vinoth Kumar, R.; Chakraborty, S. Multifaceted role of geminivirus associated betasatellite in pathogenesis. Mol. Plant Pathol. 2019, 20, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Eini, O.; Behjatnia, S.A.; Dogra, S.; Dry, I.B.; Randles, J.W.; Rezaian, M.A. Identification of sequence elements regulating promoter activity and replication of a monopartite begomovirus-associated DNA β satellite. J. Gen. Virol. 2009, 90, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Zafar, Y.; Randles, J.W.; Rezaian, M.A. A Monopartite Begomovirus-Associated DNA β Satellite Substitutes for the DNA B of a Bipartite Begomovirus to Permit Systemic Infection. J. Gen. Virol. 2007, 88, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekaran, P.; Gupta, N.; Ponnusamy, K.; Devendran, R.; George, B.; Chakraborty, S. Betasatellite-Encoded ΒC1 Protein Regulates Helper Virus Accumulation by Interfering with the ATP Hydrolysis Activity of Geminivirus-Encoded Replication Initiator Protein. J. Gen. Virol. 2023, 104, 001866. [Google Scholar] [CrossRef]
- Patil, B.L.; Fauquet, C.M. Differential Interaction between Cassava Mosaic Geminiviruses and Geminivirus Satellites. J. Gen. Virol. 2010, 91, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekaran, P.; Ponnusamy, K.; Chakraborty, S. A geminivirus betasatellite encoded βC1 protein interacts with PsbP and subverts PsbP-mediated antiviral defence in plants. Mol. Plant Pathol. 2019, 20, 943–960. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, T.; He, Y.; Su, C.; Wang, Z.; Zhou, X. N-Terminal Acetylation of the ΒC1 Protein Encoded by the Betasatellite of Tomato Yellow Leaf Curl China Virus Is Critical for Its Viral Pathogenicity. Virology 2023, 586, 1–11. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Zhao, P.; Sun, Y.; Fang, R.-X.; Ye, J. Near-Infrared Light and PIF4 Promote Plant Antiviral Defense by Enhancing RNA Interference. Plant Commun. 2024, 5, 100644. [Google Scholar] [CrossRef]
- Gupta, N.; Reddy, K.; Gnanasekaran, P.; Zhai, Y.; Chakraborty, S.; Pappu, H.R. Functional characterization of a new ORF βV1 encoded by radish leaf curl betasatellite. Front. Plant Sci. 2022, 13, 972386. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Song, Y.; Wang, Y.; Zhou, X. Functional Analysis of a Novel ΒV1 Gene Identified in a Geminivirus Betasatellite. Sci. China Life Sci. 2020, 63, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Nawaz-Ul-Rehman, M.S.; Nahid, N.; Hassan, M.A.S.; Mubin, M. Betasatellites and deltasatelliles (Tolecusatellitidae). In Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2021; pp. 239–246. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, P.; Wang, D.; Mubin, M.; Fang, R.; Ye, J. Diverse begomoviruses evolutionarily hijack plant Terpenoid-Based defense to promote whitefly performance. Cells 2022, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Yao, D.; Zhang, T.; Walling, L.L.; Yang, M.; Wang, Y.; Liu, S. Suppression of Terpenoid Synthesis in Plants by a Virus Promotes Its Mutualism with Vectors. Ecol. Lett. 2013, 16, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Weldegergis, B.T.; Li, J.; Jung, C.; Qu, J.; Sun, Y.; Qian, H.; Tee, C.; van Loon, J.J.; Dicke, M.; et al. Virulence Factors of Geminivirus Interact with MYC2 to Subvert Plant Resistance and Promote Vector Performance. Plant Cell 2014, 26, 4991–5008. [Google Scholar] [CrossRef]
- Zou, C.; Shu, Y.-N.; Yang, J.-J.; Pan, L.-L.; Zhao, J.; Chen, N.; Liu, S.-S.; Wang, X.-W. Begomovirus-Associated Betasatellite Virulence Factor ΒC1 Attenuates Tobacco Defense to Whiteflies via Interacting with Plant SKP1. Front. Plant Sci. 2020, 11, 574557. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-J.; Liu, Y.-M.; Li, H.-Y.; Liu, S.-S.; Pan, L.-L. Temporal Dynamic of the Ratio between Monopartite Begomoviruses and Their Associated Betasatellites in Plants, and Its Modulation by the Viral Gene βC1. Viruses 2023, 15, 954. [Google Scholar] [CrossRef] [PubMed]
- Venkataravanappa, V.; Kodandaram, M.; Prasanna, H.; Reddy, M.K.; Reddy, C.L. Lakshminarayana Reddy. Unraveling Different Begomoviruses, DNA Satellites and Cryptic Species of Bemisia Tabaci and Their Endosymbionts in Vegetable Ecosystem. Microb. Pathog. 2023, 174, 105892. [Google Scholar] [CrossRef] [PubMed]
- Hassan-Sheikhi, P.; Heydarnejad, J.; Massumi, H.; Kraberger, S.; Varsani, A. Novel Nanovirus and Associated Alphasatellites Identified in Milk Vetch Plants with Chlorotic Dwarf Disease in Iran. Virus Res. 2020, 276, 197830. [Google Scholar] [CrossRef]
- Zhou, X. Advances in Understanding Begomovirus Satellites. Annu. Rev. Phytopathol. 2013, 5, 357–381. [Google Scholar] [CrossRef]
- Mansoor, S.; Zafar, Y.; Briddon, R.W. Geminivirus disease complexes: The threat is spreading. Trends Plant Sci. 2006, 11, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.V.; Singh, A.K.; Singh, A.K.; Yadav, T.; Basu, S.; Kushwaha, N.; Chattopadhyay, B.; Chakraborty, S. Complexity of Begomovirus and Betasatellite Populations Associated with Chilli Leaf Curl Disease in India. J. Gen. Virol. 2015, 96, 3143–3158. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Srivastava, A.; Mishra, M.; Gaur, R.K. Chilli Leaf Curl Disease Populations in India Are Highly Recombinant, and Rapidly Segregated. 3 Biotech 2022, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Fiallo-Olivé, E.; Martínez-Zubiaur, Y.; Moriones, E.; Navas-Castillo, J. A novel class of DNA satellites associated with New World begomoviruses. Virology 2012, 426, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Anfoka, G.; Altaleb, M.; Ahmad, F.H.; Obaida, M.A. Charlock mustard (Sinapis arvensis): A weed reservoir for begomoviruses and associated betasatellite in Jordan. Can. J. Plant Pathol. 2017, 39, 325–333. [Google Scholar] [CrossRef]
- Sattar, M.N.; Khurshid, M.; El-Beltagi, H.S.; Iqbal, Z. Identification and estimation of sequence variation dynamics of Tomato Leaf curl Palampur virus and betasatellite complex infecting a new weed host. Biotechnol. Biotechnol. Equip. 2022, 36, 609–619. [Google Scholar] [CrossRef]
- Santosa, A.I.; Somowiyarjo, S. Ageratum yellow vein alphasatellite and tomato leaf curl Java betasatellite association with begomoviruses infecting crops and weeds in Indonesia. Plant Prot. Sci. 2023, 59, 317–324. [Google Scholar] [CrossRef]
- Shafiq, M.; Ondrašek, G.; Al-Sadi, A.M.; Shahid, M. Molecular Signature of a Novel Alternanthera Yellow Vein Virus Variant Infecting the Ageratum conyzoides Weed in Oman. Viruses 2023, 15, 2381. [Google Scholar] [CrossRef] [PubMed]
- Mubin, M.; Briddon, R.W.; Mansoor, S. Diverse and recombinant DNA betasatellites are associated with a begomovirus disease complex of Digera arvensis, a weed host. Virus Res. 2009, 142, 208–212. [Google Scholar] [CrossRef]
- Mubin, M.; Shahid, M.; Tahir, M.; Briddon, R.W.; Mansoor, S. Characterization of begomovirus components from a weed suggests that begomoviruses may associate with multiple distinct DNA satellites. Virus Genes 2010, 40, 452–457. [Google Scholar] [CrossRef]
- Polston, J.E.; McGovern, R.J.; Brown, L.R. Introduction of Tomato Yellow Leaf Curl Virus in Florida and Implications for the Spread of This and Other Geminiviruses of Tomato. Plant Dis. 1999, 83, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Rivera, E.A.; Rodríguez-Negrete, E.A.; Aréchiga-Carvajal, E.T.; Leyva-López, N.E.; Méndez-Lozano, J. From Metagenomics to Discovery of New Viral Species: Galium Leaf Distortion Virus, a Monopartite Begomovirus Endemic in Mexico. Front. Microbiol. 2022, 13, 843035. [Google Scholar] [CrossRef] [PubMed]
- Fiallo-Olivé, E.; Navas-Castillo, J. The role of extensive recombination in the evolution of geminiviruses. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 139–166. [Google Scholar] [CrossRef]
- Mubin, M.; Ijaz, S.; Nahid, N.; Hassan, M.; Younus, A.; Qazi, J.; Nawaz-ul-Rehman, M.S. Journey of Begomovirus Betasatellite Molecules: From Satellites to Indispensable Partners. Virus Genes 2020, 56, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Tahir, M.; Amin, I.; Mansoor, S. Amplicon-based RNAi construct targeting beta-C1 gene gives enhanced resistance against cotton leaf curl disease. 3 Biotech 2021, 11, 256. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varma, A.; Singh, M.K. The Role of Satellites in the Evolution of Begomoviruses. Viruses 2024, 16, 970. https://doi.org/10.3390/v16060970
Varma A, Singh MK. The Role of Satellites in the Evolution of Begomoviruses. Viruses. 2024; 16(6):970. https://doi.org/10.3390/v16060970
Chicago/Turabian StyleVarma, Anupam, and Manoj Kumar Singh. 2024. "The Role of Satellites in the Evolution of Begomoviruses" Viruses 16, no. 6: 970. https://doi.org/10.3390/v16060970
APA StyleVarma, A., & Singh, M. K. (2024). The Role of Satellites in the Evolution of Begomoviruses. Viruses, 16(6), 970. https://doi.org/10.3390/v16060970









