Does Prior Respiratory Viral Infection Provide Cross-Protection Against Subsequent Respiratory Viral Infections? A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
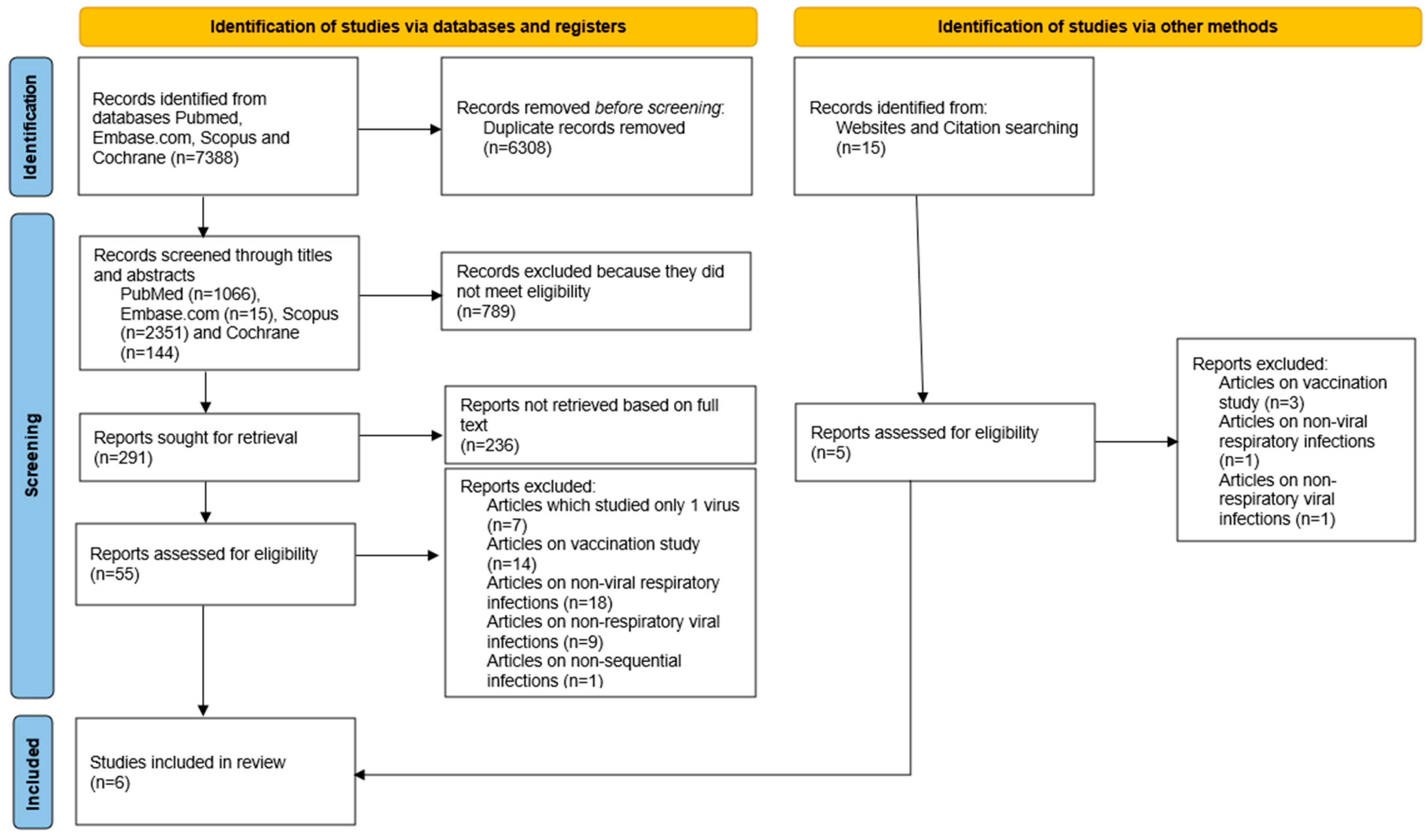
2. Materials and Methods
2.1. Search Strategy and Data Sources
2.2. Eligibility Criteria
2.3. Data Extraction and Management
2.4. Outcomes
- The prevalence of a different sequential viral infection amongst those with an initial positive swab.
- The prevalence of specific sequential viral infections is grouped by types of initial viral infection.
- The pooled estimate of the relative risk of SARS-CoV-2 infection following an initial positive viral swab compared with a negative swab.
- The pooled prevalence of a sequential viral infection in the subgroup excluding studies on SARS-CoV-2.
2.5. Statistical Methods
3. Results
3.1. Assessment of Quality of Included Studies
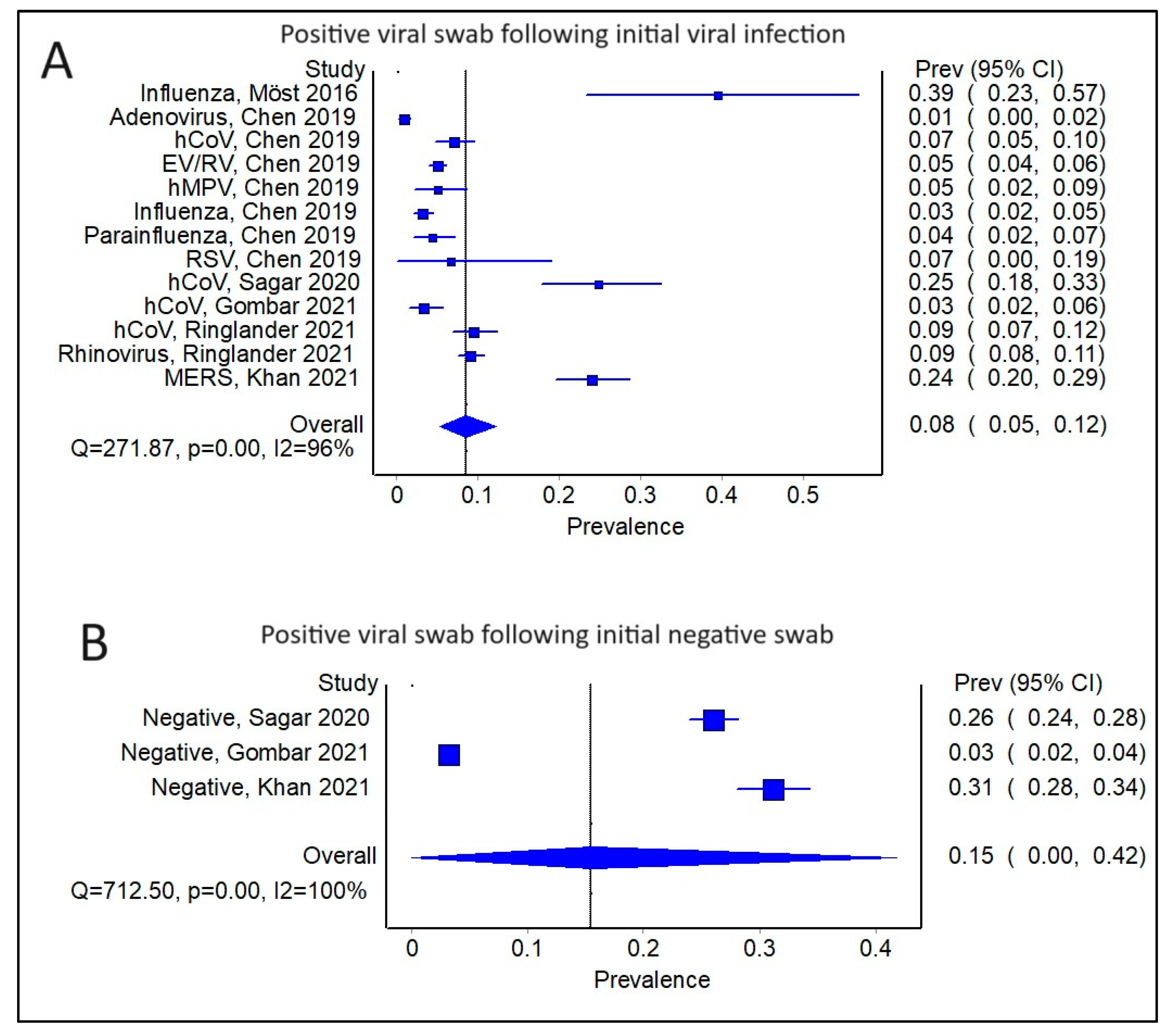
3.2. Comparing Subsequent Infections in Patients with an Initial Positive Swab versus a Negative Swab
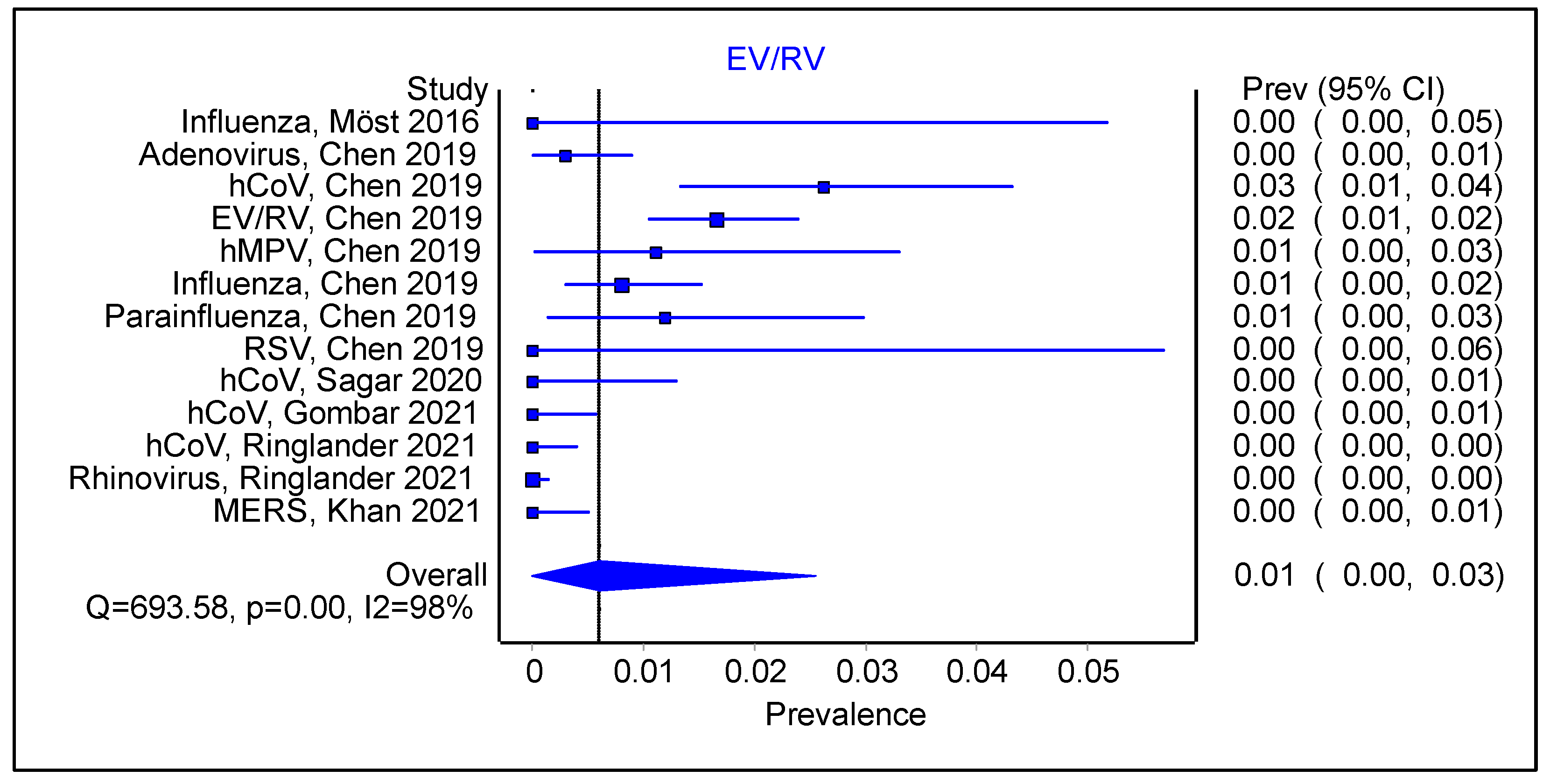
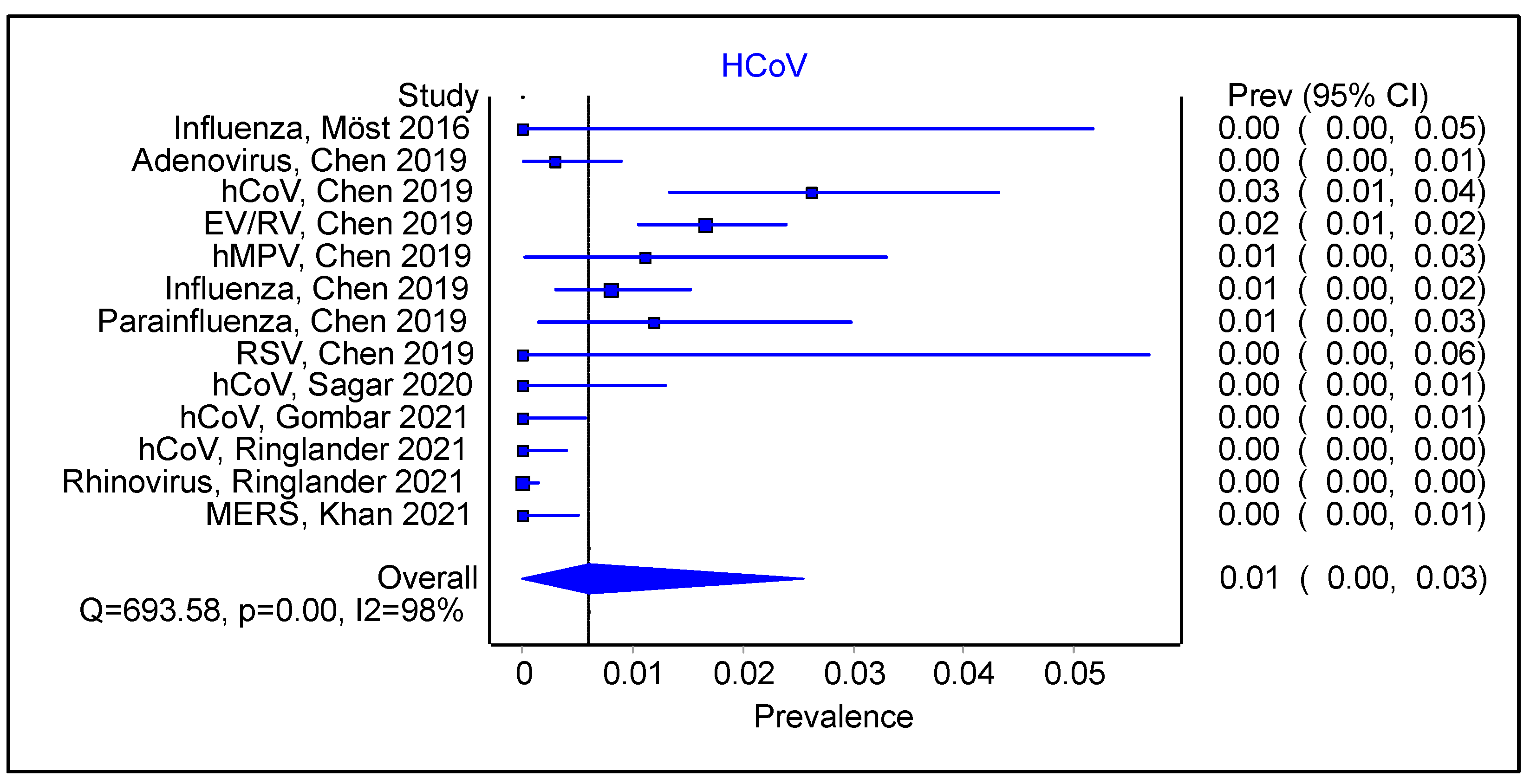
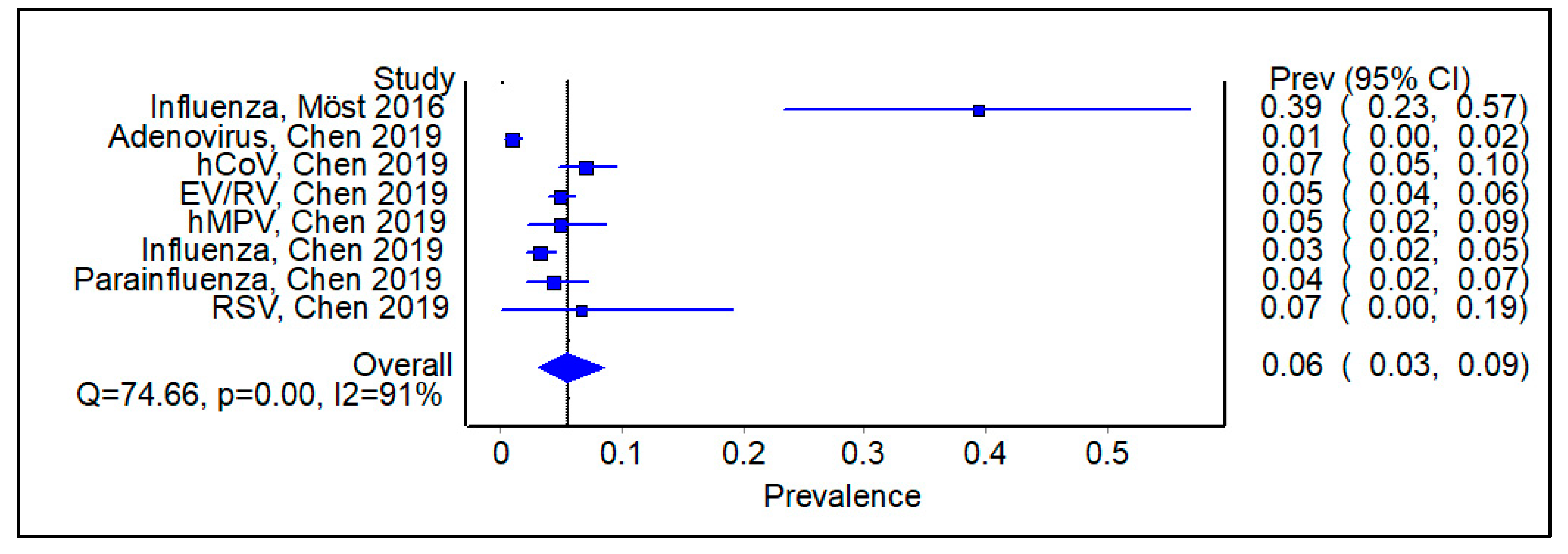
3.3. Is There an Impact of Initial Infection on the Type of Secondary/Sequential Viral Infection?
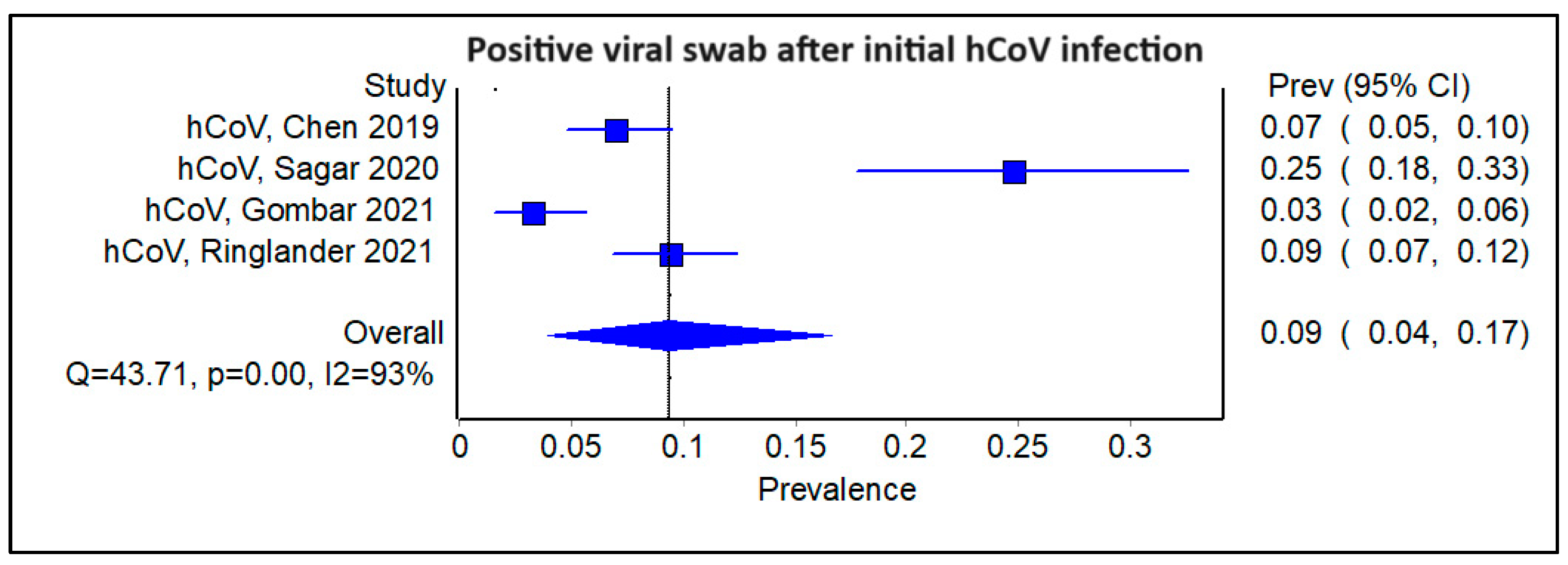
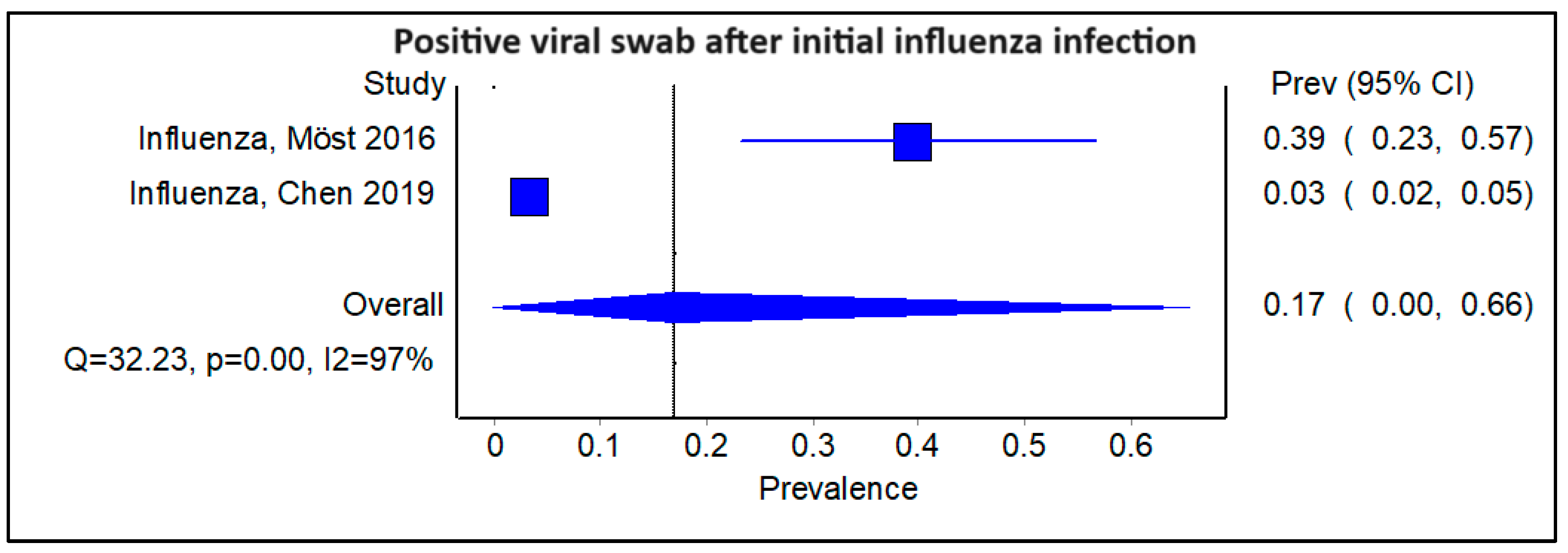
3.4. Does the Type of Initial Viral Infection Matter?
3.5. What Is the Relative Risk of SARS-CoV-2 Infection Following an Initial Positive Viral Swab Compared with a Negative Swab?
3.6. Subgroup Analysis Excluding Studies on SARS-CoV-2, Showing Pooled Prevalence of Viral Infections Following an Initial Positive Swab
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ramadan, N.; Shaib, H. Middle East respiratory syndrome coronavirus (MERS-CoV): A review. Germs 2019, 9, 35. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Dunning, J.; Thwaites, R.S.; Openshaw, P.J.M. Seasonal and pandemic influenza: 100 years of progress, still much to learn. Mucosal Immunol. 2020, 13, 566–573. [Google Scholar] [CrossRef]
- Gray, G.C.; Robie, E.R.; Studstill, C.J.; Nunn, C.L. Mitigating Future Respiratory Virus Pandemics: New Threats and Approaches to Consider. Viruses 2021, 13, 637. [Google Scholar] [CrossRef]
- Lee, V.J.; Chen, M.I.; Chan, S.P.; Wong, C.S.; Cutter, J.; Goh, K.T.; Tambyah, P.A. Influenza pandemics in Singapore, a tropical, globally connected city. Emerg. Infect. Dis. 2007, 13, 1052–1057. [Google Scholar] [CrossRef]
- World Health Organization. WHO COVID-19 Dashboard. 2020. Available online: https://covid19.who.int/ (accessed on 12 November 2022).
- Lee, S.S.; Viboud, C.; Petersen, E. Understanding the rebound of influenza in the post COVID-19 pandemic period holds important clues for epidemiology and control. Int. J. Infect. Dis. 2022, 122, 1002–1004. [Google Scholar] [CrossRef]
- Chan, C.P.; Wong, N.S.; Leung, C.C.; Lee, S.S. Positive impact of measures against COVID-19 on reducing influenza in the Northern Hemisphere. J. Travel Med. 2020, 27, taaa087. [Google Scholar] [CrossRef]
- Kim, J.; Gómez Gómez, R.E.; Hong, K.; Yum, S.; Jang, J.; Chun, B.C. Changing influenza activity in the Southern hemisphere countries during the COVID-19 pandemic. Int. J. Infect. Dis. 2021, 108, 109–111. [Google Scholar] [CrossRef]
- Itaya, T.; Furuse, Y.; Jindai, K. Does COVID-19 infection impact on the trend of seasonal influenza infection? 11 countries and regions, from 2014 to 2020. Int. J. Infect. Dis. 2020, 97, 78–80. [Google Scholar] [CrossRef]
- Chan, H.C.; Tambyah, P.A.; Tee, N.W.S.; Somani, J. Return of other respiratory viruses despite the disappearance of influenza during COVID-19 control measures in Singapore. J. Clin. Virol. 2021, 144, 104992. [Google Scholar] [CrossRef]
- Monto, A.S.; Zambon, M.; Weir, J.P. The End of B/Yamagata Influenza Transmission—Transitioning from Quadrivalent Vaccines. N. Engl. J. Med. 2024, 390, 1256–1258. [Google Scholar] [CrossRef]
- Addetia, A.; Crawford, K.H.D.; Dingens, A.; Zhu, H.; Roychoudhury, P.; Huang, M.L.; Jerome, K.R.; Bloom, J.D.; Greninger, A.L. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J. Clin. Microbiol. 2020, 58, e02107-20. [Google Scholar] [CrossRef]
- Rouse, B.T.; Sehrawat, S. Immunity and immunopathology to viruses: What decides the outcome? Nat. Rev. Immunol. 2010, 10, 514–526. [Google Scholar] [CrossRef]
- Chen, I.M.; Loh, J.P.; Chuah, C.X.P.; Gao, Q.H.C.; Sun, Y.; Ng, S.H.; Koh, W.V.; Goh, E.H.; Zhao, X.; Tambyah, P.A.; et al. Evidence for Cross-Protection against Subsequent Febrile Respiratory Illness Episodes from Prior Infections by Different Viruses among Singapore Military Recruits 2009–2014. J. Infect. Dis. 2019, 219, 1913–1923. [Google Scholar] [CrossRef]
- Stowe, J.; Tessier, E.; Zhao, H.; Guy, R.; Muller-Pebody, B.; Zambon, M.; Andrews, N.; Ramsay, M.; Lopez Bernal, J. Interactions between SARS-CoV-2 and influenza, and the impact of coinfection on disease severity: A test-negative design. Int. J. Epidemiol. 2021, 50, 1124–1133. [Google Scholar] [CrossRef]
- Vennila Gopal, P.T.; Ngiam, N.; Seong, J.T.C. Systematic Review and Meta-Analysis of Prior Viral Infection and Cross-Protection Against Subsequent Respiratory Viral infections. 2022. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022295380 (accessed on 17 October 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; O’Connell, B.S.D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2021. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 17 September 2022).
- Piret, J.; Boivin, G. Viral Interference between Respiratory Viruses. Emerg. Infect. Dis. 2022, 28, 273–281. [Google Scholar] [CrossRef]
- Cabán, M.; Rodarte, J.V.; Bibby, M.; Gray, M.D.; Taylor, J.J.; Pancera, M.; Boonyaratanakornkit, J. Cross-protective antibodies against common endemic respiratory viruses. Nat. Commun. 2023, 14, 798. [Google Scholar] [CrossRef]
- Anestad, G.; Vainio, K.; Hungnes, O. Interference between outbreaks of epidemic viruses. Scand. J. Infect. Dis. 2007, 39, 653–654. [Google Scholar] [CrossRef]
- Greer, R.M.; McErlean, P.; Arden, K.E.; Faux, C.E.; Nitsche, A.; Lambert, S.B.; Nissen, M.D.; Sloots, T.P.; Mackay, I.M. Do rhinoviruses reduce the probability of viral co-detection during acute respiratory tract infections? J. Clin. Virol. 2009, 45, 10–15. [Google Scholar] [CrossRef]
- Karppinen, S.; Toivonen, L.; Schuez-Havupalo, L.; Waris, M.; Peltola, V. Interference between respiratory syncytial virus and rhinovirus in respiratory tract infections in children. Clin. Microbiol. Infect. 2016, 22, e1–e208. [Google Scholar] [CrossRef]
- Achten, N.B.; Wu, P.; Bont, L.; Blanken, M.O.; Gebretsadik, T.; Chappell, J.D.; Wang, L.; Yu, C.; Larkin, E.K.; Carroll, K.N.; et al. Interference between respiratory syncytial virus and human rhinovirus infection in infancy. J. Infect. Dis. 2017, 215, 1102–1106. [Google Scholar] [CrossRef]
- Ringlander, J.; Martner, A.; Nilsson, S.; Westin, J.; Lindh, M.; Hellstrand, K. Incidence and Severity of COVID-19 in Patients with and without Previously Verified Infections with Common Cold Coronaviruses. J. Infect. Dis. 2021, 223, 1831–1832. [Google Scholar] [CrossRef]
- Gombar, S.; Bergquist, T.; Pejaver, V.; Hammarlund, N.E.; Murugesan, K.; Mooney, S.; Shah, N.; Pinsky, B.A.; Banaei, N. SARS-CoV-2 infection and COVID-19 severity in individuals with prior seasonal coronavirus infection. Diagn. Microbiol. Infect. Dis. 2021, 100, 115338. [Google Scholar] [CrossRef]
- Sagar, M.; Reifler, K.; Rossi, M.; Miller, N.S.; Sinha, P.; White, L.F.; Mizgerd, J.P. Recent endemic coronavirus infection is associated with less-severe COVID-19. J. Clin. Investig. 2021, 131, e143380. [Google Scholar] [CrossRef]
- Khan, A.A.; Alahmari, A.A.; Almuzaini, Y.; Alamri, F.; Alsofayan, Y.M.; Aburas, A.; Al-Muhsen, S.; Van Kerkhove, M.; Yezli, S.; Ciottone, G.R.; et al. Potential Cross-Reactive Immunity to COVID-19 Infection in Individuals With Laboratory-Confirmed MERS-CoV Infection: A National Retrospective Cohort Study From Saudi Arabia. Front. Immunol. 2021, 12, 727989. [Google Scholar] [CrossRef]
- Selin, L.K.; Brehm, M.A.; Naumov, Y.N.; Cornberg, M.; Kim, S.K.; Clute, S.C.; Welsh, R.M. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol. Rev. 2006, 211, 164–181. [Google Scholar] [CrossRef]
- Walzl, G.; Tafuro, S.; Moss, P.; Openshaw, P.J.; Hussell, T. Influenza virus lung infection protects from respiratory syncytial virus-induced immunopathology. J. Exp. Med. 2000, 192, 1317–1326. [Google Scholar] [CrossRef]
- Poulain, M.; Chambre, D.; Pes, G.M. Centenarians exposed to the Spanish flu in their early life better survived to COVID-19. Aging 2021, 13, 21855–21865. [Google Scholar] [CrossRef]
- Goulding, J.; Snelgrove, R.; Saldana, J.; Didierlaurent, A.; Cavanagh, M.; Gwyer, E.; Wales, J.; Wissinger, E.L.; Hussell, T. Respiratory infections: Do we ever recover? Proc. Am. Thorac. Soc. 2007, 4, 618–625. [Google Scholar] [CrossRef]


| Database: Medline (PubMed) Date of Search: 13 August 2022 No. of Results: 1066 Limits and filters applied (referring to PRISMA-S item 9 & 10): nil |
| Search Strategy (copy and paste as run) (((respiratory tract infections[MeSH Terms]) OR (“Respiratory tract infection*”[Title/Abstract] OR “Respiratory viral infection*”[Title/Abstract] OR “Viral respiratory infection*”[Title/Abstract] OR Influenza[Title/Abstract] OR Flu[Title/Abstract] OR H1N1[Title/Abstract] OR H3N2[Title/Abstract] OR “Respiratory Syncytial virus*”[Title/Abstract] OR RSV[Title/Abstract] OR Adenovirus[Title/Abstract] OR Parainfluenza[Title/Abstract] OR Enterovirus[Title/Abstract] OR Rhinovirus[Title/Abstract] OR Coronavirus*[Title/Abstract] OR COVID-19[Title/Abstract] OR SARS CoV-2[Title/Abstract])) AND ((cross protection[MeSH Terms]) OR (“Cross-protect*”[Title/Abstract] OR “cross protect*”[Title/Abstract] OR co-infect*[Title/Abstract] OR “virus cross-protect*”[Title/Abstract] OR “virus cross protect*”[Title/Abstract] OR “cross-reacti*”[Title/Abstract]))) AND ((((((“observational stud*”[Publication Type]) OR (observation[MeSH Terms])) OR (cohort studies[MeSH Terms])) OR (cohort analyses[MeSH Terms])) OR (case control studies[MeSH Terms])) OR (case control studies[MeSH Terms])) |
| Database: Embase.com Date of Search: 13 August 2022 No. of Results: 15 Limits and filters applied (referring to PRISMA-S item 9 & 10): nil |
| Search Strategy (copy and paste as run) (‘viral respiratory tract infection’/exp OR (‘respiratory tract infection’:ab,ti OR ‘respiratory tract viral infection’:ab,ti OR ‘respiratory tract virus infection’:ab,ti OR ‘respiratory viral infection’:ab,ti OR ‘viral respiratory disease’:ab,ti OR ‘viral respiratory tract infection’:ab,ti)) AND (‘heterologous immunity’/exp OR (‘cross immunity’:ab,ti OR ‘cross protection immunity’:ab,ti OR ‘cross serotype immunity’:ab,ti OR ‘cross serovar immunity’:ab,ti OR ‘cross variant immunity’:ab,ti OR ‘cross-protective immunity’:ab,ti OR ‘cross-reactive immunity’:ab,ti OR ‘cross-strain immunity’:ab,ti OR ‘crossprotective immunity;’:ab,ti OR ‘crossreactive immunity’:ab,ti OR ‘hetero-subtype immunity’:ab,ti OR ‘hetero-subtypic immunity’:ab,ti OR ‘heterogeneous immunity’:ab,ti OR ‘heterogenous immunity’:ab,ti OR ‘heterospecific immunity’:ab,ti OR ‘heterosubtype immunity’:ab,ti OR ‘heterosubtypic immunity’:ab,ti OR ‘heterovariant immunity’:ab,ti OR ‘immunity, heterologous’:ab,ti)) |
| Database: Scopus Date of Search: 13 August 2022 No. of Results: 2351 Limits and filters applied (referring to PRISMA-S item 9 & 10): nil |
| Search Strategy (copy and paste as run) (TITLE-ABS-KEY(Cross-protection OR “cross protection” OR “heterologous immunity” OR “Cross-protect*” OR “cross protect*” OR co-infect* OR “virus cross-protect*” OR “virus cross protect*” OR “cross-reacti*”)) AND (TITLE-ABS-KEY(“Respiratory tract infection*” OR “Respiratory viral infection*” OR “Viral respiratory tract infection” OR “Viral respiratory infection*” OR Influenza OR Flu OR H1N1 OR H3N2 OR “Respiratory Syncytial virus*” OR RSV OR Adenovirus OR Parainfluenza OR Enterovirus OR Rhinovirus OR Coronavirus* OR COVID-19 OR SARS-CoV-2)) |
| Database: Cochrane Library Date of Search: 13 August 2022 No. of Results: 144 Limits and filters applied (referring to PRISMA-S item 9 & 10): nil |
| Search Strategy (copy and paste as run) #1 MeSH descriptor: [Respiratory Tract Infections] explode all trees 18,339 #2 (“Respiratory tract infection*” OR “Respiratory viral infection*” OR “Viral respiratory tract infection” OR “Viral respiratory infection*” OR Influenza OR Flu OR H1N1 OR H3N2 OR “Respiratory Syncytial virus*” OR RSV OR Adenovirus OR Parainfluenza OR Enterovirus OR Rhinovirus OR Coronavirus* OR COVID-19 OR SARS-CoV-2):ti,ab,kw 30,577 #3 MeSH descriptor: [Cross Protection] explode all trees 20 #4 (Cross-protection OR “cross protection” OR “heterologous immunity” OR “Cross-protect*” OR “cross protect*” OR co-infect* OR “virus cross-protect*” OR “virus cross protect*” OR “cross-reacti*”):ti,ab,kw 1125 #5 (#1 OR #2) AND (#3 OR #4) 144 |
| First Author | Year | Country | Study Type | Total Samples | 1° Pathogen | 2° Pathogen | P+/S+ * | Interval Between 1° & 2° Pathogen | P+/ S− * | P−/ S+ * | P−/ S− * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gombar | 2021 | USA | Retrospective | 2768 | HCoV | SARS-CoV-2 | 10 | NS | 292 | 77 | 2389 |
| Ringlander | 2021 | Sweden | Retrospective | 434 | HCoV | SARS-CoV-2 | 41 | NS | 393 | 0 | 0 |
| 1242 | RV | SARS-CoV-2 | 113 | NS | 1129 | 0 | 0 | ||||
| Chen | 2019 | Singapore | Prospective | 677 | ADV | ADV | 2 | Days: Median 34 (IQR 21–55) | 675 | 0 | 0 |
| 677 | ADV | HCoV | 1 | 676 | 0 | 0 | |||||
| 677 | ADV | EV/RV | 3 | 674 | 0 | 0 | |||||
| 458 | HCoV | ADV | 5 | 453 | 0 | 0 | |||||
| 458 | HCoV | HCoV | 7 | 451 | 0 | 0 | |||||
| 458 | HCoV | EV/RV | 11 | 447 | 0 | 0 | |||||
| 458 | HCoV | HMV | 3 | 455 | 0 | 0 | |||||
| 458 | HCoV | Flu | 2 | 456 | 0 | 0 | |||||
| 458 | HCoV | HPIV | 4 | 454 | 0 | 0 | |||||
| 1449 | EV/RV | ADV | 15 | 1434 | 0 | 0 | |||||
| 1449 | EV/RV | HCoV | 16 | 1433 | 0 | 0 | |||||
| 1449 | EV/RV | EV/RV | 24 | 1425 | 0 | 0 | |||||
| 1449 | EV/RV | HMV | 3 | 1446 | 0 | 0 | |||||
| 1449 | EV/RV | Flu | 8 | 1441 | 0 | 0 | |||||
| 1449 | EV/RV | HPIV | 4 | 1445 | 0 | 0 | |||||
| 1449 | EV/RV | RSV | 2 | 1447 | 0 | 0 | |||||
| 181 | HMV | ADV | 1 | 180 | 0 | 0 | |||||
| 181 | HMV | HCoV | 2 | 179 | 0 | 0 | |||||
| 181 | HMV | EV/RV | 4 | 177 | 0 | 0 | |||||
| 181 | HMV | Flu | 1 | 180 | 0 | 0 | |||||
| 181 | HMV | HPIV | 1 | 180 | 0 | 0 | |||||
| 870 | Flu | ADV | 4 | 866 | 0 | 0 | |||||
| 870 | Flu | HCoV | 8 | 862 | 0 | 0 | |||||
| 870 | Flu | EV/RV | 8 | 862 | 0 | 0 | |||||
| 870 | Flu | Flu | 5 | 865 | 0 | 0 | |||||
| 870 | Flu | HPIV | 3 | 867 | 0 | 0 | |||||
| 254 | HPIV | ADV | 1 | 253 | 0 | 0 | |||||
| 254 | HPIV | HCoV | 2 | 252 | 0 | 0 | |||||
| 254 | HPIV | EV/RV | 3 | 251 | 0 | 0 | |||||
| 254 | HPIV | Flu | 3 | 251 | 0 | 0 | |||||
| 254 | HPIV | HPIV | 2 | 252 | 0 | 0 | |||||
| 30 | RSV | EV/RV | 1 | 29 | 0 | 0 | |||||
| 30 | RSV | Flu | 1 | 29 | 0 | 0 | |||||
| Khan | 2021 | Saudi Arabia | Retrospective | 1176 | MERS-CoV | SARS-CoV-2 | 82 | Years: Median 3.4 (IQR 3.6) | 260 | 260 | 574 |
| Sagar | 2020 | USA | Retrospective | 1812 | HCoV | SARS-CoV-2 | 33 | Days: Median 121 (IQR 69–440) | 100 | 437 | 1242 |
| Möst | 2016 | Austria | Retrospective | 33 | Flu A | Flu B | 13 | Days: Mean 50 | 20 | 0 | 0 |
| NOS Items | Gombar | Ringlander | Chen | Khan | Sagar | Most |
|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | 1 | 1 | 1 | 1 | 0 | 1 |
| Selection of the non-exposed cohort | 1 | 1 | 1 | 1 | 1 | 1 |
| Ascertainment of exposure | 1 | 1 | 1 | 1 | 1 | 1 |
| Demonstration that outcome of interest was not present at the start of the study | 1 | 1 | 1 | 1 | 1 | 1 |
| Comparability of cohorts on the basis of the design or analysis | 1 | 1 | 1 | 1 | 1 | 1 |
| Assessment of outcome | 1 | 1 | 1 | 1 | 1 | 1 |
| Follow-up was long enough for outcomes to occur | 1 | 1 | 1 | 1 | 0 | 0 |
| Adequate of follow-up of cohorts | 1 | 1 | 1 | 1 | 0 | 0 |
| Total Score | 8 | 8 | 8 | 8 | 5 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gopal, V.; Koh, M.C.Y.; Ngiam, J.N.; Hang-Cheng, O.; Somani, J.; Tambyah, P.A.; Tey, J. Does Prior Respiratory Viral Infection Provide Cross-Protection Against Subsequent Respiratory Viral Infections? A Systematic Review and Meta-Analysis. Viruses 2024, 16, 982. https://doi.org/10.3390/v16060982
Gopal V, Koh MCY, Ngiam JN, Hang-Cheng O, Somani J, Tambyah PA, Tey J. Does Prior Respiratory Viral Infection Provide Cross-Protection Against Subsequent Respiratory Viral Infections? A Systematic Review and Meta-Analysis. Viruses. 2024; 16(6):982. https://doi.org/10.3390/v16060982
Chicago/Turabian StyleGopal, Vennila, Matthew Chung Yi Koh, Jinghao Nicholas Ngiam, Ong Hang-Cheng, Jyoti Somani, Paul Anatharajah Tambyah, and Jeremy Tey. 2024. "Does Prior Respiratory Viral Infection Provide Cross-Protection Against Subsequent Respiratory Viral Infections? A Systematic Review and Meta-Analysis" Viruses 16, no. 6: 982. https://doi.org/10.3390/v16060982
APA StyleGopal, V., Koh, M. C. Y., Ngiam, J. N., Hang-Cheng, O., Somani, J., Tambyah, P. A., & Tey, J. (2024). Does Prior Respiratory Viral Infection Provide Cross-Protection Against Subsequent Respiratory Viral Infections? A Systematic Review and Meta-Analysis. Viruses, 16(6), 982. https://doi.org/10.3390/v16060982





