Exploring the Complexities of Long COVID
Abstract
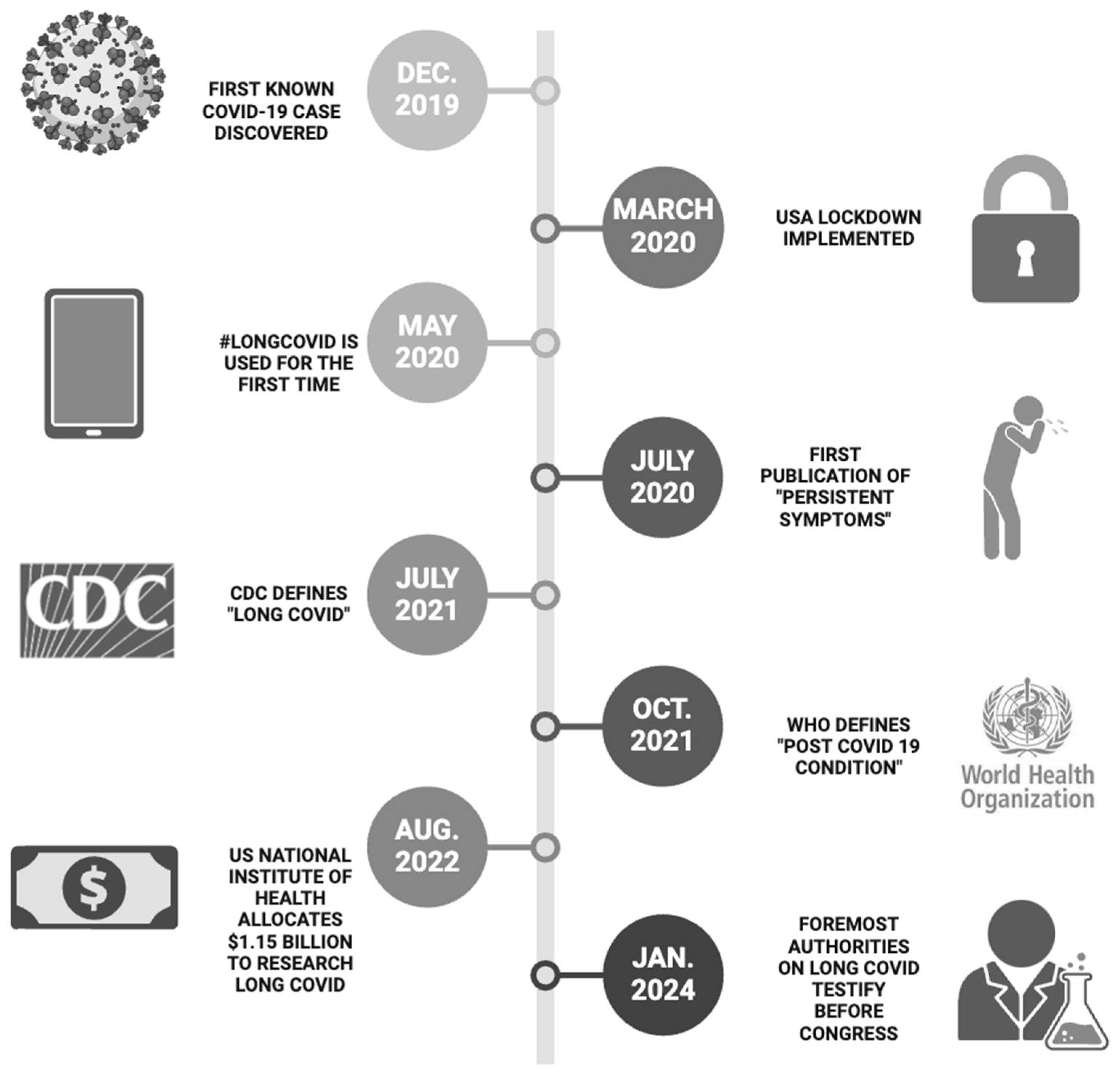
1. Introduction
2. Major Findings
2.1. Overview of Long COVID
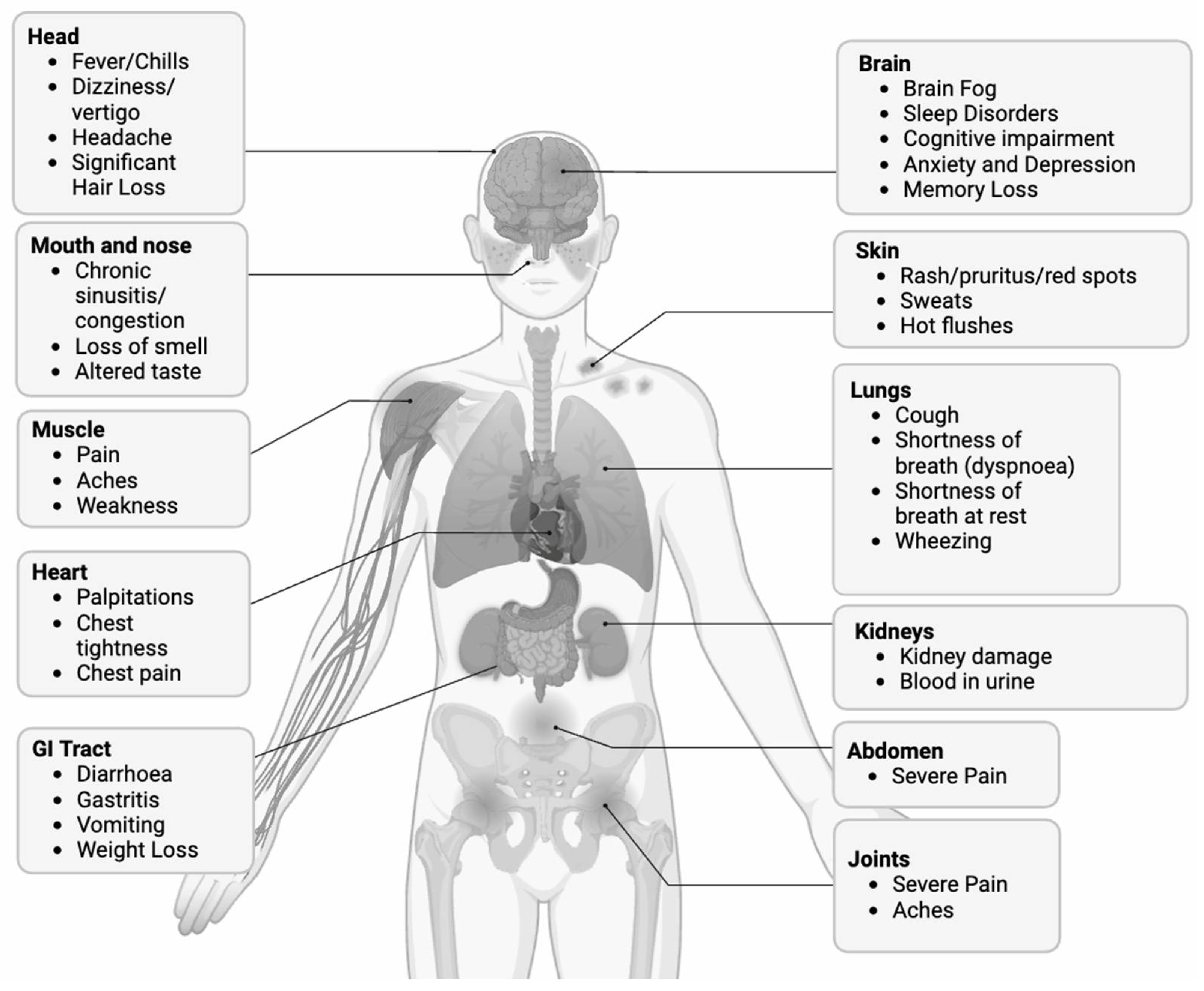
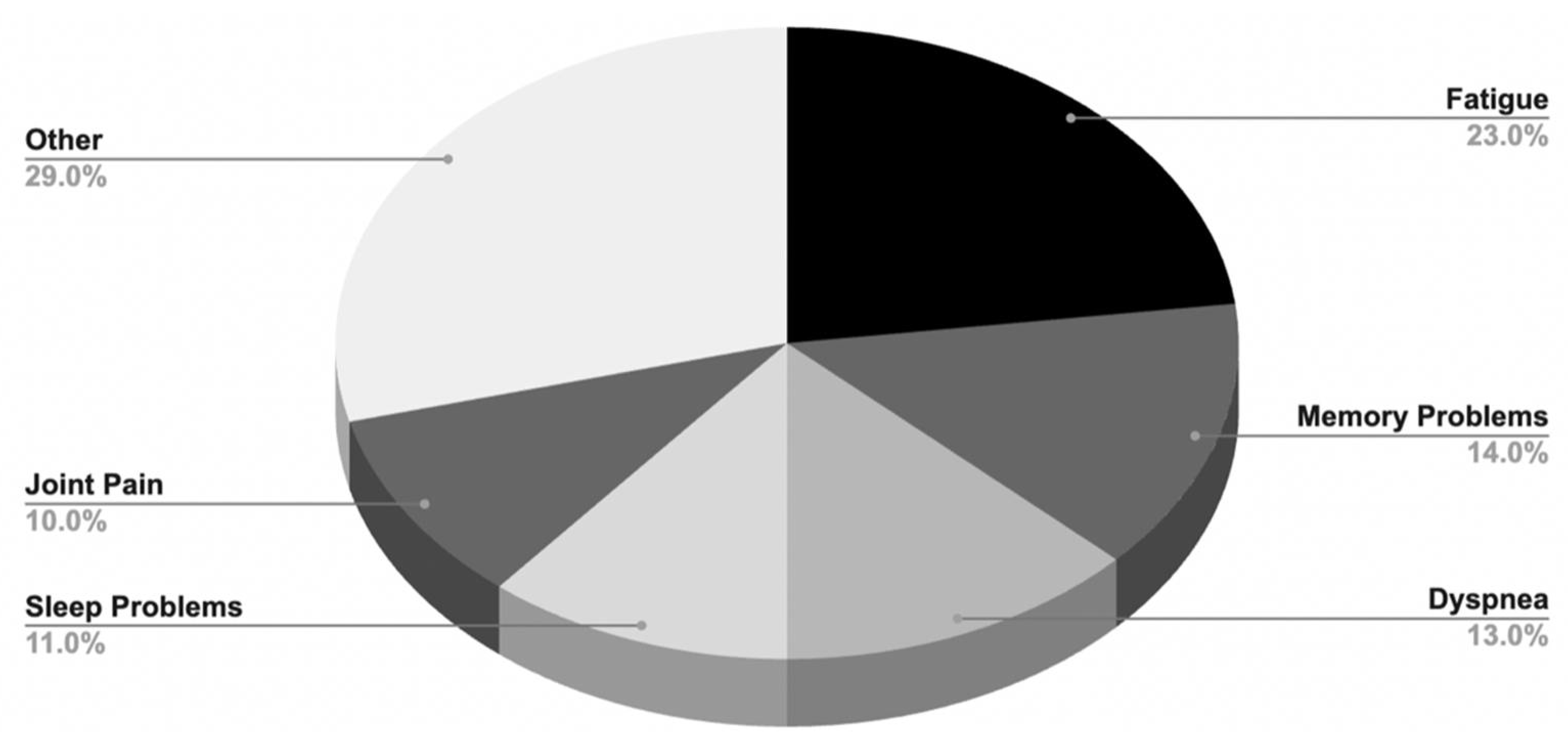
2.1.1. Symptoms and Prevalence
2.1.2. Long COVID Duration
2.2. Demographic Findings
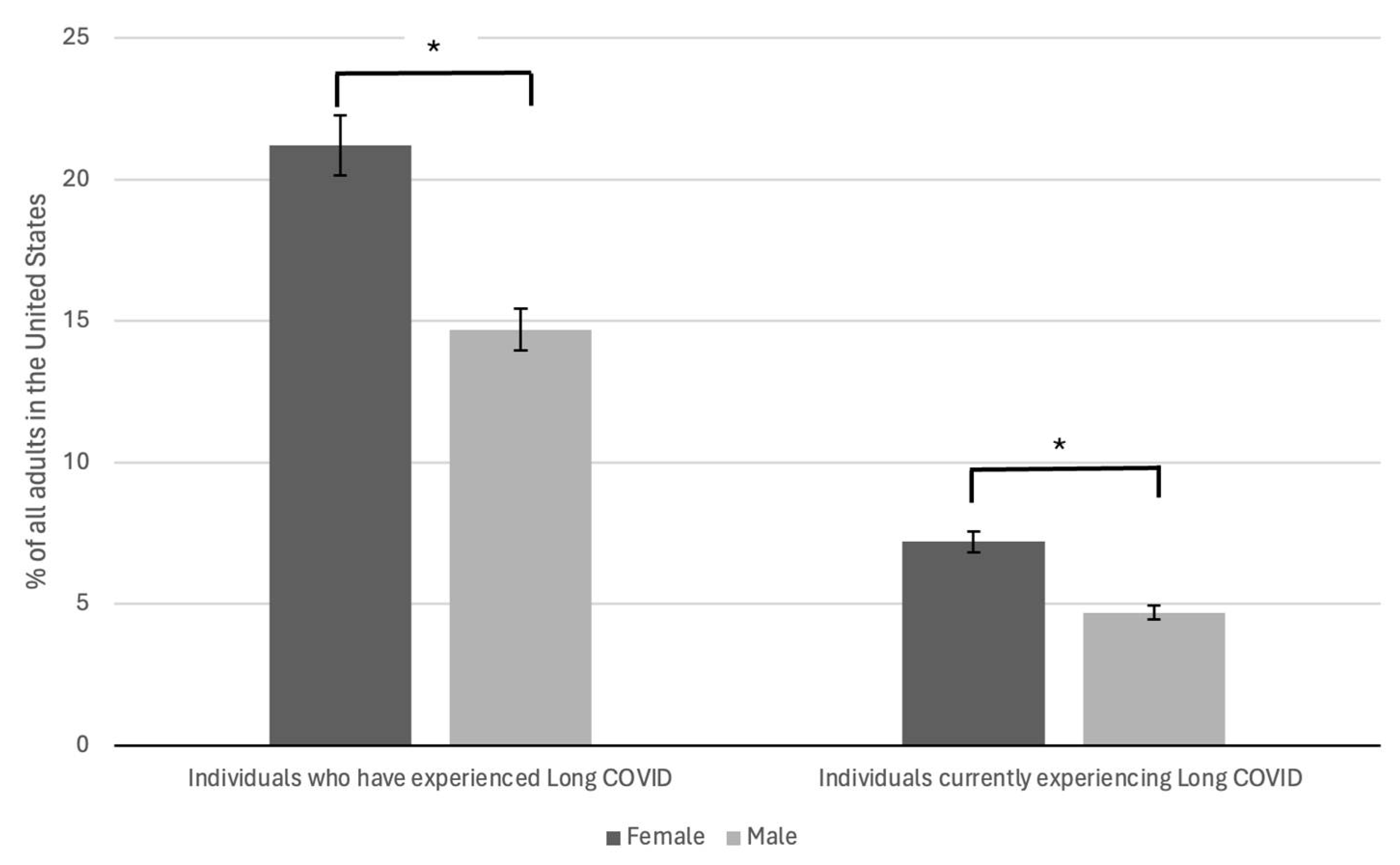
2.2.1. Female Sex
2.2.2. Comorbidities
2.2.3. Age-Related Risk Factors
2.2.4. Socioeconomic Status
2.2.5. Vaccine Status
2.3. Immunological Signature and Biomarkers
2.4. Pathophysiologic Considerations
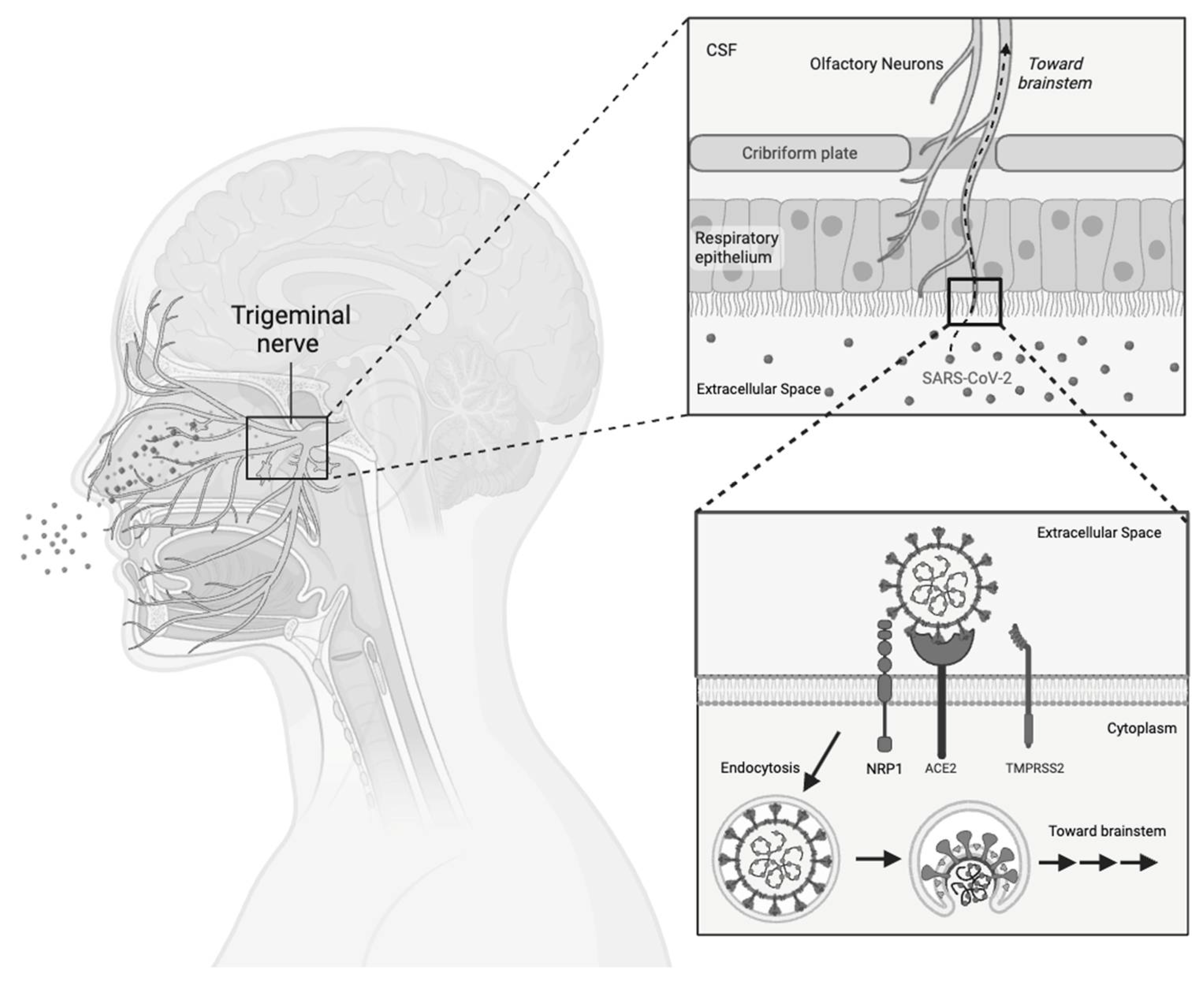
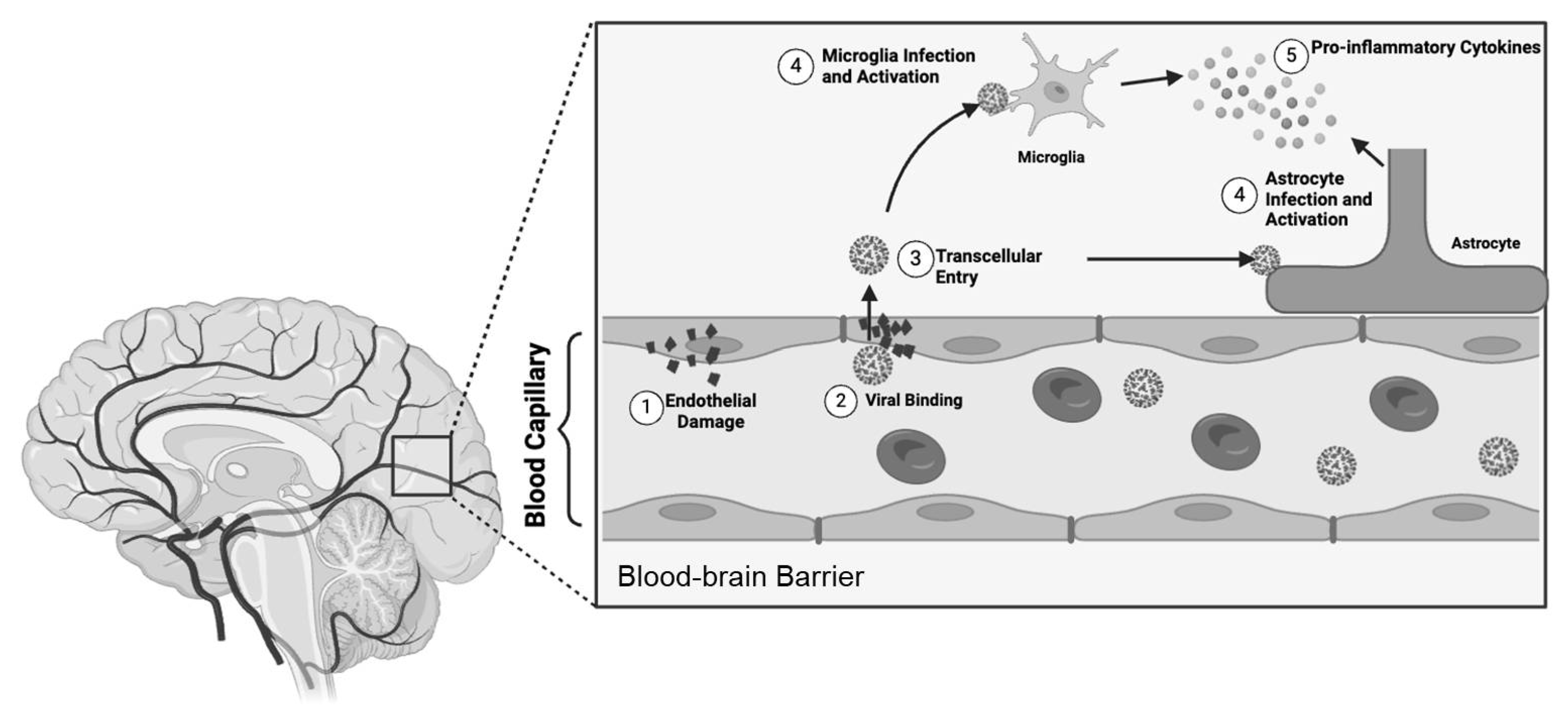
2.4.1. Nervous System
2.4.2. Cardiovascular System
2.4.3. Respiratory System
2.4.4. Gut Microbiome Alterations
2.5. Treatment
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sadeghi Dousari, A.; Taati Moghadam, M.; Satarzadeh, N. COVID-19 (Coronavirus Disease 2019): A New Coronavirus Disease. Infect. Drug Resist. 2020, 13, 2819–2828. [Google Scholar] [CrossRef]
- Yakusheva, O.; van den Broek-Altenburg, E.; Brekke, G.; Atherly, A. Lives saved and lost in the first six month of the US COVID-19 pandemic: A retrospective cost-benefit analysis. PLoS ONE 2022, 17, e0261759. [Google Scholar] [CrossRef]
- Callard, F.; Perego, E. How and why patients made Long COVID. Soc. Sci. Med. 2021, 268, 113426. [Google Scholar] [CrossRef]
- Carfi, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients after Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Ford, N.D.; Slaughter, D.; Edwards, D.; Dalton, A.; Perrine, C.; Vahratian, A.; Saydah, S. Long COVID and Significant Activity Limitation Among Adults, by Age—United States, June 1–13, 2022, to June 7–19, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 866–870. [Google Scholar] [CrossRef]
- Srikanth, S.; Boulos, J.R.; Dover, T.; Boccuto, L.; Dean, D. Identification and diagnosis of long COVID-19: A scoping review. Prog. Biophys. Mol. Biol. 2023, 182, 1–7. [Google Scholar] [CrossRef]
- Morrow, A.; Gray, S.R.; Bayes, H.K.; Sykes, R.; McGarry, E.; Anderson, D.; Boiskin, D.; Burke, C.; Cleland, J.G.F.; Goodyear, C.; et al. Prevention and early treatment of the long-term physical effects of COVID-19 in adults: Design of a randomised controlled trial of resistance exercise-CISCO-21. Trials 2022, 23, 660. [Google Scholar] [CrossRef]
- Barker, K.K.; Whooley, O.; Madden, E.F.; Ahrend, E.E.; Greene, R.N. The long tail of COVID and the tale of long COVID: Diagnostic construction and the management of ignorance. Sociol. Health Illn. 2024, 46 (Suppl. S1), 189–207. [Google Scholar] [CrossRef] [PubMed]
- Munblit, D.; O’Hara, M.E.; Akrami, A.; Perego, E.; Olliaro, P.; Needham, D.M. Long COVID: Aiming for a consensus. Lancet Respir. Med. 2022, 10, 632–634. [Google Scholar] [CrossRef]
- Conrad, P.; Barker, K.K. The social construction of illness: Key insights and policy implications. J. Health Soc. Behav. 2010, 51 (Suppl. S1), S67–S79. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Thaweethai, T.; Jolley, S.E.; Karlson, E.W.; Levitan, E.B.; Levy, B.; McComsey, G.A.; McCorkell, L.; Nadkarni, G.N.; Parthasarathy, S.; Singh, U.; et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA 2023, 329, 1934–1946. [Google Scholar] [CrossRef]
- Yang, J.; Markus, K.; Andersen, K.M.; Rudolph, A.E.; McGrath, L.J.; Nguyen, J.L.; Kyaw, M.H.; Whittle, I.; Blazos, V.; Heron, L.; et al. Definition and measurement of post-COVID-19 conditions in real-world practice: A global systematic literature review. BMJ Open 2024, 14, e077886. [Google Scholar] [CrossRef]
- Hastie, C.E.; Lowe, D.J.; McAuley, A.; Mills, N.L.; Winter, A.J.; Black, C.; Scott, J.T.; O’Donnell, C.A.; Blane, D.N.; Browne, S.; et al. True prevalence of long-COVID in a nationwide, population cohort study. Nat. Commun. 2023, 14, 7892. [Google Scholar] [CrossRef]
- Bosworth, M.; Pawelek, P.; Ayoubkhani, D. Prevalence of Ongoing Symptoms following Coronavirus (COVID-19) Infection in the UK: 2 February 2023. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/2february2023 (accessed on 10 April 2024).
- National Center for Health Statistics. U.S. Census Bureau, H.P.S. 2022–2023. Long COVID. Available online: https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm (accessed on 14 January 2024).
- Pela, G.; Goldoni, M.; Solinas, E.; Cavalli, C.; Tagliaferri, S.; Ranzieri, S.; Frizzelli, A.; Marchi, L.; Mori, P.A.; Majori, M.; et al. Sex-Related Differences in Long-COVID-19 Syndrome. J. Womens Health 2022, 31, 620–630. [Google Scholar] [CrossRef]
- Sykes, D.L.; Van der Feltz-Cornelis, C.M.; Holdsworth, L.; Hart, S.P.; O’Halloran, J.; Holding, S.; Crooks, M.G. Examining the relationship between inflammatory biomarkers during COVID-19 hospitalization and subsequent long-COVID symptoms: A longitudinal and retrospective study. Immun. Inflamm. Dis. 2023, 11, e1052. [Google Scholar] [CrossRef]
- Prevalence of Ongoing Symptoms following Coronavirus (COVID-19) Infection in the UK: 30 March 2023. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/30march2023 (accessed on 10 April 2024).
- Tran, V.T.; Porcher, R.; Pane, I.; Ravaud, P. Course of post COVID-19 disease symptoms over time in the ComPaRe long COVID prospective e-cohort. Nat. Commun. 2022, 13, 1812. [Google Scholar] [CrossRef]
- Pertynska-Marczewska, M.; Pertynski, T. Premenopausal and postmenopausal women during the COVID-19 pandemic. Prz. Menopauzalny 2022, 21, 200–206. [Google Scholar] [CrossRef]
- Teilmann, S.C.; Clement, C.A.; Thorup, J.; Byskov, A.G.; Christensen, S.T. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J. Endocrinol. 2006, 191, 525–535. [Google Scholar] [CrossRef]
- Phiel, K.L.; Henderson, R.A.; Adelman, S.J.; Elloso, M.M. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol. Lett. 2005, 97, 107–113. [Google Scholar] [CrossRef]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Fedotcheva, N.I.; Shimanovsky, N.L. Progesterone as an Anti-Inflammatory Drug and Immunomodulator: New Aspects in Hormonal Regulation of the Inflammation. Biomolecules 2022, 12, 1299. [Google Scholar] [CrossRef]
- Ghandehari, S.; Matusov, Y.; Pepkowitz, S.; Stein, D.; Kaderi, T.; Narayanan, D.; Hwang, J.; Chang, S.; Goodman, R.; Ghandehari, H.; et al. Progesterone in Addition to Standard of Care vs. Standard of Care Alone in the Treatment of Men Hospitalized with Moderate to Severe COVID-19: A Randomized, Controlled Pilot Trial. Chest 2021, 160, 74–84. [Google Scholar] [CrossRef]
- Tramontana, F.; Battisti, S.; Napoli, N.; Strollo, R. Immuno-Endocrinology of COVID-19: The Key Role of Sex Hormones. Front. Endocrinol. 2021, 12, 726696. [Google Scholar] [CrossRef]
- Kalidhindi, R.S.R.; Borkar, N.A.; Ambhore, N.S.; Pabelick, C.M.; Prakash, Y.S.; Sathish, V. Sex steroids skew ACE2 expression in human airway: A contributing factor to sex differences in COVID-19? Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L843–L847. [Google Scholar] [CrossRef]
- Moreno-Perez, O.; Merino, E.; Alfayate, R.; Torregrosa, M.E.; Andres, M.; Leon-Ramirez, J.M.; Boix, V.; Gil, J.; Pico, A. Male pituitary-gonadal axis dysfunction in post-acute COVID-19 syndrome-Prevalence and associated factors: A Mediterranean case series. Clin. Endocrinol. 2022, 96, 353–362. [Google Scholar] [CrossRef]
- Jacobsen, H.; Klein, S.L. Sex Differences in Immunity to Viral Infections. Front. Immunol. 2021, 12, 720952. [Google Scholar] [CrossRef]
- Sharma, C.; Bayry, J. High risk of autoimmune diseases after COVID-19. Nat. Rev. Rheumatol. 2023, 19, 399–400. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.R.; Dhodapkar, R.M.; Lu, P.; Gehlhausen, J.R.; Tabachnikova, A.; Greene, K.; Tabacof, L.; Malik, A.A.; et al. Distinguishing features of long COVID identified through immune profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef]
- Lott, N.; Gebhard, C.E.; Bengs, S.; Haider, A.; Kuster, G.M.; Regitz-Zagrosek, V.; Gebhard, C. Sex hormones in SARS-CoV-2 susceptibility: Key players or confounders? Nat. Rev. Endocrinol. 2023, 19, 217–231. [Google Scholar] [CrossRef]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef]
- Burno, A.M.; Zang, C.; Xu, Z.; Wang, F.; Weiner, M.G.; Guthe, N.; Fitzgerald, M.; Kaushal, R.; Carton, T.W.; Metz, T.D. Association between acquiring SARS-CoV-2 during pregnancy and post-acute sequelae of SARS-CoV-2 infection: RECOVER electronic health record cohort analysis. Lancet 2024, 24, 73. [Google Scholar] [CrossRef]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated with Post-COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef]
- Zadeh, F.H.; Wilson, D.R.; Agrawal, D.K. Long COVID: Complications, Underlying Mechanisms, and Treatment Strategies. Arch. Microbiol. Immunol. 2023, 7, 36–61. [Google Scholar]
- Zheng, Y.B.; Zeng, N.; Yuan, K.; Tian, S.S.; Yang, Y.B.; Gao, N.; Chen, X.; Zhang, A.Y.; Kondratiuk, A.L.; Shi, P.P.; et al. Prevalence and risk factor for long COVID in children and adolescents: A meta-analysis and systematic review. J. Infect. Public Health 2023, 16, 660–672. [Google Scholar] [CrossRef]
- Rao, S.; Gross, R.S.; Mohandas, S.; Stein, C.R.; Case, A.; Dreyer, B.; Pajor, N.M.; Bunnell, H.T.; Warburton, D.; Berg, E.; et al. Postacute Sequelae of SARS-CoV-2 in Children. Pediatrics 2024, 153, e2023062570. [Google Scholar] [CrossRef]
- Fujita-Rohwerder, N.; Beckmann, L.; Zens, Y.; Verma, A. Diagnostic accuracy of rapid point-of-care tests for diagnosis of current SARS-CoV-2 infections in children: A systematic review and meta-analysis. BMJ Evid. Based Med. 2022, 27, 274–287. [Google Scholar] [CrossRef]
- Khafaja, S.; Youssef, N.; El Zein, Z.; Boutros, C.F.; Bou Karroum, S.; Abdel-Halim, N.; Salameh, R.; Hodroj, D.; El Meski, N.; Nasrallah, O.; et al. Multisystem inflammatory syndrome in children (MIS-C) and “Near MIS-C”: A continuum? Front. Pediatr. 2022, 10, 988706. [Google Scholar] [CrossRef]
- Mansell, V.; Hall Dykgraaf, S.; Kidd, M.; Goodyear-Smith, F. Long COVID and older people. Lancet Healthy Longev. 2022, 3, e849–e854. [Google Scholar] [CrossRef]
- Muller, L.; Di Benedetto, S. From aging to long COVID: Exploring the convergence of immunosenescence, inflammaging, and autoimmunity. Front. Immunol. 2023, 14, 1298004. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Al-Aly, Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat. Commun. 2021, 12, 6571. [Google Scholar] [CrossRef]
- Shabnam, S.; Razieh, C.; Dambha-Miller, H.; Yates, T.; Gillies, C.; Chudasama, Y.V.; Pareek, M.; Banerjee, A.; Kawachi, I.; Lacey, B.; et al. Socioeconomic inequalities of Long COVID: A retrospective population-based cohort study in the United Kingdom. J. R. Soc. Med. 2023, 116, 263–273. [Google Scholar] [CrossRef]
- Hill-Briggs, F.; Adler, N.E.; Berkowitz, S.A.; Chin, M.H.; Gary-Webb, T.L.; Navas-Acien, A.; Thornton, P.L.; Haire-Joshu, D. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care 2020, 44, 258–279. [Google Scholar] [CrossRef]
- Descatha, A.; Evanoff, B.A.; Fadel, M. Post-COVID condition or “long COVID”, return-to work, and occupational health research. Scand. J. Work Environ. Health 2023, 49, 165–169. [Google Scholar] [CrossRef]
- Hair, N.L.; Urban, C. Association of Severe COVID-19 and Persistent COVID-19 Symptoms with Economic Hardship among US Families. JAMA Netw. Open 2023, 6, e2347318. [Google Scholar] [CrossRef]
- Nusbaum, N.J. Long COVID, Disability, and the Workplace. South. Med. J. 2023, 116, 718–720. [Google Scholar] [CrossRef]
- Chakraborty, C.; Bhattacharya, M.; Dhama, K. SARS-CoV-2 Vaccines, Vaccine Development Technologies, and Significant Efforts in Vaccine Development during the Pandemic: The Lessons Learned Might Help to Fight against the Next Pandemic. Vaccines 2023, 11, 682. [Google Scholar] [CrossRef]
- MacCallum-Bridges, C.; Hirschtick, J.L.; Patel, A.; Orellana, R.C.; Elliott, M.R.; Fleischer, N.L. The impact of COVID-19 vaccination prior to SARS-CoV-2 infection on prevalence of long COVID among a population-based probability sample of Michiganders, 2020–2022. Ann. Epidemiol. 2024, 92, 17–24. [Google Scholar] [CrossRef]
- Puhach, O.; Adea, K.; Hulo, N.; Sattonnet, P.; Genecand, C.; Iten, A.; Jacquerioz, F.; Kaiser, L.; Vetter, P.; Eckerle, I.; et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat. Med. 2022, 28, 1491–1500. [Google Scholar] [CrossRef]
- Lundberg-Morris, L.; Leach, S.; Xu, Y.; Martikainen, J.; Santosa, A.; Gisslen, M.; Li, H.; Nyberg, F.; Bygdell, M. COVID-19 vaccine effectiveness against post-COVID-19 condition among 589,722 individuals in Sweden: Population based cohort study. BMJ 2023, 383, e076990. [Google Scholar] [CrossRef]
- Catala, M.; Mercade-Besora, N.; Kolde, R.; Trinh, N.T.H.; Roel, E.; Burn, E.; Rathod-Mistry, T.; Kostka, K.; Man, W.Y.; Delmestri, A.; et al. The effectiveness of COVID-19 vaccines to prevent long COVID symptoms: Staggered cohort study of data from the UK, Spain, and Estonia. Lancet Respir. Med. 2024, 12, 225–236. [Google Scholar] [CrossRef]
- Krishna, B.A.; Lim, E.Y.; Metaxaki, M.; Jackson, S.; Mactavous, L.; BioResource, N.; Lyons, P.A.; Doffinger, R.; Bradley, J.R.; Smith, K.G.C.; et al. Spontaneous, persistent, T cell-dependent IFN-gamma release in patients who progress to Long COVID. Sci. Adv. 2024, 10, eadi9379. [Google Scholar] [CrossRef]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef]
- Schultheiss, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1beta, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [CrossRef]
- Wong, A.C.; Devason, A.S.; Umana, I.C.; Cox, T.O.; Dohnalova, L.; Litichevskiy, L.; Perla, J.; Lundgren, P.; Etwebi, Z.; Izzo, L.T.; et al. Serotonin reduction in post-acute sequelae of viral infection. Cell 2023, 186, 4851–4867.e20. [Google Scholar] [CrossRef]
- Chen, B.; Julg, B.; Mohandas, S.; Bradfute, S.B.; RECOVER Mechanistic Pathways Task Force. Viral persistence, reactivation, and mechanisms of long COVID. eLife 2023, 12, e86015. [Google Scholar] [CrossRef]
- Ghafari, M.; Hall, M.; Golubchik, T.; Ayoubkhani, D.; House, T.; MacIntyre-Cockett, G.; Fryer, H.R.; Thomson, L.; Nurtay, A.; Kemp, S.A.; et al. Prevalence of persistent SARS-CoV-2 in a large community surveillance study. Nature 2024, 626, 1094–1101. [Google Scholar] [CrossRef]
- Salman, M.A.; Mallah, S.I.; Khalid, W.; Ryan Moran, L.; Abousedu, Y.A.I.; Jassim, G.A. Characteristics of Patients with SARS-CoV-2 Positive Cerebrospinal Fluid: A Systematic Review. Int. J. Gen. Med. 2021, 14, 10385–10395. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, H.; Lee, S.W.; Kwon, R.; Rhee, S.Y.; Lee, J.A.; Koyanagi, A.; Smith, L.; Fond, G.; Boyer, L.; et al. Long-Term Autoimmune Inflammatory Rheumatic Outcomes of COVID-19: A Binational Cohort Study. Ann. Intern. Med. 2024, 177, 291–302. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Saidara, E.; Maes, M. Persistent SARS-CoV-2 Infection, EBV, HHV-6 and Other Factors May Contribute to Inflammation and Autoimmunity in Long COVID. Viruses 2023, 15, 400. [Google Scholar] [CrossRef]
- Gold, J.E.; Okyay, R.A.; Licht, W.E.; Hurley, D.J. Investigation of Long COVID Prevalence and Its Relationship to Epstein-Barr Virus Reactivation. Pathogens 2021, 10, 763. [Google Scholar] [CrossRef]
- Reiss, A.B.; Greene, C.; Dayaramani, C.; Rauchman, S.H.; Stecker, M.M.; De Leon, J.; Pinkhasov, A. Long COVID, the Brain, Nerves, and Cognitive Function. Neurol. Int. 2023, 15, 821–841. [Google Scholar] [CrossRef]
- Chou, S.H.; Beghi, E.; Helbok, R.; Moro, E.; Sampson, J.; Altamirano, V.; Mainali, S.; Bassetti, C.; Suarez, J.I.; McNett, M.; et al. Global Incidence of Neurological Manifestations Among Patients Hospitalized with COVID-19-A Report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw. Open 2021, 4, e2112131. [Google Scholar] [CrossRef]
- Chen, S.; Wang, S. The immune mechanism of the nasal epithelium in COVID-19-related olfactory dysfunction. Front. Immunol. 2023, 14, 1045009. [Google Scholar] [CrossRef]
- Malik, J.R.; Acharya, A.; Avedissian, S.N.; Byrareddy, S.N.; Fletcher, C.V.; Podany, A.T.; Dyavar, S.R. ACE-2, TMPRSS2, and Neuropilin-1 Receptor Expression on Human Brain Astrocytes and Pericytes and SARS-CoV-2 Infection Kinetics. Int. J. Mol. Sci. 2023, 24, 8622. [Google Scholar] [CrossRef]
- Motta, C.S.; Torices, S.; da Rosa, B.G.; Marcos, A.C.; Alvarez-Rosa, L.; Siqueira, M.; Moreno-Rodriguez, T.; Matos, A.d.R.; Caetano, B.C.; Martins, J.S.C.d.C.; et al. Human Brain Microvascular Endothelial Cells Exposure to SARS-CoV-2 Leads to Inflammatory Activation through NF-kappaB Non-Canonical Pathway and Mitochondrial Remodeling. Viruses 2023, 15, 745. [Google Scholar] [CrossRef]
- Schweitzer, F.; Goereci, Y.; Franke, C.; Silling, S.; Bosl, F.; Maier, F.; Heger, E.; Deiman, B.; Pruss, H.; Onur, O.A.; et al. Cerebrospinal Fluid Analysis Post-COVID-19 Is Not Suggestive of Persistent Central Nervous System Infection. Ann. Neurol. 2022, 91, 150–157. [Google Scholar] [CrossRef]
- Fernandez-Castaneda, A.; Lu, P.; Geraghty, A.C.; Song, E.; Lee, M.H.; Wood, J.; O’Dea, M.R.; Dutton, S.; Shamardani, K.; Nwangwu, K.; et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 2022, 185, 2452–2468.e16. [Google Scholar] [CrossRef]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef]
- Shrestha, A.B.; Mehta, A.; Pokharel, P.; Mishra, A.; Adhikari, L.; Shrestha, S.; Yadav, R.S.; Khanal, S.; Sah, R.; Nowrouzi-Kia, B.; et al. Long COVID Syndrome and Cardiovascular Manifestations: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 491. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, K.O.; Lin, A.; Rodriguez, J.B.C. Cardiac Manifestations of Post-Acute COVID-19 Infection. Curr. Cardiol. Rep. 2022, 24, 1775–1783. [Google Scholar] [CrossRef]
- Writing, C.; Gluckman, T.J.; Bhave, N.M.; Allen, L.A.; Chung, E.H.; Spatz, E.S.; Ammirati, E.; Baggish, A.L.; Bozkurt, B.; Cornwell, W.K., 3rd; et al. 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-Acute Sequelae of SARS-CoV-2 Infection, and Return to Play: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 79, 1717–1756. [Google Scholar]
- Gyongyosi, M.; Alcaide, P.; Asselbergs, F.W.; Brundel, B.; Camici, G.G.; Martins, P.D.C.; Ferdinandy, P.; Fontana, M.; Girao, H.; Gnecchi, M.; et al. Long COVID and the cardiovascular system-elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: A joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial and Pericardial Diseases. Cardiovasc. Res. 2023, 119, 336–356. [Google Scholar] [PubMed]
- Osiaevi, I.; Schulze, A.; Evers, G.; Harmening, K.; Vink, H.; Kumpers, P.; Mohr, M.; Rovas, A. Persistent capillary rarefication in long COVID syndrome. Angiogenesis 2023, 26, 53–61. [Google Scholar] [CrossRef]
- Querfeld, U.; Mak, R.H.; Pries, A.R. Microvascular disease in chronic kidney disease: The base of the iceberg in cardiovascular comorbidity. Clin. Sci. 2020, 134, 1333–1356. [Google Scholar] [CrossRef]
- Ma, L.; Sahu, S.K.; Cano, M.; Kuppuswamy, V.; Bajwa, J.; McPhatter, J.; Pine, A.; Meizlish, M.L.; Goshua, G.; Chang, C.H.; et al. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. Sci. Immunol. 2021, 6, eabh2259. [Google Scholar] [CrossRef]
- El-Rhermoul, F.Z.; Fedorowski, A.; Eardley, P.; Taraborrelli, P.; Panagopoulos, D.; Sutton, R.; Lim, P.B.; Dani, M. Autoimmunity in Long COVID and POTS. Oxf. Open Immunol. 2023, 4, iqad002. [Google Scholar] [CrossRef]
- Marques, K.C.; Quaresma, J.A.S.; Falcao, L.F.M. Cardiovascular autonomic dysfunction in “Long COVID”: Pathophysiology, heart rate variability, and inflammatory markers. Front. Cardiovasc. Med. 2023, 10, 1256512. [Google Scholar] [CrossRef] [PubMed]
- Ormiston, C.K.; Swiatkiewicz, I.; Taub, P.R. Postural orthostatic tachycardia syndrome as a sequela of COVID-19. Heart Rhythm. 2022, 19, 1880–1889. [Google Scholar] [CrossRef]
- Seeley, M.C.; Gallagher, C.; Ong, E.; Langdon, A.; Chieng, J.; Bailey, D.; Page, A.; Lim, H.S.; Lau, D.H. High Incidence of Autonomic Dysfunction and Postural Orthostatic Tachycardia Syndrome in Patients with Long COVID: Implications for Management and Health Care Planning. Am. J. Med. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Daines, L.; Zheng, B.; Pfeffer, P.; Hurst, J.R.; Sheikh, A. A clinical review of long-COVID with a focus on the respiratory system. Curr. Opin. Pulm. Med. 2022, 28, 174–179. [Google Scholar] [CrossRef] [PubMed]
- She, Y.X.; Yu, Q.Y.; Tang, X.X. Role of interleukins in the pathogenesis of pulmonary fibrosis. Cell Death Discov. 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, L.; Wei, R.; Dai, W.; Zeng, R.; Luo, D.; Jiang, R.; Zhuo, Z.; Yang, Q.; Li, J.; et al. Risks of digestive diseases in long COVID: Evidence from a population-based cohort study. BMC Med. 2024, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kuang, D.; Li, D.; Yang, J.; Yan, J.; Xia, Y.; Zhang, F.; Cao, H. Roles of the gut microbiota in severe SARS-CoV-2 infection. Cytokine Growth Factor. Rev. 2022, 63, 98–107. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, G.L.V.; Oliveira, C.N.S.; Pinzan, C.F.; de Salis, L.V.V.; Cardoso, C.R.B. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front. Immunol. 2021, 12, 635471. [Google Scholar] [CrossRef] [PubMed]
- Plummer, A.M.; Matos, Y.L.; Lin, H.C.; Ryman, S.G.; Birg, A.; Quinn, D.K.; Parada, A.N.; Vakhtin, A.A. Gut-brain pathogenesis of post-acute COVID-19 neurocognitive symptoms. Front. Neurosci. 2023, 17, 1232480. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.W. Paxlovid as a potential treatment for long COVID. Expert. Opin. Pharmacother. 2023, 24, 1839–1843. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, K.O. RECOVER-VITAL: Platform Protocol, Appendix to Measure the Effects of Paxlovid on Long COVID Symptoms (RECOVER-VITAL). Available online: https://clinicaltrials.gov/study/NCT05965726 (accessed on 22 June 2024).
- Durstenfeld, M.S.; Peluso, M.J.; Lin, F.; Peyser, N.D.; Isasi, C.; Carton, T.W.; Henrich, T.J.; Deeks, S.G.; Olgin, J.E.; Pletcher, M.J.; et al. Association of nirmatrelvir for acute SARS-CoV-2 infection with subsequent Long COVID symptoms in an observational cohort study. J. Med. Virol. 2024, 96, e29333. [Google Scholar] [CrossRef]
- Congdon, S.; Narrowe, Z.; Yone, N.; Gunn, J.; Deng, Y.; Nori, P.; Cowman, K.; Islam, M.; Rikin, S.; Starrels, J. Nirmatrelvir/ritonavir and risk of long COVID symptoms: A retrospective cohort study. Sci. Rep. 2023, 13, 19688. [Google Scholar] [CrossRef]
- Bramante, C.T.; Buse, J.B.; Liebovitz, D.M.; Nicklas, J.M.; Puskarich, M.A.; Cohen, K.; Belani, H.K.; Anderson, B.J.; Huling, J.D.; Tignanelli, C.J.; et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): A multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect. Dis. 2023, 23, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Isman, A.; Nyquist, A.; Strecker, B.; Harinath, G.; Lee, V.; Zhang, X.; Zalzala, S. Low-dose naltrexone and NAD+ for the treatment of patients with persistent fatigue symptoms after COVID-19. Brain Behav. Immun. Health 2024, 36, 100733. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.E.; Nguyen, T.M.; Segal, D.; MacDonald, J.K.; Chande, N. Low dose naltrexone for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 4, CD010410. [Google Scholar] [CrossRef] [PubMed]
- Sultani, G.; Samsudeen, A.F.; Osborne, B.; Turner, N. NAD(+): A key metabolic regulator with great therapeutic potential. J. Neuroendocrinol. 2017, 29, e12508. [Google Scholar] [CrossRef]
- Lau, R.I.; Su, Q.; Lau, I.S.F.; Ching, J.Y.L.; Wong, M.C.S.; Lau, L.H.S.; Tun, H.M.; Mok, C.K.P.; Chau, S.W.H.; Tse, Y.K.; et al. A synbiotic preparation (SIM01) for post-acute COVID-19 syndrome in Hong Kong (RECOVERY): A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2024, 24, 256–265. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donald, J.; Bilasy, S.E.; Yang, C.; El-Shamy, A. Exploring the Complexities of Long COVID. Viruses 2024, 16, 1060. https://doi.org/10.3390/v16071060
Donald J, Bilasy SE, Yang C, El-Shamy A. Exploring the Complexities of Long COVID. Viruses. 2024; 16(7):1060. https://doi.org/10.3390/v16071060
Chicago/Turabian StyleDonald, Jackson, Shymaa E. Bilasy, Catherine Yang, and Ahmed El-Shamy. 2024. "Exploring the Complexities of Long COVID" Viruses 16, no. 7: 1060. https://doi.org/10.3390/v16071060
APA StyleDonald, J., Bilasy, S. E., Yang, C., & El-Shamy, A. (2024). Exploring the Complexities of Long COVID. Viruses, 16(7), 1060. https://doi.org/10.3390/v16071060





