IgA Anti-β2-Glycoprotein I Antibodies as Markers of Thrombosis and Severity in COVID-19 Patients
Abstract
:1. Introduction
2. Patients and Methods
- Demographic variables: cardiovascular risk factors, cardiovascular disease, thrombophilia, malignancy, connective tissue disease, lung or kidney diseases, pregnancy, or puerperium and treatment before admission;
- Clinical features: fever, cough, dyspnoea, diarrhoea, anosmia and ageusia;
- Routine laboratory tests: complete blood cell count, coagulation test, D-dimer, creatinine, lactate dehydrogenase, C-reactive protein, ferritin and interleukin-6 levels;
- Severity scales: Brescia-COVID Respiratory Severity Scale, CURB-65 and neutrophil-to-lymphocyte ratio (NLR);
- PaO2/FiO2 (arterial oxygen partial pressure (mmHg) to fractional inspired oxygen ratio) and SO2/FiO2 (oxygen saturation to fractional inspired oxygen ratio);
- Percentage of chest affected according to X-ray;
- Complications during hospital stay: deep vein thrombosis (DVT), pulmonary embolism (PE), other thromboses and death;
- Treatment during hospital stay: corticosteroids, corticosteroid pulses, anakinra, tocilizumab and low-molecular-weight heparin (LMWH);
- Admission to ICU.
2.1. Antiphospholipid Antibodies Quantification
2.2. Statistical Analysis
3. Results
3.1. Presence of Antiphospholipid Antibodies
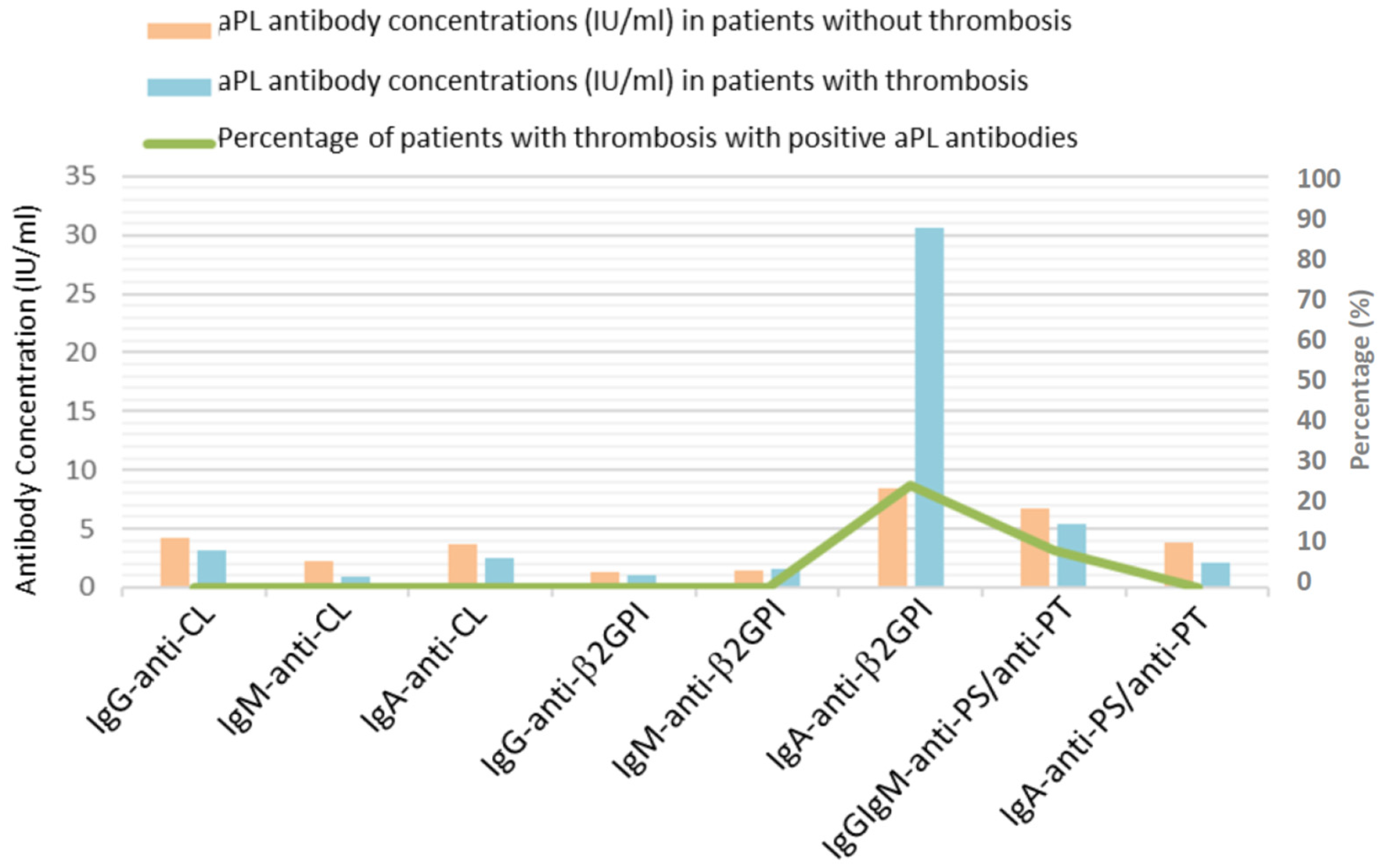
3.2. Antiphospholipid Antibodies, Thrombosis and Coagulation Test in Patients with COVID-19
3.3. Antiphopholipid Antibodies and Severity of COVID-19
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, M.A.; Spinler, S.A. COVID-19 and thrombosis: From bench to bedside. Trends Cardiovasc. Med. 2021, 31, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020, 55, 2000607. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Mendes, M.C.; Martins, A.P.C.; Borges, N.H.; Godoy, T.M.; Dos Santos Miggiolaro, A.F.R.; Dos Santos Dezidério, F.; Machado-Souza, C.; De Noronha, L. Endothelial Dysfunction and Thrombosis in Patients With COVID-19—Brief Report. Arter. Thromb. Vasc. Biol. 2020, 40, 2404–2407. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.; Palomo, M.; Moreno-Castaño, A.B.; Fernández, S.; Torramadé-Moix, S.; Pascual, G.; Martinez-Sanchez, J.; Richardson, E.; Téllez, A.; Nicolas, J.M.; et al. Is the Endothelium the Missing Link in the Pathophysiology and Treatment of COVID-19 Complications? Cardiovasc. Drugs Ther. 2022, 36, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Barbhaiya, M.; Zuily, S.; Naden, R.; Hendry, A.; Manneville, F.; Amigo, M.; Amoura, Z.; Andrade, D.; Andreoli, L.; Artim-Esen, B.; et al. The 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria. Arthritis Rheumatol. 2023, 75, 1687–1702. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Pinto, C.; García-Carrasco, M.; Cervera, R. Role of Infectious Diseases in the Antiphospholipid Syndrome (Including Its Catastrophic Variant). Curr. Rheumatol. Rep. 2018, 20, 62. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Talathi, S.; Lopez-Olivo, M.A.; Suarez-Almazor, M.E. Risk of developing antiphospholipid antibodies following viral infection: A systematic review and meta-analysis. Lupus 2018, 27, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Blank, M.; Asherson, R.A.; Cervera, R.; Shoenfeld, Y. Antiphospholipid syndrome infectious origin. J. Clin. Immunol. 2004, 24, 12–23. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef]
- Martínez-Urbistondo, M.; Gutiérrez-Rojas, A.; Andrés, A.; Gutiérrez, I.; Escudero, G.; García, S.; Gutiérrez, A.; Sánchez, E.; Herráiz, J.; De La Fuente, S.; et al. Severe lymphopenia as a predictor of COVID-19 mortality in immunosuppressed patients. J. Clin. Med. 2021, 10, 3595. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Kim, G.H.J.; Park, S.-B.; Lee, S.-I.; Koh, J.S.; Brown, M.S.; Abtin, F.; McNitt-Gray, M.F.; Goldin, J.G.; Lee, J.S. Quantitative Computed Tomography Lung COVID Scores with Laboratory Markers: Utilization to Predict Rapid Progression and Monitor Longitudinal Changes in Patients with Coronavirus 2019 (COVID-19) Pneumonia. Biomedicines 2024, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Gatto, M.; Perricone, C.; Tonello, M.; Bistoni, O.; Cattelan, A.M.; Bursi, R.; Cafaro, G.; De Robertis, E.; Mencacci, A.; Bozza, S.; et al. Frequency and clinical correlates of antiphospholipid antibodies arising in patients with SARS-CoV-2 infection: Findings from a multicentre study on 122 cases. Clin. Exp. Rheumatol. 2020, 38, 754–759. [Google Scholar] [PubMed]
- Pineton de Chambrun, M.; Frere, C.; Miyara, M.; Amoura, Z.; Martin-Toutain, I.; Mathian, A.A.; Hekimian, G.; Combes, A. High frequency of antiphospholipid antibodies in critically ill COVID-19 patients: A link with hypercoagulability? J. Intern. Med. 2021, 289, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Devreese, K.; Linskens, E.A.; Benoit, D.; Peperstraete, H. Antiphospholipid antibodies in patients with COVID-19: A relevant observation? J. Thromb. Haemost. 2020, 18, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Siguret, V.; Voicu, S.; Neuwirth, M.; Delrue, M.; Gayat, E.; Stépanian, A.; Mégarbane, B. Are antiphospholipid antibodies associated with thrombotic complications in critically ill COVID-19 patients? Thromb. Res. 2020, 195, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Borghi, M.O.; Beltagy, A.; Garrafa, E.; Curreli, D.; Cecchini, G.; Bodio, C.; Grossi, C.; Blengino, S.; Tincani, A.; Franceschini, F.; et al. Anti-Phospholipid Antibodies in COVID-19 are different from those detectable in the anti-Phospholipid syndrome. Front. Immunol. 2020, 11, 584241. [Google Scholar] [CrossRef]
- Harzallah, I.; Debliquis, A.; Drénou, B. Lupus anticoagulant is frequent in patients with Covid-19. J. Thromb. Haemost. 2020, 18, 2064–2065. [Google Scholar] [CrossRef] [PubMed]
- Gendron, N.; Dragon-Durey, M.A.; Chocron, R.; Darnige, L.; Jourdi, G.; Philippe, A.; Chenevier-Gobeaux, C.; Hadjadj, J.; Duchemin, J.; Khider, L.; et al. Lupus anticoagulant single positivity during the acute phase of COVID-19 is not associated with venous thromboembolism or in-hospital mortality. Arthritis Rheumatol. 2021, 73, 1976–1985. [Google Scholar] [CrossRef]
- Taha, M.; Samavati, L. Antiphospholipid antibodies in COVID-19: A meta-analysis and systematic review. RMD Open 2021, 7, e001580. [Google Scholar] [CrossRef]
- Kanduc, D.; Shoenfeld, Y. On the molecular determinants of the SARS-CoV-2 attack. Clin. Immunol. 2020, 215, 108426. [Google Scholar] [CrossRef]
- Hasan Ali, O.; Bomze, D.; Risch, L.; Brugger, S.D.; Paprotny, M.; Weber, M.; Thiel, S.; Kern, L.; Albrich, W.C.; Kohler, P.; et al. Severe COVID-19 is associated with elevated serum IgA and antiphospholipid IgA-antibodies. Clin. Infect. Dis. 2021, 73, e2869–e2874. [Google Scholar] [CrossRef]
- Vila, P.; Hernandez, M.C.; Lopez-Fernandez, M.F.; Batlle, J. Prevalence, follow-up and clinical significance of the anticardiolipin antibodies in normal subjects. Thromb. Haemost. 1994, 72, 209–213. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, Y.; Zhang, S.; Qin, X.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; et al. Antiphospholipid Antibodies in Critically Ill Patients With COVID-19. Arthritis Rheumatol. 2020, 72, 1998–2004. [Google Scholar] [CrossRef]
- Melayah, S.; Omrani, N.; Alouini, H.; Ghozzi, M.; Mrad, S.; Boussarsar, M.; Chaouch, H.; Hachfi, W.; Letaief, A.; Mankaï, A.; et al. IgA is the predominant isotype of anti-β2 glycoprotein I in patients with COVID-19. Lab. Med. 2024, 55, 373–379. [Google Scholar] [CrossRef]
- Serrano, M.; Espinosa, G.; Lalueza, A.; Bravo-Gallego, L.Y.; Diaz-Simón, R.; Bs, S.G.; Bs, J.G.; Moises, J.; Bs, L.N.; Prieto-González, S.; et al. Beta-2-glycoprotein-i deficiency could precipitate an antiphospholipid syndrome-like prothrombotic situation in patients with coronavirus disease 2019. ACR Open Rheumatol. 2021, 3, 267–276. [Google Scholar] [CrossRef]
- Zervou, F.N.; Louie, P.; Stachel, A.; Zacharioudakis, I.M.; Ortiz-Mendez, Y.; Thomas, K.; Aguero-Rosenfeld, M.E. SARS-CoV-2 antibodies: IgA correlates with severity of disease in early COVID-19 infection. J. Med. Virol. 2021, 93, 5409–5415. [Google Scholar]
- Zuo, Y.; Estes, S.K.; Ali, R.A.; Gandhi, A.A.; Yalavarthi, S.; Shi, H.; Sule, G.; Gockman, K.; Madison, J.A.; Zuo, M.; et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020, 12, eabd3876. [Google Scholar] [CrossRef]
- Amezcua-Guerra, L.M.; Rojas-Velasco, G.; Brianza-Padilla, M.; Vázquez-Rangel, A.; Márquez-Velasco, R.; Baranda-Tovar, F.; Springall, R.; Gonzalez-Pacheco, H.; Juárez-Vicuña, Y.; Tavera-Alonso, C.; et al. Presence of antiphospholipid antibodies in COVID-19: A case series study. Ann. Rheum. Dis. 2021, 80, e73. [Google Scholar] [CrossRef]
- Pengo, V.; Tripodi, A.; Reber, G.; Rand, J.H.; Ortel, T.L.; Galli, M.; De Groot, P.G. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J. Thromb. Haemost. 2009, 7, 1737–1740. [Google Scholar] [CrossRef]
- Platton, S.; Bowles, L.; Pasi, K.J. Lupus Anticoagulant in Patients with Covid-19. Reply. N. Engl. J. Med. 2020, 383, 1893–1894. [Google Scholar]
- Tortosa, C.; Cabrera-Marante, O.; Serrano, M.; Martínez-Flores, J.A.; Pérez, D.; Lora, D.; Morillas, L.; Paz-Artal, E.; Morales, J.M.; Pleguezuelo, D.; et al. Incidence of thromboembolic events in asymptomatic carriers of IgA anti ß2 glycoprotein-I antibodies. PLoS ONE 2017, 12, e0178889. [Google Scholar] [CrossRef]
- Sweiss, N.J.; Bo, R.; Kapadia, R.; Manst, D.; Mahmood, F.; Adhikari, T.; Volkov, S.; Badaracco, M.; Smaron, M.; Chang, A.; et al. IgA Anti-β2-Glycoprotein I Autoantibodies Are Associated with an Increased Risk of Thromboembolic Events in Patients with Systemic Lupus Erythematosus. PLoS ONE 2010, 5, e12280. [Google Scholar] [CrossRef]
- Cabrera-Marante, O.; de Frías, E.R.; Serrano, M.; Morillo, F.L.; Naranjo, L.; Gil-Etayo, F.J.; Paz-Artal, E.; Pleguezuelo, D.E.; Serrano, A. The Weight of IgA Anti-β2glycoprotein I in the Antiphospholipid Syndrome Pathogenesis: Closing the Gap of Seronegative Antiphospholipid Syndrome. Int. J. Mol. Sci. 2020, 21, 8972. [Google Scholar] [CrossRef] [PubMed]
- Lakos, G.; Favaloro, E.J.; Harris, E.N.; Meroni, P.L.; Tincani, A.; Wong, R.C.; Pierangeli, S.S. International consensus guidelines on anticardiolipin and anti-beta2-glycoprotein I testing: Report from the 13th International Congress on Antiphospholipid Antibodies. Arthritis Rheum. 2012, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Le Joncour, A.; Frere, C.; Martin-Toutain, I.; Gougis, P.; Ghillani-Dalbin, P.; Maalouf, G.; Vieira, M.; Marcelin, A.-G.; Salem, J.-E.; Allenbach, Y.; et al. Antiphospholipid antibodies and thrombotic events in COVID-19 patients hospitalized in medicine ward. Autoimmun. Rev. 2021, 20, 102729. [Google Scholar] [CrossRef]
- Garcia-Arellano, G.; Camacho-Ortiz, A.; Moreno-Arquieta, I.A.; la Garza, J.A.C.-D.; Rubio-Torres, D.C.; Garza-Gonzalez, E.; Bocanegra-Ibarias, P.; Galarza-Delgado, D.A. Anticardiolipin and anti-beta-2 glycoprotein I antibodies in patients with moderate or severe COVID-19. Am. J. Med. Sci. 2023, 365, 215–217. [Google Scholar] [CrossRef]
- de Ocáriz, X.G.L.; Quismondo, N.C.; Guerrero, E.V.; Rodríguez, M.R.; Díaz, R.A.; López, J.M. Thrombosis and antiphospholipid antibodies in patients with SARS-COV-2 infection (COVID-19). Int. J. Lab. Hematol. 2020, 42, e280–e282. [Google Scholar]


| General data | |||
| Men | 74.2% (n = 118) | Weight (kg) | 84.0 ± 15.8 |
| Women | 25.8% (n = 41) | Height (cm) | 168.8 ± 10.6 |
| Age (years) | 63.1 ± 12.3 | BMI (kg/cm2) | 29.4 ± 4.7 |
| Comorbidities | |||
| Hypertension | 41.5% (n = 66) | Cardiovascular disease | 16.5% (n = 26) |
| Diabetes | 19.5% (n = 31) | Cerebrovascular disease | 5.7% (n = 9) |
| Dyslipidemia | 28.9% (n = 46) | Connective tissue diseases | 2.5% (n = 4) |
| Metabolic syndrome | 11.3% (n = 18) | Lung disease | 18.9% (n = 30) |
| Thrombophilia | 0.6% (n = 1) | Kidney disease | 5.0% (n = 8) |
| Smokers | 17.0% (n = 27) | Immunosuppression | 8.8% (n = 14) |
| Neoplasia | 15.7% (n = 25) | Pregnant/Puerperal women | 0.0% (n = 0) |
| Treatment prior to admission | |||
| ASA/Clopidogrel | 14.5% (n = 23) | Hydroxychloroquine | 0.6% (n = 1) |
| Anticoagulation | 8.2% (n = 13) | Contraception | 0.0% (n = 0) |
| Clinical features | |||
| Fever | 95.0% (n = 151) | Diarrhoea | 22.6% (n = 36) |
| Cough | 74.8% (n = 119) | Anosmia | 8.8% (n = 14) |
| Dyspnea | 61.6% (n = 98) | Ageusia | 8.8% (n = 14) |
| Analytical values | |||
| PaO2/FiO2 | 191.9 ± 102.2 | PT (s) | 17.0 ± 9.6 |
| SO2/FiO2 | 269.9 ± 121.9 | APTT (s) | 47.3 ± 24 |
| LDH (U/L) | 443.9 ± 207.3 | Fibrinogen (mg/dl) | 406.4 ± 183.8 |
| Ferritin (ng/mL) | 1348.8 ± 1417.7 | Lymphocytes (/μL) | 936.5 ± 1441.2 |
| D-dimer (μg/mL) | 4.1 ± 10.3 | IL-6 (pg/mL) | 206.6 ± 493.3 |
| Platelets (103/μL) | 195.1 ± 99.9 | C-Reactive Protein (mg/L) | 155.0 ± 79.6 |
| NLR | 17.6 ± 58.8 | ||
| Percentage of chest affected according to X-ray | |||
| 75% | 28.9% (n = 46) | 50% | 52.2% (n = 83) |
| 25% | 18.9% (n = 30) | ||
| Thrombotic complications during hospital stay | |||
| DVT | 3.1% (n = 5) | Other thrombosis | 1.3% (n = 2) |
| PE | 4.4% (n = 7) | TEE in total | 7.5% (n = 12) |
| Treatments during hospital stay | |||
| Corticosteroids | 74.8% (n = 119) | Anakinra | 1.9 (n = 3) |
| Corticosteroid boluses | 38.4% (n = 61) | LMWH | 84.2 (n = 134) |
| Tocilizumab | 39.6% (n = 63) | ||
| Evolution | |||
| Death | 6.9% (n = 11) | Readmission | 8.8% (n = 14) |
| ICU | 6.9% (n = 11) | ||
| Presence of aPL | Concentration of aPL (IU/mL) | ||||||
| Positive aPL | COVID-19 | Healthy Donors | aPL | COVID-19 | Healthy Donors | ||
| anti-CL | IgG | 3.8% (n = 6) | 1.3% (n = 1) | anti-CL | IgG | 4.1 ± 4.4 | 2.3 ± 4.7 |
| IgM | 2.5% (n = 4) | 2.5% (n = 2) | IgM | 2.1 ± 3.4 | 2.7 ± 4.2 | ||
| IgA | 0.6% (n = 1) | 0.0% (n = 0) | IgA | 3.6 ± 3.2 | 3.6 ± 2.1 | ||
| IgG/IgA | 0.6% (n = 1) | 0.0% (n = 0) | |||||
| Total | 7.6% (n = 12) | 3.8% (n = 3) | |||||
| anti-β2GPI | IgG | 0.0% (n = 0) | 1.3% (n = 1) | anti-β2GPI | IgG | 1.2 ± 0.9 | 3.3 ± 4.5 |
| IgM | 1.3% (n = 2) | 3.8% (n = 3) | IgM | 1.5 ± 1.9 | 6.7 ± 20.2 | ||
| IgA | 6.3% (n = 10) | 0.0% (n = 0) | IgA | 10.2 ± 45.3 | 1.8 ± 1.4 | ||
| Total | 7.6% (n = 12) | 5.1% (n = 4) | |||||
| anti-PS/anti-PT | IgG/IgM | 3.8% (n = 6) | 12.5% (n = 10) | anti-PS/anti-PT | IgG/IgM | 6.6 ± 4.4 | 5.2 ± 6.1 |
| IgA | 3.8% (n = 6) | 0.0% (n = 0) | IgA | 3.7 ± 4.2 | 0.7 ± 0.5 | ||
| IgG/IgM/IgA | 0.6% (n = 1) | 0.0% (n = 0) | |||||
| Total | 8.2% (n = 13) | 12.5% (n = 10) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mellor-Pita, S.; Tutor-Ureta, P.; Velasco, P.; Plaza, A.; Diego, I.; Vázquez-Comendador, J.; Vionnet, A.P.; Durán-del Campo, P.; Moreno-Torres, V.; Vargas, J.A.; et al. IgA Anti-β2-Glycoprotein I Antibodies as Markers of Thrombosis and Severity in COVID-19 Patients. Viruses 2024, 16, 1071. https://doi.org/10.3390/v16071071
Mellor-Pita S, Tutor-Ureta P, Velasco P, Plaza A, Diego I, Vázquez-Comendador J, Vionnet AP, Durán-del Campo P, Moreno-Torres V, Vargas JA, et al. IgA Anti-β2-Glycoprotein I Antibodies as Markers of Thrombosis and Severity in COVID-19 Patients. Viruses. 2024; 16(7):1071. https://doi.org/10.3390/v16071071
Chicago/Turabian StyleMellor-Pita, Susana, Pablo Tutor-Ureta, Paula Velasco, Aresio Plaza, Itziar Diego, José Vázquez-Comendador, Ana Paula Vionnet, Pedro Durán-del Campo, Víctor Moreno-Torres, Juan Antonio Vargas, and et al. 2024. "IgA Anti-β2-Glycoprotein I Antibodies as Markers of Thrombosis and Severity in COVID-19 Patients" Viruses 16, no. 7: 1071. https://doi.org/10.3390/v16071071
APA StyleMellor-Pita, S., Tutor-Ureta, P., Velasco, P., Plaza, A., Diego, I., Vázquez-Comendador, J., Vionnet, A. P., Durán-del Campo, P., Moreno-Torres, V., Vargas, J. A., & Castejon, R. (2024). IgA Anti-β2-Glycoprotein I Antibodies as Markers of Thrombosis and Severity in COVID-19 Patients. Viruses, 16(7), 1071. https://doi.org/10.3390/v16071071






