SARS-CoV-2 Variants and COVID-19 in Bangladesh—Lessons Learned
Abstract
:1. Introduction
2. The Will Power for Vaccination
3. Human Behavior in the COVID-19 Pandemic
4. The SARS-CoV-2 Curriculum Vitae
5. SARS-CoV-2 Variants Evolved Mechanisms of Host Cell Entry and Immune Evasion That Led to Superior Fitness over the Original Strain
6. COVID-19 in Bangladesh—A Timeline of Infection, Morbidity, Deaths and Vaccination Coverage
7. SARS-CoV-2 Variants in Bangladesh—Life-Threatening Menace or Mild Problem?
8. Challenges and Lessons
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/data (accessed on 10 March 2023).
- Bangladesh Directorate General of Health Services, Ministry of Health and Family Welfare. Coronavirus COVID-19 Dashboard. Available online: http://103.247.238.92/webportal/pages/covid19.php (accessed on 1 June 2023).
- Cegolon, L.; Magnano, G.; Negro, C.; Filon, F.L.; on behalf of the ORCHESTRA Working Group. SARS-CoV-2 Reinfections in Health-Care Workers, 1 March 2020–31 January 2023. Viruses 2023, 15, 1551. [Google Scholar] [CrossRef] [PubMed]
- Cameron-Blake, E.; Tatlow, H.; Andretti, B.; Boby, T.; Green, K.; Hale, T.; Petherick, A.; Phillips, T.; Pott, A.; Wade, A.; et al. A panel dataset of COVID-19 vaccination policies in 185 countries. Nat. Hum. Behav. 2023, 7, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: A prospective, longitudinal, cohort study. Lancet Infect. Dis. 2022, 22, 183–195. [Google Scholar] [CrossRef]
- Woodridge, Y.; Amit, S.; Huppert, A.; Kopelman, M. Viral load dynamics of SARS-CoV-2 Delta and Omicron variants following multiple vaccine doses and previous infections. Nat. Commun. 2022, 13, 6706–6711. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Wyka, K.; White, T.M.; Picchio, C.A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Ratzan, S.C.; Kamarulzaman, A.; El-Mohandes, A. A survey of COVID-19 vaccine acceptance across 23 countries in 2022. Nat. Med. 2022, 29, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J. Widespread Public Misunderstanding of Pivotal Trials for COVID-19 Vaccines May Damage Public Confidence in All Vaccines. Frontiers 2022, 10, 847658. [Google Scholar] [CrossRef]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’byrne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Jalkanen, P.; Kolehmainen, P.; Häkkinen, H.K.; Huttunen, M.; Tähtinen, P.A.; Lundberg, R.; Maljanen, S.; Reinholm, A.; Tauriainen, S.; Pakkanen, S.H.; et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat. Commun. 2021, 12, 3991. [Google Scholar] [CrossRef] [PubMed]
- Mahla, R.S.; Dustin, L.B. Searching for escape-resistant anti–SARS-CoV-2 neutralizing antibodies. J. Clin. Investig. 2022, 132, e157416. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; COVID-19 Genomics UK Consortium; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef]
- Townsend, J.P.; Hassler, H.B.; Sah, P.; Galvani, A.P.; Dornburg, A. The durability of natural infection and vaccine-induced immunity against future infection by SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2022, 119, e2204336119. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. SARS-CoV-2 T Cell Responses Elicited by COVID-19 Vaccines or Infection Are Expected to Remain Robust against Omicron. Viruses 2022, 14, 79. [Google Scholar] [CrossRef]
- Johnson, A.G.; Amin, A.B.; Ali, A.R.; Hoots, B.; Cadwell, B.L.; Arora, S.; Avoundjian, T.; Awofeso, A.O.; Barnes, J.; Bayoumi, N.S.; et al. COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence—25 U.S. Jurisdictions, 4 April–25 December 2021. MMWR Morb. Mortal Wkly. Rep. 2022, 71, 132–138. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by COVID-19 Vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef]
- Kirsebom, F.C.M.; Andrews, N.; Stowe, J.; Toffa, S.; Sachdeva, R.; Gallagher, E.; Groves, N.; O’Connell, A.-M.; Chand, M.; Ramsay, M.; et al. COVID-19 vaccine effectiveness against the omicron (BA.2) variant in England. Lancet Infect. Dis. 2022, 22, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Solante, R.; Alvarez-Moreno, C.; Burhan, E.; Chariyalertsak, S.; Chiu, N.-C.; Chuenkitmongkol, S.; Dung, D.V.; Hwang, K.-P.; Ibarra, J.O.; Kiertiburanakul, S.; et al. Expert review of global real-world data on COVID-19 vaccine booster effectiveness and safety during the omicron-dominant phase of the pandemic. Expert Rev. Vaccines 2023, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, N.E.; Eskola, J.; Liang, X.; Chaudhuri, M.; Dube, E.; Gellin, B.; Goldstein, S.; Larson, H.; Manzo, M.L.; Reingold, A.; et al. Vaccine Hesitancy: Definition, Scope and Determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Lamuda, P.A.; Azar, A.; Taylor, B.G.; Balawajder, E.F.; Pollack, H.A.; Schneider, J.A. Latent class analysis of medical mistrust and COVID-19 vaccine hesitancy among adults in the United States just prior to FDA emergency use authorization. Vaccine 2023, 41, 2671–2679. [Google Scholar] [CrossRef]
- Steinert, J.I.; Sternberg, H.; Prince, H.; Fasolo, B.; Galizzi, M.M.; Büthe, T.; Veltri, G.A. COVID-19 vaccine hesitancy in eight European countries: Prevalence, determinants, and heterogeneity. Sci. Adv. 2022, 8, eabm9825. [Google Scholar] [CrossRef]
- WHO Vaccine Tracker and Landscape. Available online: https://www.who.int/teams/blueprint/covid-19/covid-19-vaccine-tracker-and-landscape (accessed on 30 March 2023).
- New York Times Tracking Coronavirus Vaccinations around the World. Available online: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html (accessed on 13 March 2023).
- Surokkha. Available online: https://play.google.com/store/apps/details?id=com.codersbucket.surokkha_app&hl=en (accessed on 1 June 2023).
- Ali, M.; Hossain, A. What is the extent of COVID-19 vaccine hesitancy in Bangladesh? A cross-sectional rapid national survey. Br. Med. J. 2021, 11, e050303. [Google Scholar] [CrossRef]
- Mahmud-Al-Rafat, A.; Hewins, B.; Mannan, A.; Kelvin, D.J.; Billah, M. COVID-19 vaccine inequity, dependency, and production capability in low-income and middle-income countries: The case of Bangladesh. Lancet Infect. Dis. 2022, 22, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Hasan, M.; Nasreen, W.; Tushar, I.; Bhuiyan, M.A. The COVID-19 vaccination experience in Bangladesh: Findings from a cross-sectional study. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211065628. [Google Scholar] [CrossRef] [PubMed]
- Nazmunnahar, N.; Ahamed, B.; Haque, A.; Tanbir; Roknuzzaman, A.S.M.; Sarker, R.; Islam, R. COVID-19 vaccination success in Bangladesh: Key strategies were prompt response, early drives for vaccines, and effective awareness campaigns. Health Sci. Rep. 2023, 6, e1281. [Google Scholar] [CrossRef] [PubMed]
- Sarker, N.M.; Ghosh, S. A Study on the Key Elements of Bangladesh’s Vaccine Diplomacy During COVID-19. J. Asian Afr. Stud. 2023, 00219096231179659. [Google Scholar] [CrossRef]
- Bento, A.I.; Nguyen, T.; Wing, C.; Lozano-Rojas, F.; Ahn, Y.; Simon, K. Evidence from internet search data shows information-seeking responses to news of local COVID-19 cases. Proc. Natl. Acad. Sci. USA 2020, 117, 11220–11222. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, M.; Kronbichler, A.; Shin, J.I.; Mayer, G.; Tilg, H.; Perco, P. Association of the COVID-19 pandemic with Internet Search Volumes: A Google TrendsTM Analysis. Int. J. Infect. Dis. 2020, 95, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Bromme, R.; Mede, N.G.; Thomm, E.; Kremer, B.; Ziegler, R. An anchor in troubled times: Trust in science before and within the COVID-19 pandemic. PLoS ONE 2022, 17, e0262823. [Google Scholar] [CrossRef]
- Axfors, C.; Schmitt, A.M.; Janiaud, P.; Hooft, J.V.; Abd-Elsalam, S.; Abdo, E.F.; Abella, B.S.; Akram, J.; Amaravadi, R.K.; Angus, D.C.; et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat. Commun. 2021, 12, 2349. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Mafham, M.; Linsell, L.; Bell, J.L.; Staplin, N.; Emberson, J.R.; Wiselka, M.; Ustianowski, A.; Elmahi, E.; et al. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2020, 383, 2030–2040. [Google Scholar] [CrossRef]
- Owens, B. Excitement around hydroxychloroquine for treating COVID-19 causes challenges for rheumatology. Lancet Rheumatol. 2020, 2, e257. [Google Scholar] [CrossRef]
- Mahmud, R.; Rahman, M.; Alam, I.; Ahmed, K.G.U.; Kabir, A.H.; Sayeed, S.J.B.; Rassel, M.A.; Monayem, F.B.; Islam, S.; Islam, M.M.; et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: A randomized trial. J. Int. Med. Res. 2021, 49, 3000605211013550. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J.; COVID-19 Systematic Urgent Review Group Effort (SURGE) Study Authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–2020. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Hoque, E.; Alam, R.; Rouf, A.; Khan, S.I.; Xu, H.; Ramakrishna, S. Face Masks to Combat Coronavirus (COVID-19)—Processing, Roles, Requirements, Efficacy, Risk and Sustainability. Polymers 2022, 14, 1296. [Google Scholar] [CrossRef]
- Jennings, W.; Stoker, G.; Bunting, H.; Valgarðsson, V.O.; Gaskell, J.; Devine, D.; McKay, L.; Mills, M.C. Lack of Trust, Conspiracy Beliefs, and Social Media Use Predict COVID-19 Vaccine Hesitancy. Vaccines 2021, 9, 593. [Google Scholar] [CrossRef] [PubMed]
- Horton, R. Offline: Boris Johnson and COVID-19—More light than heat. Lancet 2023, 402, 2277. [Google Scholar] [CrossRef] [PubMed]
- Caceres, M.M.F.; Sosa, J.P.; Lawrence, J.A.; Sestacovschi, C.; Tidd-Johnson, A.; Rasool, M.H.U.; Gadamidi, V.K.; Ozair, S.; Pandav, K.; Cuevas-Lou, C.; et al. The impact of misinformation on the COVID-19 pandemic. AIMS Public Health 2022, 9, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Khanam, M.; Shuchi, N.S. COVID-19 Pandemic in Bangladesh. A scoping review of governance issues affecting response in public sector. Public Health Pract. 2024, 7, 1004572024. [Google Scholar] [CrossRef] [PubMed]
- Ara, T.; Ferdous, Z.; Mahi, M.; Amin, E.; Chowdhury, S.B.; Rahman, S.; Rahman, L.; Rahman, M. Assessment of COVID-19 management and its consequences on healthcare professionals: A cross-sectional study from Bangladesh. Br. Med. J. 2023, 13, e068633. [Google Scholar] [CrossRef] [PubMed]
- Okada, P.; Buathong, R.; Phuygun, S.; Thanadachakul, T.; Parnmen, S.; Wongboot, W.; Waicharoen, S.; Wacharapluesadee, S.; Uttayamakul, S.; Vachiraphan, A.; et al. Early transmission patterns of coronavirus disease 2019 (COVID-19) in travellers from Wuhan to Thailand, January 2020. Eurosurveillance 2020, 25, 8–13. [Google Scholar] [CrossRef]
- World Health Organization. Tracking SARS-CoV-2 Variants. June 2020. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 1 June 2023).
- World Health Organization. Guidance for Surveillance of SARS-CoV-2 Variants: Interim Guidance, 9 August 2021. Available online: https://www.who.int/publications/i/item/WHO_2019-nCoV_surveillance_variants (accessed on 1 June 2023).
- World Health Organization. WHO SAGE Roadmap on Uses of COVID-19 Vaccines in the Context of OMICRON and Substantial Population Immunity. 20 March 2023. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-good-practice-statement-second-booster (accessed on 1 June 2023).
- Focosi, D.; Quiroga, R.; McConnell, S.; Johnson, M.C.; Casadevall, A. Convergent Evolution in SARS-CoV-2 Spike Creates a Variant Soup from Which New COVID-19 Waves Emerge. Int. J. Mol. Sci. 2023, 24, 2264. [Google Scholar] [CrossRef]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827.e19. [Google Scholar] [CrossRef]
- Global Initiative On sharing All Influenza Data (GISAID) EpiCoV. Available online: https://gisaid.org/hcov19-variants/ (accessed on 12 June 2023).
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, J.R.C.; van Schalkwyk, C.; Govender, N.; von Gottberg, A.; Cohen, C.; Groome, M.J.; Dushoff, J.; Mlisana, K.; Moultrie, H. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. medRxiv 2021. [Google Scholar] [CrossRef]
- Dhawan, M.; Sharma, A.; Priyanka; Thakur, N.; Rajkhowa, T.K.; Choudhary, O.P. Delta variant (B.1.617.2) of SARS-CoV-2: Mutations, impact, challenges and possible solutions. Hum. Vaccines Immunother. 2022, 18, 2068883. [Google Scholar] [CrossRef]
- Tareq, A.M.; Bin Emran, T.; Dhama, K.; Dhawan, M.; Tallei, T.E. Impact of SARS-CoV-2 delta variant (B.1.617.2) in surging second wave of COVID-19 and efficacy of vaccines in tackling the ongoing pandemic. Hum. Vaccines Immunother. 2021, 17, 4126–4127. [Google Scholar] [CrossRef]
- Elliott, P.; Eales, O.; Steyn, N.; Tang, D.; Bodinier, B.; Wang, H.; Elliott, J.; Whitaker, M.; Atchison, C.; Diggle, P.J.; et al. Twin peaks: The Omicron SARS-CoV-2 BA.1 and BA.2 epidemics in England. Science 2022, 376, eabq4411. [Google Scholar] [CrossRef]
- Tamura, T.; Ito, J.; Uriu, K.; Zahradnik, J.; Kida, I.; Anraku, Y.; Nasser, H.; Shofa, M.; Oda, Y.; Lytras, S.; et al. Virological characteristics of the SARS-CoV-2 XBB variant derived from recombination of two Omicron subvariants. Nat. Commun. 2023, 14, 2800. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Editorial: The XBB.1.5 (‘Kraken’) Subvariant of Omicron SARS-CoV-2 and its Rapid Global Spread. Med. Sci. Monit. 2023, 29, e939580. [Google Scholar] [CrossRef]
- Uriu, K.; Ito, J.; Zahradnik, J.; Fujita, S.; Kosugi, Y.; Schreiber, G.; Sato, K. Enhanced transmissibility, infectivity, and immune resistance of the SARS-CoV-2 omicron XBB.1.5 variant. Lancet Infect. Dis. 2023, 23, 280–281. [Google Scholar] [CrossRef]
- Fraser, B.J.; Beldar, S.; Seitova, A.; Hutchinson, A.; Mannar, D.; Li, Y.; Kwon, D.; Tan, R.; Wilson, R.P.; Leopold, K.; et al. Structure and activity of human TMPRSS2 protease implicated in SARS-CoV-2 activation. Nat. Chem. Biol. 2022, 18, 963–971. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; COVID-19 Genomics UK (COG-UK) Consortium; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Kepp, K.P. Structure and Mutations of SARS-CoV-2 Spike Protein: A Focused Overview. ACS Infect. Dis. 2022, 8, 29–58. [Google Scholar] [CrossRef] [PubMed]
- Daria, A.A.; Assaduzzaman, M.S.; Islam, M.R. Bangladesh reported delta variant of coronavirus among its citizen: Actionable items to tackle the potential massive third wave. Infect. Prev. Pract. 2021, 3, 100159–100160. [Google Scholar]
- Rahman, M.; Shirin, T.; Rahman, S.; Rahman, M.M.; Hossain, M.E.; Hossain, M.K.; Rahman, M.Z.; El Arifeen, S.; Ahmed, T. The emergence of SARS-CoV-2 variants in Dhaka city, Bangladesh. Transbound. Emerg. Dis. 2021, 1, 1–2. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, Y.; Wang, F.; Huang, Z.; Qiu, W.; Mao, A. Case fatality rates of COVID-19 during epidemic periods of variants of concern: A meta-analysis by continents. Int. J. Infect. Dis. 2024, 141, 106950. [Google Scholar] [CrossRef]
- Bari, M.; Hossain, M.; Akhter, S.; Emran, T. Delta variant and black fungal invasion: A bidirectional assault might worsen the massive second/third stream of COVID-19 outbreak in South-Asia. Ethic- Med. Public Health 2021, 19, 100722. [Google Scholar] [CrossRef]
- Hossain, M.S.; Khan, J.R.; Al Mamun, S.M.A.; Islam, M.T.; Raheem, E. Excess mortality during the COVID-19 pandemic (2020–2021) in an urban community of Bangladesh. PLoS Glob. Public Health 2023, 3, e0002176. [Google Scholar] [CrossRef]
- Lytton, S.D.; Ghosh, A.K.; Herr, M.; Duchmann, H.; Shumi, Y.; Sharif, M.; Nafisa, T.; Landt, O.; Shamsuzzaman, A.K.; Islam, A. SARS-CoV-2 Nucleocapsid detection in Bangladeshi Covid-19 is not affected by Dengue infection. Pathogens 2021, 10, 637–652. [Google Scholar] [CrossRef]
- Cowley, L.A.; Afrad, M.H.; Rahman, S.I.A.; Al Mamun, M.; Chin, T.; Mahmud, A.; Rahman, M.Z.; Billah, M.M.; Khan, M.H.; Sultana, S.; et al. Genomics, social media and mobile phone data enable mapping of SARS-CoV-2 lineages to inform health policy in Bangladesh. Nat. Microbiol. 2021, 6, 1271–1278. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Kaiser, M.; Molla, M.A.; Nafisa, T.; Yeasmin, M.; Ratul, R.H.; Sharif, M.; Akram, A.; Hosen, N.; Mamunur, R.; et al. Molecular and Serological Characterization of the SARS-CoV-2 Delta Variant in Bangladesh in 2021. Viruses 2021, 13, 2310. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Landt, O.; Yeasmin, M.; Sharif, M.; Ratul, R.H.; Molla, M.A.; Nafisa, T.; Mosaddeque, M.B.; Hosen, N.; Bulbul, R.H.; et al. Clinical Presentation of COVID-19 and Antibody Responses in Bangladeshi Patients Infected with the Delta or Omicron Variants of SARS-CoV-2. Vaccines 2022, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Bhattacharya, M.; Chopra, H.; Islam, A.; Saikumar, G.M.; Dhama, K.M. The SARS-CoV-2 Omicron recombinant subvariants XBB, XBB.1, and XBB.1.5 are expanding rapidly with unique mutations, antibody evasion, and immune escape properties—An alarming global threat of a surge in COVID-19 cases again? Int. J. Surg. 2023, 109, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.A.; Islam, S.; Sana, S.; Mondal, P.; Meem, R.I.; Rana, S.; Mondol, D.; Sana, M.; Hossain, S.I.; Joarder, T.; et al. A review of the genome, epidemiology, clinical features, prevention, and treatment scenario of COVID-19: Bangladesh aspects. Egypt J. Bronchol. 2021, 15, 8. [Google Scholar] [CrossRef]
- Saha, S.; Tanmoy, A.M.; Hooda, Y.; Tanni, A.A.; Goswami, S.; Al Sium, S.M.; Sajib, M.S.I.; Malaker, R.; Islam, S.; Rahman, H.; et al. COVID-19 rise in Bangladesh correlates with increasing detection of B.1.351 variant. BMJ Glob. Health 2021, 6, e006012. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Rocha, I.C.N.; Ramos, K.G.; Cedeño, T.D.D.; Dos Santos Costa, A.C.; Tsagkaris, C.; Billah, M.; Ahmad, S.; Essar, M.Y. Emergence of highly infectious SARS-CoV-2 variants in Bangladesh: The need for systematic genetic surveillance as a public health strategy. Trop. Med. Health BMC 2021, 49, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Lytton, S.D.; Ghosh, A.K.; Bulbul, R.H.; Nafisa, T.; Mamunur, R.; Meier, C.; Landt, O.; Kaiser, M. The severe acute respiratory syndrome coronavirus-2 (SARS CoV-2) omicron sub-variants in Bangladesh cause mild COVID-19 and associate with similar antibody responses irrespective of natural infection or vaccination history. Heliyon 2024, 10, e31011. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286.e8. [Google Scholar] [CrossRef]
- Lytton, S.D.; Bulbul, R.H.; Barua, K.; Begum, M.C.; Chowdhury, B.; Islam, Z.; Faiyaz, K.I.; Chandan, S.K.; Shakeel, A.; Landt, O.; et al. Hepatitis E Virus Capsid Antigen (HEV-Ag)—A practical diagnostic biomarker in the HEV outbreak scenario. J. Clin. Virol. 2020, 134, 104862. [Google Scholar] [CrossRef]
- Islam, S.R.U.; Akther, T.; Sultana, S.; Deb, P.; Ghosh, A.K.; Nasif, A.O.; Bhuiyan, A.H.; Jahan, M.; Nessa, A.; Munshi, S.U.; et al. Challenges in the establishment of a biosafety testing laboratory for COVID-19 in Bangladesh. J. Infect. Dev. Ctries. 2021, 15, 1833–1837. [Google Scholar] [CrossRef]
- Concern Launches Coronavirus Screening Booths in Bangladesh. Available online: https://www.concern.org.uk/news/concern-launches-coronavirus-screening-booths-bangladesh (accessed on 1 June 2023).
- Cousins, S. Bangladesh’s COVID-19 testing criticised. Lancet 2022, 396, 591–592. [Google Scholar] [CrossRef]
- Serván-Mori, E.; Islam, D.; Kaplan, W.A.; Thrasher, R.; Wirtz, V.J. Out-of-pocket expenditure on medicines in Bangladesh: An analysis of the national household income and expenditure survey 2016–2017. PLoS ONE 2022, 17, e0274671. [Google Scholar] [CrossRef]
- Molla, A.A.; Chi, C. Who pays for healthcare in Bangladesh? An analysis of progressivity in health systems financing. Int. J. Equity Health 2017, 16, 167. Available online: http://creativecommons.org/publicdomain/zero/1.0/ (accessed on 1 June 2023). [CrossRef]
- Chowdhury, P.B.; Hossain, S.; Biswas, R.K. A combination of COVID-19 and dengue fever in Bangladesh: Preparedness of Bangladesh. J. Glob. Health 2020, 10, 020314. [Google Scholar] [CrossRef]
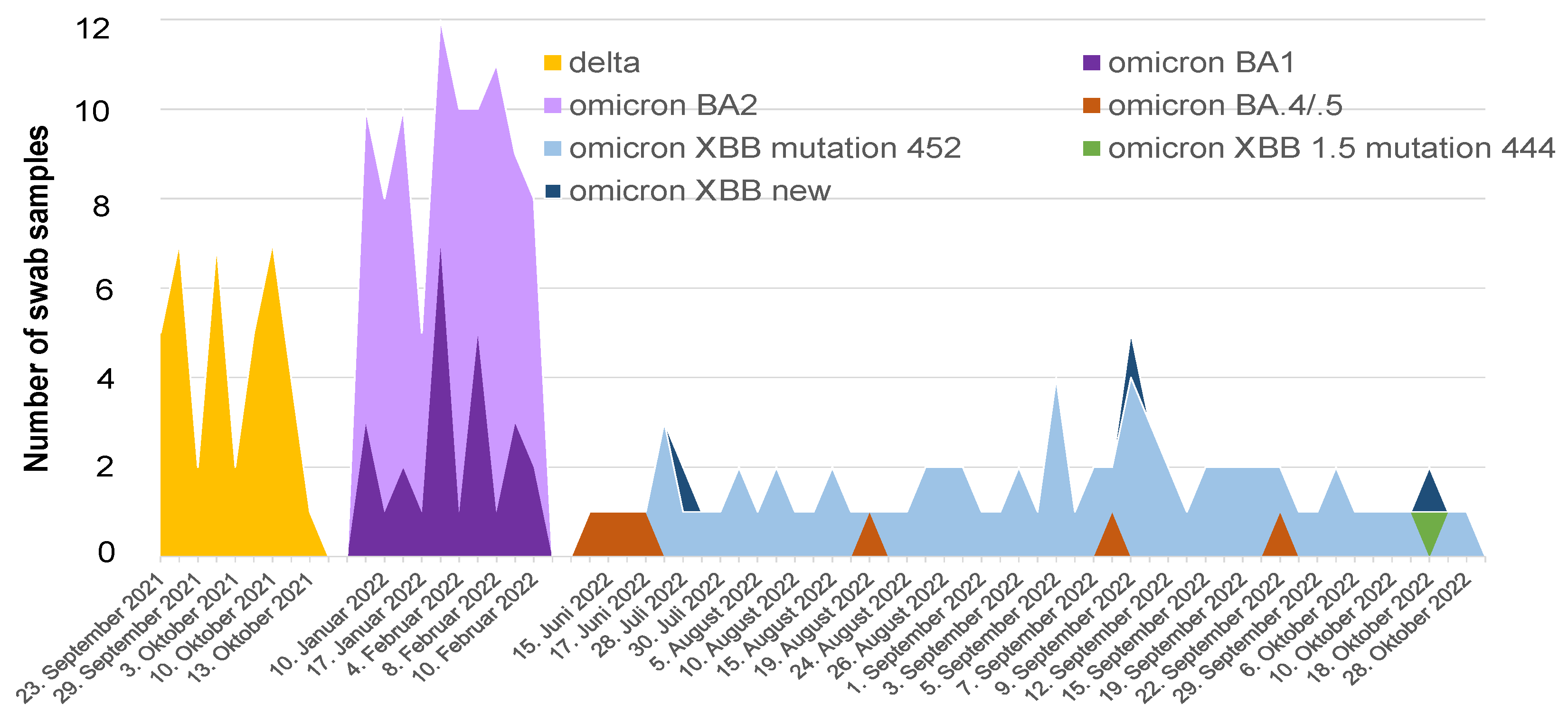
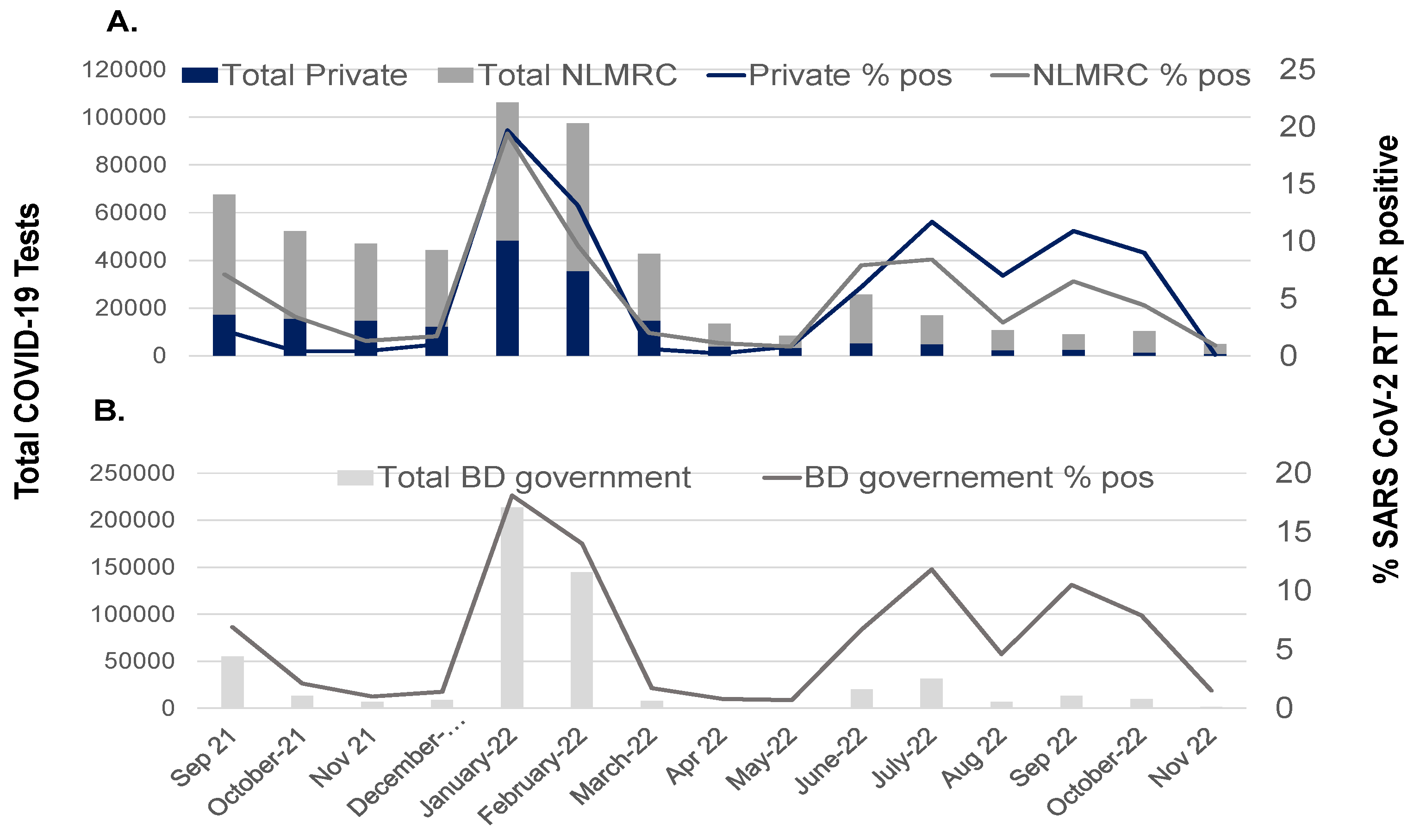
| COVID-19 Pandemic Period | SARS-CoV-2 | Vaccination | COVID-19 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dominant VOCs | % Population Receiving Doses | Hospital Bed Occupancy | Deaths | ||||||
| 1 | 2 | ≥3 | % ICU Beds | Nr (%) | |||||
| April 2020–January 2021 | Wuhan-like | 0 | 0 | 0 | NA | 8050 (1.5) | |||
| February 2021–March 2021 | Alpha | O | 3.2 | 0 | 0 | NA | 991 (1.27) | ||
| April 2021–May 2021 | Beta | 3.4 | 2.46 | 0 | 47.6 | 3573 (1.9) | |||
| June 2021–September 2021 | Delta | 19.8 | 10 | 0 | 57.8 | 14,891 (2) | |||
| October 2021–December 2021 | 60.5 | 40.5 | 0.03 | 15.7 | 560 (1.9) | ||||
| January 2022–February 2022 | BA.2 | 90.2 | 59.8 | 2.3 | 20 | 965 (0.27) | |||
| March 2022–May 2022 | 93 | 79 | 9 | 6.1 | 93 (0.94) | ||||
| June 2022–August 2022 | BA.4/.5 | XBB | 93.7 | 81.8 | 25.8 | 5.9 | 193 (0.33) | ||
| September 2022–November 2022 | xxxxxxxxxxx | 100 | 84.2 | 35 | 4.6 | 110 (0.44) | |||
| December 2022–February 2023 | 100 | 91.2 | 41.6 | 3.1 | 12 (0.97) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lytton, S.D.; Ghosh, A.K. SARS-CoV-2 Variants and COVID-19 in Bangladesh—Lessons Learned. Viruses 2024, 16, 1077. https://doi.org/10.3390/v16071077
Lytton SD, Ghosh AK. SARS-CoV-2 Variants and COVID-19 in Bangladesh—Lessons Learned. Viruses. 2024; 16(7):1077. https://doi.org/10.3390/v16071077
Chicago/Turabian StyleLytton, Simon D., and Asish Kumar Ghosh. 2024. "SARS-CoV-2 Variants and COVID-19 in Bangladesh—Lessons Learned" Viruses 16, no. 7: 1077. https://doi.org/10.3390/v16071077





