Abstract
Morbillivirus canis (canine distemper virus (CDV)) is recognized as a multihost pathogen responsible for a transmissible disease affecting both domestic and wild animals. A considerable portion of wildlife populations remain unvaccinated due to a lack of safety and immunogenicity data on existing vaccines for the prevention of CDV infection in these species. This review aimed to assess the current state of CDV vaccination research for both domestic and wild animals and to explore novel vaccine candidates through in vivo studies. It also sought to synthesize the scattered information from the extensive scientific literature on CDV vaccine research, identify key researchers in the field, and highlight areas where research on CDV vaccination is lacking. A scoping review was conducted across four databases following the PRISMA-ScR protocol, with information analyzed using absolute and relative frequencies and 95% confidence intervals (CIs) for study number proportions. Among the 2321 articles retrieved, 68 met the inclusion criteria and focused on CDV vaccines in various animal species, such as dogs, ferrets, minks, and mice. Most of the scientific community involved in this research was in the USA, Canada, France, and Denmark. Various vaccine types, including MLV CDV, recombinant virus, DNA plasmids, inactivated CDV, and MLV measles virus (MeV), were identified, along with diverse immunization routes and schedules employed in experimental and commercial vaccines. Safety and efficacy data were summarized. Notably, 37 studies reported postimmunization CDV challenge, primarily in dogs, revealing the survival rates of vaccinated animals. In summary, CDV vaccines generally demonstrate an acceptable safety profile in dogs and show promise as a means of controlling CDV. However, significant gaps in vaccine research persist, particularly concerning wildlife reservoirs, indicating the need for further investigation.
1. Introduction
Morbillivirus canis, commonly known as canine distemper virus (CDV), causes a highly contagious disease in domestic and wildlife animals, and CDV infection has been reported in more than 8 orders and 20 families worldwide [1]. The host range of CDV predominantly includes species within the order Carnivora, spanning diverse families such as Canidae Procyonidae, Mustelidae, Ursidae, Ailuridae, and Felidae, and, to a lesser extent, other notable families from different orders, namely Artiodactyla, Primates, Rodentia, and Proboscidea [2,3,4]. Given the extensive spectrum of species affected by CDV, different studies have explored its cross-species transmission among both wild and domesticated animals, investigating not only their interactions to elucidate the molecular diversity but also strategies such as vaccination to control CDV’s spread in domestic and wild animals [1,5].
Canine distemper disease (CDD) manifests with a range of clinical symptoms, such as fever, as well as respiratory, gastrointestinal, and neurological issues, mainly in domestic dogs [6]. The clinical signs in wildlife are mainly associated with neurological disorders [7]. CDV is included in the Paramyxoviridae family, genus Morbillivirus, and has a single-stranded negative-sense RNA genome with six transcription units that encode for eight proteins [1]. Among these proteins, the H and F proteins are essential, because of their function in diverse essential viral processes [8]. Both proteins are involved as the main antigenic determinants of CDV due to their viral particle localization [9]. Moreover, they have been employed in CDV vaccine alternatives [10], helper T-cell epitopes from the F protein have been identified [11], and, finally, H and F peptides have been recovered in higher proportions than other viral proteins from dog major histocompatibility complex (MHC) molecules [12].
The H protein is considered the most variable protein among all lineages [13], and, to date, based on its entire sequence, diverse genotypes have been described, such as America-1, America-2 to -5, Arctic-like, Rockborn-like, Asia-1 to -4, Africa-1 to -2, European Wildlife, Europe/South America-1, South America-2 and -3, and South/North America-4 [14,15]. The re-emergence of different CDV infections in domestic and wildlife animals has demonstrated the importance of updating the vaccine strains and examining the status of CDV vaccination, since commercial vaccines are not fully employed in wildlife animals due to the lack of relevant evidence of their safety and efficacy [16]. Diverse circulating lineages, geographically distributed with amino acid changes in viral proteins involved in the host immune response and viral neutralization, could explain the vaccine failure in response to wild-type emerging strains, cross-species transmission, and the increased virulence of emerging CDV strains in domestic dogs and wildlife animals [17]. Additionally, case reports associated with CDV infection in wild animals have recently been published [3], with CDV being a threat to a wide range of in-danger species [16,18].
The transmission dynamics of CDV between domestic and wild animals are not fully understood. Therefore, examining these transmission dynamics and identifying CDV reservoirs holds significant promise for the formulation of effective strategies to safeguard endangered species [17]. Some reports suggest that vaccinating wildlife may play a pivotal role in preventing the extinction of at-risk populations due to CDV [5,18]. Thus, immunizing animals and ensuring safety in both domestic and wildlife populations represent a field of study that warrants extensive efforts to ensure the health of companion animals and those endangered by CDV infection.
Regarding the current state of vaccination, MLV vaccines based on traditional America-1 strains (i.e., the Onderstepoort strain) and Snyder Hill, Convac, Rockborn, or CDV3 strains [19] are commercially available. However, these strains no longer circulate around the world [20]. Recombinant CDV vaccines have been developed by integrating the CDV F and H proteins into a canarypox virus vector, and these vaccines have demonstrated a safe profile across various susceptible species, including dogs, European ferrets (Mustela putorius furo), giant pandas (Ailuropoda melanoleuca), fennec foxes, meerkats (Suricata suricatta), and Siberian polecats (Mustela eversmanni) [21,22,23,24]. Although recombinant canarypox vaccines exhibit safety and moderate efficacy across a spectrum of species, they induce a less robust immune response than MLV vaccines, primarily due to replicative limitations [25]. However, this characteristic underscores their suitability for the immunization of young dogs in the presence of maternal antibodies [9]. Nevertheless, it has become imperative not only to develop new vaccine strategies to control CDV infection but also to assess the efficacy and safety of the currently available vaccines, since there is limited experimental evidence regarding these vaccines in wildlife animals. Other alternative strategies include experimental assays, including a recombinant bivalent vaccine, which employs a rabies virus that expresses the H and F CDV proteins, evaluated in domestic dogs and ferrets [19].
Based on the limited spectrum of current vaccines, different researchers worldwide have investigated new vaccines against CDV through in vivo studies involving diverse animal species, such as domestic dogs, BALB/c mice, minks, and ferrets, including recombinant viruses [26,27,28,29], chimeric measles virus constructs expressing CDV proteins [30], DNA vaccines encoding the main antigenic determinants of CDV [31,32,33], the H and F CDV proteins isolated as antigens [34], recombinant mouse adenovirus 1 expressing CDV antigens [35], and a novel vaccine formulation based on bacterium-like particles presenting CDV antigens [19]. However, the state of CDV vaccination research, development, and efficacy evaluation have yet to be explored and summarized in order to establish different vaccination schemes to control CDV’s transmission, dissemination, and disease course.
Therefore, this scoping review aims to assess the current state of research and advancements in CDV vaccination for both domestic and wild animals. This review encompasses the evaluation of existing vaccines in various species and the investigation of novel vaccine candidates through in vivo studies. Given the proliferation of reports on new vaccination strategies for CDV, this inquiry is warranted to target both wildlife and domestic canine populations. Furthermore, this scoping review seeks to consolidate the fragmented information scattered throughout the extensive scientific literature regarding CDV vaccine research. Additionally, this study aims to identify key researchers in this field and pinpoint regions where research on CDV vaccination is lacking.
2. Materials and Methods
2.1. Type of Study
Following the recommendations of the PRISMA-ScR [36] and the Joanna Briggs Institute, a systematic scoping review on CDV vaccination and development for domestic and wildlife animals was conducted. The scoping review was registered at https://osf.io/n9sed (accessed on 22 May 2024) and the PRISMA-ScR checklist is available in Table S1.
2.2. Search and Study Selection
The data were searched in multidisciplinary repositories such as PubMed, SciELO, ScienceDirect, and Scopus. For term selection, we used the Descriptors in Health Sciences (DeCS), Medical Subject Heading (MeSH), and both known names for CDV, with truncated terms vacc* AND canine distemper virus and vacc* AND canine morbillivirus, in all databases. A total of 8 searches were performed as follows: SciELO: (ti:(canine distemper virus)) AND (ti:(vacc*)) AND (ab:(canine distemper virus)) AND (ab:(vacc*)) and (ti:(canine morbillivirus)) AND (ti:(vacc*)) AND (ab:(canine morbillivirus)) AND (ab:(vacc*)); Scopus: (TITLE-ABS-KEY (canine AND distemper AND virus) AND TITLE-ABS-KEY (vacc*)) and (TITLE-ABS-KEY (canine AND morbillivirus) AND TITLE-ABS-KEY (vacc*)); PubMed: (vacc*[Title/Abstract]) AND (canine distemper virus[Title/Abstract]) AND (vacc*[Title/Abstract]) AND (canine morbillivirus[Title/Abstract]); and, finally, ScienceDirect: canine distemper virus AND vacc? and canine morbillivirus AND vacc?. The articles retrieved from each search (restricted to title, abstract, and/or keywords) were saved in a common file in Zotero to eliminate duplicates.
2.3. Screening and Eligibility
We screened studies published historically until 2024 (the last update was performed on 9 January 2024), including original research (eliminating reviews, editorials, protocols, and book chapters) that was conducted on CDV vaccination and development for domestic and wildlife animals in vivo, and the language was also considered (including English, Spanish, and Portuguese). These criteria were applied by two researchers independently. During the eligibility phase, studies were excluded if they did not include safety information, such as weight changes, temperature, survival, the immunization route, or efficacy, measured as either specific or neutralizing CDV antibodies.
2.4. Data Extraction
The following variables were extracted from the selected studies: title; authors; year of publication; country of publication; sample size; species; immunogen type; immunization route; vaccine safety measured by temperature, weight, behavior, leukocyte count, and survival; and efficacy through specific antibody (Ab) production measured either with neutralization assays or with ELISA. In studies where viral challenge was reported, the inoculation route and challenge survival were also retrieved. All information regarding the included studies was synthesized in figures and tables.
2.5. Data Charting Process
The data charting process was independently developed by two researchers to ensure concordance across all phases of the search, study selection, and data extraction processes. A third investigator resolved any discrepancies through consensus and further examination.
2.6. Analysis of the Information
The description of different aspects of the included studies was performed with absolute and relative frequencies and 95% confidence intervals (CIs). A scientific collaboration network in this research field was created with Gephi. The difference in survival between vaccinated and nonvaccinated animals was calculated using the Z test or confidence interval for the difference in proportions. The frequency analyses and Z tests were performed with EPIDAT version 4.2.
3. Results
3.1. Study Selection and Prisma Flow Chart
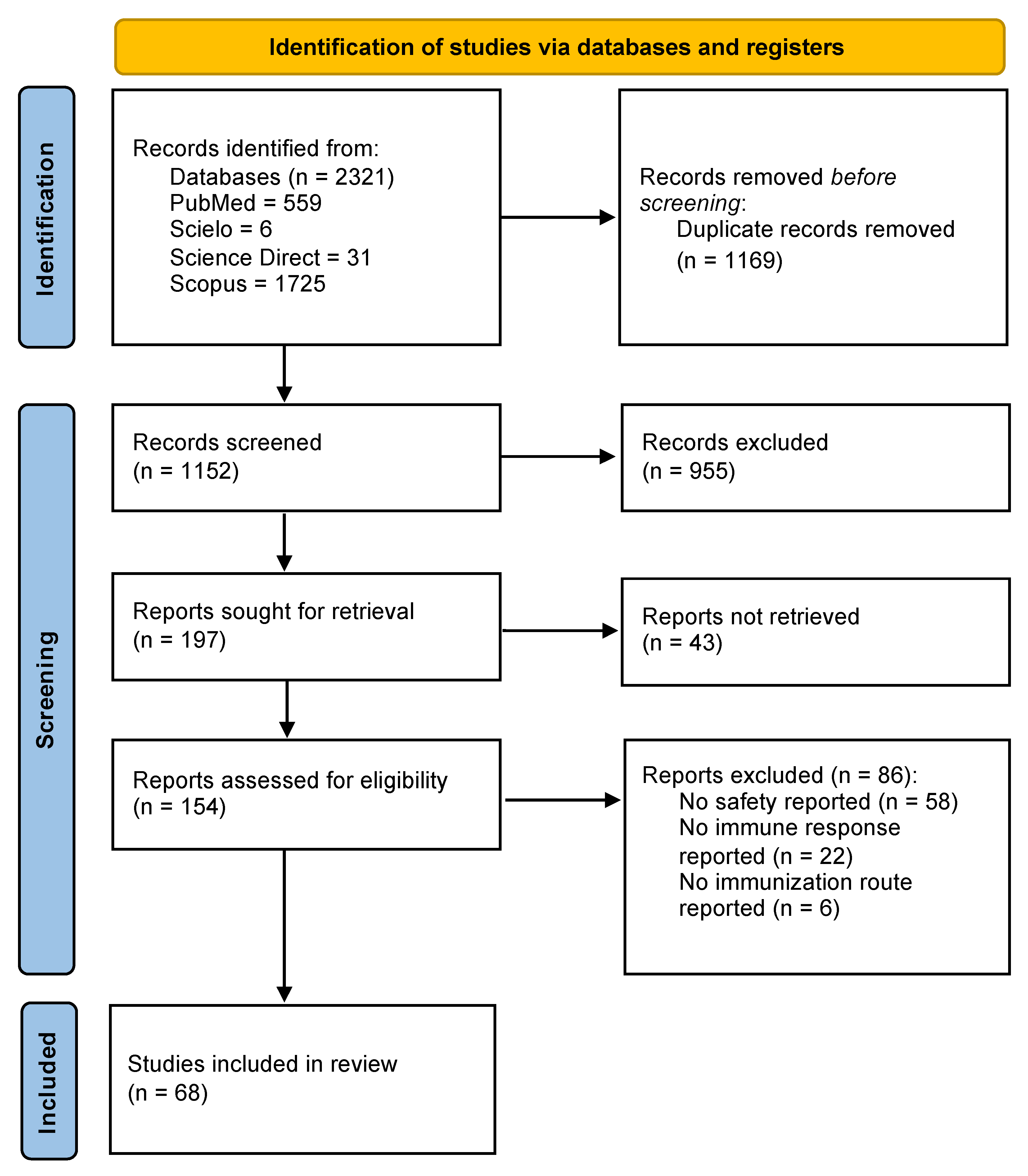
Initially, 2321 studies were identified in PubMed, SciELO, Science Direct, and Scopus. After duplicates were removed, the number of articles for screening decreased to 1552. Then, the inclusion criteria were applied, and 154 studies were included. Finally, 68 studies were included in this scoping review after the application of the exclusion criteria (Figure 1).
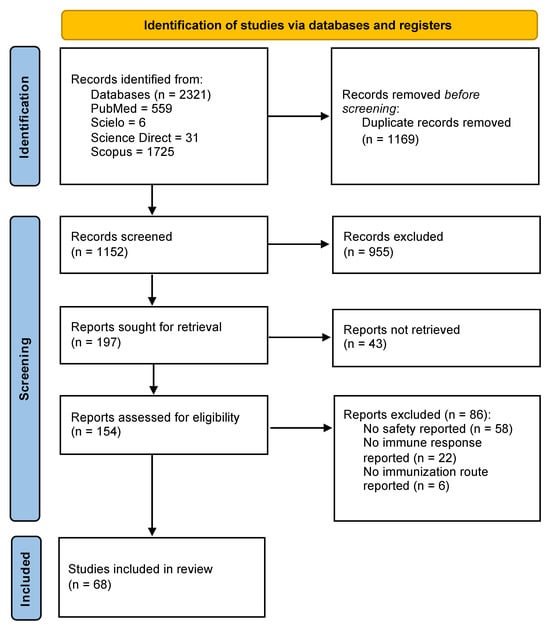
Figure 1.
Study selection algorithm based on PRISMA guidelines.
3.2. International Cooperation and Researchers
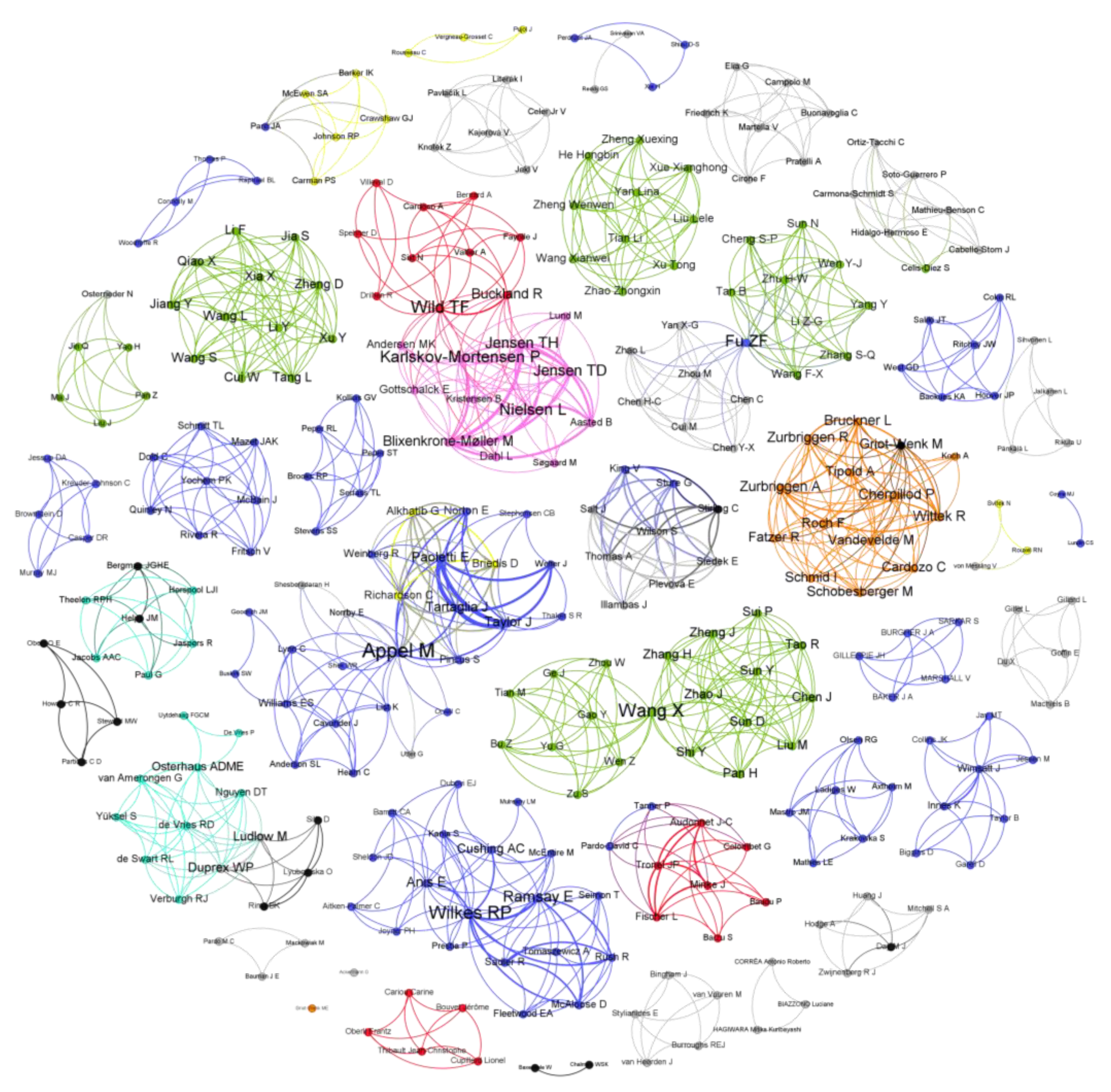
To understand the level of international cooperation in the development of CDV vaccines, a scientific collaboration network analysis of researchers was carried out, considering their countries of affiliation. The scientific community related to CDV vaccination in domestic and wildlife animals comprised 308 researchers from diverse countries, including the United States of America, China, France, the Netherlands, Switzerland, Canada, the United Kingdom, and Denmark (Figure 2). Notably, there are few researchers from different countries who collaborate in CDV vaccine research, as evidenced by the presence of mostly individual nodes in the collaboration networks shown in Figure 2. Collaboration was evidenced only between researchers from the USA and Canada and between researchers from France and Denmark. In the figure, the size of the author’s name denotes the importance of CDV vaccine development since it represents the presence of these authors in different studies. Among the authors who have contributed greatly to CDV vaccination research, in the USA, Rebecca Wilkes has performed different studies in wild animals, and Max J.G. Appel contributed to initial MLV CDV vaccines in dogs, especially those based on the Onderstepoort strain. In France, T. Fabian Wild has also contributed considerably, including studies in mice and minks, employing recombinant and DNA vaccines (Table 1).
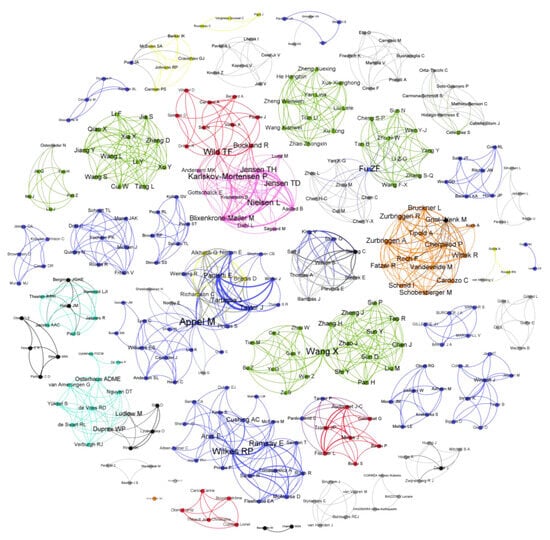
Figure 2.
Scientific collaboration network related to research on CDV vaccination development. Relationships among researchers based on country of affiliation. The United States of America is marked in blue, China in green, France in red, the Netherlands in cyan, Switzerland in orange, Canada in yellow, the United Kingdom in black, and Denmark in pink. Low-frequency countries are marked in gray. The diameter of each node denotes the importance of this researcher in CDV vaccine development. The graph was created with Gephi.

Table 1.
Studies’ main characteristics.
3.3. Included Studies and Countries Contributing to CDV Vaccine Development
A total of 68 studies were included in the scoping review. The main characteristics of the included articles are summarized in Table 1. The geographic distribution included studies from the United States of America (n = 31); China (n = 7); France (n = 5); Belgium, Canada, Denmark, the Netherlands, and the United Kingdom (n = 3 in each country); Switzerland (n = 2); and Australia, Brazil, the Czech Republic, Finland, Germany, India, Italy, and South Africa (n = 1 in each country). The United States of America had the greatest number of in vivo CDV vaccine studies, followed by China and France, which correlates with the authors’ contributions as reported in Figure 2.
3.4. Domestic and Wild Animals in CDV Vaccine Trials
As shown in Table 2, 37.3% of the studies were carried out in dogs, followed by ferrets, mice, minks, African wild dogs, tigers, and cats, with only one study on other species (Table 2), shown as a relative frequency (RF). A total of 2363 individuals were included in all studies on diverse animal species; however, considering the vast array of species affected by CDV, there is still a lack of in vivo CDV vaccine studies focused on the control of CDD and its dissemination among domestic and wildlife animals, due to the lower number of studies corresponding to either domestic or laboratory animals.

Table 2.
Domestic and wildlife species included in CDV vaccine trials.
3.5. CDV Vaccine Characteristics in Included Stidies
The different immunogenic approaches employed in the included studies and their characteristics are summarized in Table 3. The most frequent vaccine types were MLV CDV (n = 25) and recombinant virus (n = 25), with the same number of studies, as well as DNA plasmids (n = 13), inactivated CDV (n = 6), MLV MeV (n = 3), and others (n = 4). On the other hand, different immunization schemes were employed with one (n = 25), two (n = 31), three (n = 16), and four (n = 5) doses (Table 3), where the two-dose scheme had the highest percentage, at 40.3% of the total number of studies. Moreover, regarding the inoculation route, most studies employed subcutaneous immunization (n = 36), followed by intramuscular (n = 30), oral (n = 6), and other methods (n = 11).

Table 3.
Characteristics of vaccines used in CDV studies.
3.6. Safety and Efficacy of CDV Vaccines from Included Studies
The main characteristics of vaccines are safety and efficacy. Although there are diverse alternatives to evaluate safety, in the included studies, the clinical signs (n = 65), temperature (n = 26), weight loss (n = 21), survival (n = 14), animal behaviors (n = 6), and leucocyte counts (n = 5) were the primary indicators. In addition, neutralizing antibodies (n = 66) and specific antibodies such as IgG (n = 15), detected by ELISA, were reported as measures of the humoral immune response to immunization with CDV vaccines (Table 4). Overall, 54.4% of the included studies involved infectious virus challenge (n = 37) with different CDV lineages, and intranasal challenge was the most common route (n = 22), followed by intravenous challenge (n = 2) and other methods (n = 17), as shown in Table 4.

Table 4.
Measures of safety, immunogenicity, and viral challenge with route in CDV vaccine studies.
As mentioned before, a total of thirty-seven studies reported postimmunization virus challenge (Table 4) on different species. Seventeen studies included dogs, eight included ferrets, five included minks, four included mice, one study included foxes, one included raccoons, one included Siberian polecats, and one included hybrid ferret WITH Siberian polecats. One study included two animal populations, mice and foxes, totaling thirty-eight animal populations.
3.7. Postimmunization Challenge and Efficacy for CDV Vaccine Trials
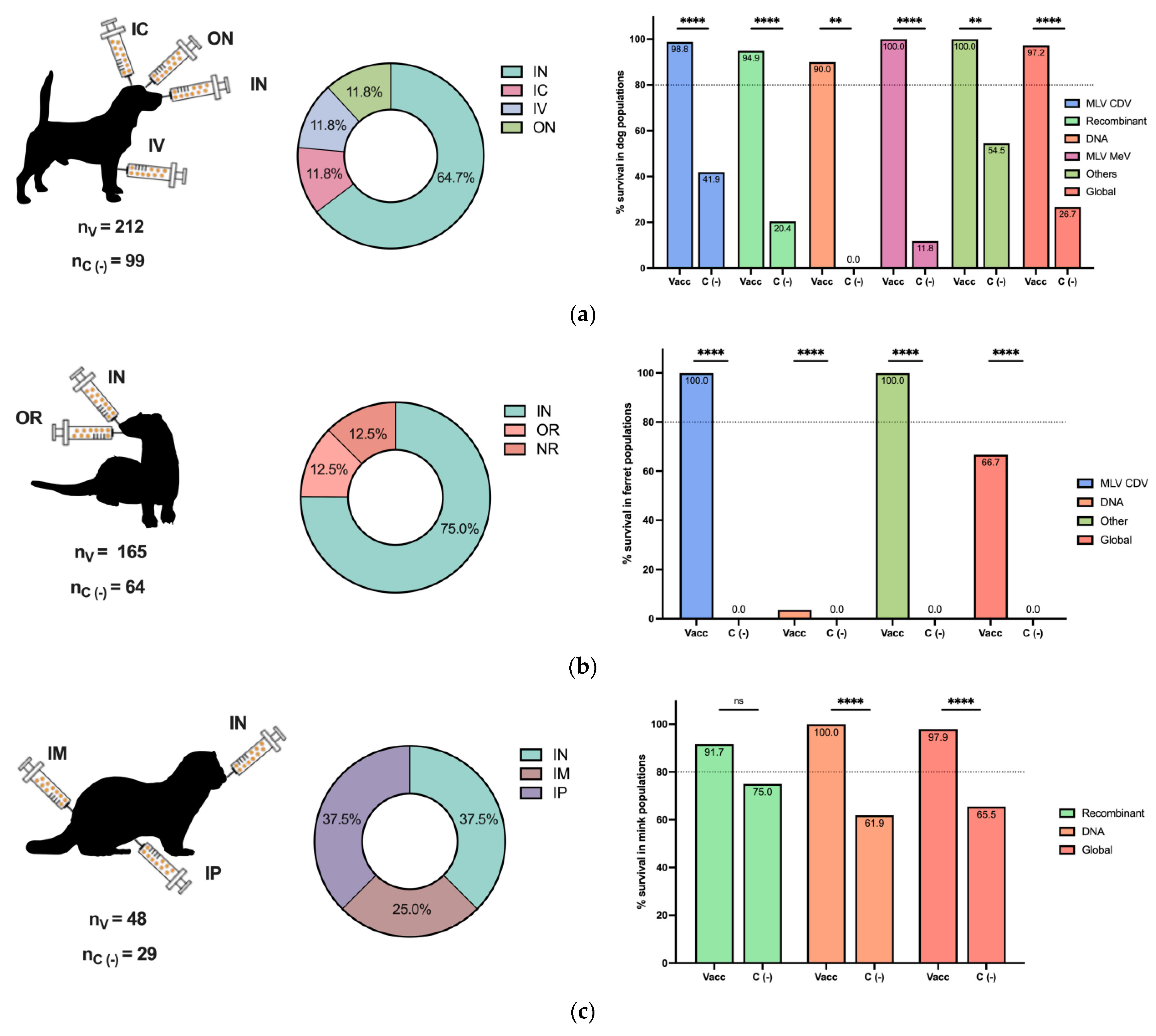
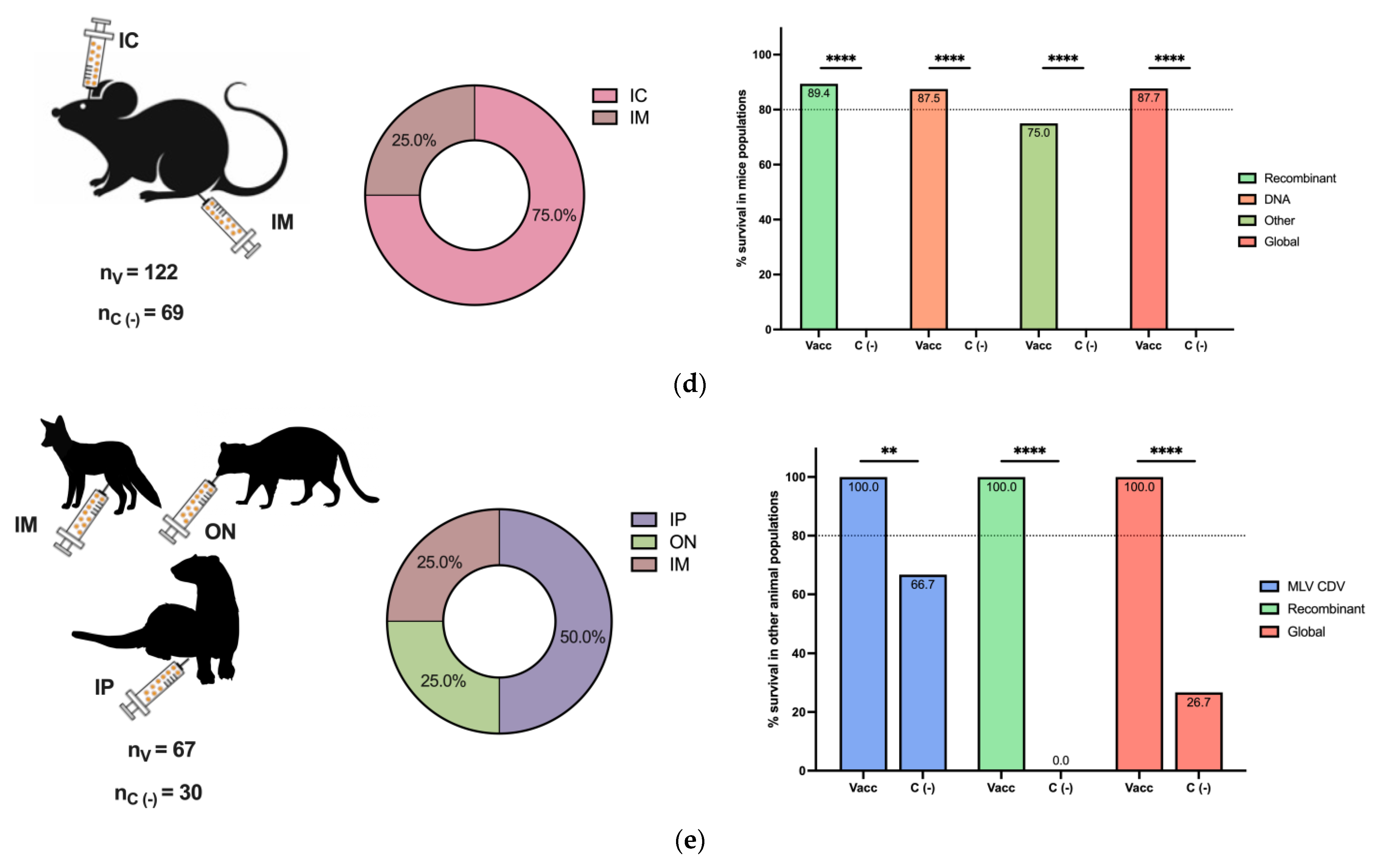
The efficacy of the CDV challenge is reported in Figure 3 for different animal populations. As expected, the dog was the most common animal included in vaccine and challenge studies, followed by ferrets and mice (Figure 3a–e, left panels). Intranasal, oral, and oronasal CDV inoculation was broadly employed in dogs, ferrets, and minks (Figure 3a–c, middle panels), since this route could simulate a natural CDV infection, in contrast to other routes, such as IM, IC, and IP, among others, used for mice and other animals (Figure 3d,e, middle panels).
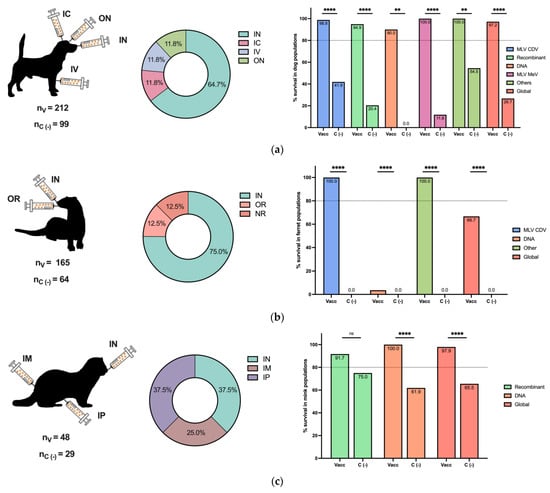
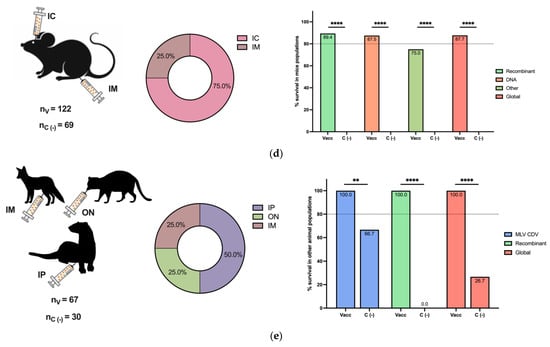
Figure 3.
Postimmunization CDV challenge in different animal populations. The numbers of included vaccinated (nV) and negative vaccination control (nc (−)) animals of all challenged species are reported. The inoculation routes are shown, as well as the percentage of survival based on the vaccine type for (a) dog populations, (b) ferret populations, (c) mink populations, and (d) mouse populations. (e) Other populations, such as foxes, raccoons, Siberian polecats, and hybrid ferrets WITH Siberian polecats, are described. Other vaccines included MLV MeV, inactivated CDV, ISCOMs, and purified H and F CDV proteins in dogs; chimeric MeV-expressing CDV antigens in ferrets; and peptide-based vaccines in mice. IN: intranasal, IC: intracranial, SC: subcutaneous, IP: intraperitoneal, IM: intramuscular, ON: oculonasal, IV: intravenous, OR: oral, ID: intradermal, IDU: intraduodenal, NR: not reported. Z test for the difference in proportions with ** p < 0.01, **** p < 0.0001 and ns: not significance.
For each animal species, the overall survival percentage was evaluated as the cumulative survival, including all vaccine platforms colored in red (Figure 3a–e, right panels). For dogs and minks, all employed vaccines exhibited a survival percentage greater than 80%, and the global survival rates of the whole population were 97.2% and 97.9%, respectively (Figure 3a,c, right panels). Similarly, for the grouped populations of foxes, raccoons, Siberian polecats, and hybrid ferrets WITH Siberian polecats, the survival percentage was greater than 80%, and the global percentage was 100% (Figure 3e, right panel). In contrast, for ferrets, 100% of the animals that received MLVs and chimeric MeV-expressing CDV survived, with a global survival rate of 66.7%, since DNA vaccines were not effective enough (Figure 3b, right panel). Finally, in mice, a peptide-based vaccine resulted in 75% survival; however, the global survival rate was 87.7% (Figure 3d, right panel). Overall, the employed vaccine platforms have demonstrated moderate to high protection against CDV infection, considering that, for all animals, survival in negative vaccination control individuals in populations such as mice and ferrets was 0.0%, and all differences were statistically significant. On the other hand, the MLV CDV vaccine demonstrated approximately 100% protection in all animal populations, indicating the importance of this type of vaccination platform; however, for other alternatives, such as DNA vaccines, their efficacy depends on the animal species (Figure 3a–e, right panels).
4. Discussion
Currently, there are different CDV vaccine alternatives for domestic dogs, including MLV vaccines, recombinant viruses, and multivalent vaccines [1,20,79]. This scoping review provides valuable insights into the safety and efficacy of CDV vaccines in vivo across domestic and wildlife animal populations, as reported in scientific databases. Through a systematic examination of the literature, this review synthesizes key findings, identifies knowledge gaps, and outlines implications for future research and vaccination strategies, since there are many species that could be affected by CDV, and most of them lack structured research on CDV vaccines.
In total, we included sixty-eight in vivo studies on CDV vaccine development (Figure 1) and research, where the United States of America, China, and France had the highest numbers of in vivo CDV vaccine trials in domestic and wildlife animals, which coincides with the authors’ affiliations (Figure 2). As reported, collaboration among researchers from different countries seems low in CDV vaccine development, as shown in Figure 2. There is a need for collaborative research in wildlife animal vaccination, new-generation effective and safe vaccines, and population-based immunization strategies to control CDV’s transmission and dissemination. This is essential in advancing scientific knowledge and addressing complex global challenges such as CDV infection for domestic and wildlife animal conservation. By pooling diverse expertise, resources, and perspectives, international collaborations foster innovation and accelerate scientific breakthroughs. Such partnerships enable the sharing of data, methodologies, and best practices, leading to more robust and reproducible findings [93]. Moreover, collaboration across borders enhances the cultural understanding and promotes mutual respect among scientists, laying the foundation for long-term partnerships that transcend geopolitical boundaries [94]. In an increasingly interconnected world, where many scientific questions require multidisciplinary approaches, international collaboration is not only beneficial but also essential in pushing the boundaries of human knowledge and tackling the pressing issues facing human and animal health [95]. Although conducting vaccination studies within wildlife populations is resource-intensive, both financially and logistically, and is influenced by various factors, including the vaccination policy recommendations of the respective countries, there is an urgent need for CDV vaccination in wildlife populations in several biodiverse regions of Asia, South America, and Africa.
Considering that CDV can affect a vast array of domestic and wildlife animals [2,3], there is a small number of species for which there are in vivo CDV vaccine studies, which are shown in Table 2, demonstrating the necessity of exploring CDV vaccines for the broad range of wildlife animals affected by CDV. This is crucial as controlling CDV transmission and dissemination in endangered wildlife animal species may contribute to the conservation of these threatened animals [16,96,97]. As expected, most studies were conducted on dogs, ferrets, and mice (Table 2). The presence of only a few studies in other species indicates an essential limitation in CDV vaccine development since there are reports about the transmission of CDV in different species [17]. The circulation of CDV lineages in domestic dogs that have been disseminated only to wild animals has been reported, indicating the importance of controlling CDV infection in domestic and wild animals [15].
There are different commercially available vaccines for CDV infection in dogs, ferrets, and minks based on MLVs and recombinant vaccines [16]. Here, MLV CDV vaccines were studied more frequently than other vaccine alternatives. Notably, recombinant viruses have been widely employed for CDV infection in different animal species (Table 3). Nevertheless, there are some experimental vaccines for CDV based on new alternatives, such as more recombinant viruses, primarily based on the canarypox backbone [26,27,28,29], recombinant mouse adenovirus 1 expressing CDV antigens [35], and formulations based on bacterium-like particles presenting CDV antigens [19]. This review indicates that the development of CDV vaccines has become an important research field since the emergence of geographically distributed CDV lineages has led to new challenges in CDV infection control and dissemination. This is because the current vaccines are based on the Onderstepoort strain, which has not circulated for many years [20] and differs from the current circulating lineages worldwide by more than 10% [14].
On the other hand, it is well known that CDV has a remarkable ability to cross species barriers [5], since the mutations affecting the CDV H protein, which is essential for virus attachment to host cell receptors and the humoral immune response, impair CDV control and facilitate the emergence of novel strains, for which the current vaccines are not completely effective [1]. The presence of antigenic variations among some of the examined CDV wild-type isolates, as well as disparities between these isolates and the vaccine strain Onderstepoort, which is presently employed worldwide, was demonstrated by a cross-neutralization assay, indicating the necessity of developing revised CDV vaccines based on the virus’ genetic and antigenic variations [98].
The safety profile of a vaccine emerges as a focal point when a new vaccine has been developed or tested in other species [99]. While the vaccines reported in this review generally exhibited an acceptable safety profile, varying degrees of adverse reactions, including local injection site reactions, temperature changes, weight loss, behavior changes, and clinical signs, could be reported, as shown in Table 4. The safety and efficacy measures underscore the importance of ongoing vaccine trials, particularly in diverse animal populations, in which the vaccine responses may differ. There are many species for which there are no data about vaccine safety and efficacy, as demonstrated in this review, based on the small number of studies in other species compared to studies in dogs, ferrets, and even minks (Table 2 and Table 4). This is a notable gap identified in this scoping review because of the limited data on CDV vaccine safety and efficacy in wildlife species. Although vaccination efforts have traditionally focused on domestic dogs, wildlife reservoirs play a significant role in CDV’s transmission dynamics [16]. The lack of comprehensive data on vaccine responses in wild animals underscores the urgency of expanding the research efforts in this area. Technical limitations must be overcome, such as the application of vaccination strategies in the field, the use of drones or immunogen bait, vaccine storage and transportation in forest zones, and the monitoring of safety and efficacy, among others, because vaccine evaluation in these animal populations has been possible only in captive animals. Moreover, this scoping review highlights that the humoral immune response has been considered an essential measure of vaccine efficacy, as has been demonstrated for diverse viral infection limitations [100], since most studies have measured nAb (Table 4).
Postimmunization challenge has been employed as an effective measure in vaccine development because it simulates the natural infection protection under controlled conditions with circulating virus strains [19]. As reported in Table 4, approximately half of the included studies investigated CDV challenge in vaccinated animals such as dogs, ferrets, minks, mice, foxes, raccoons, Siberian polecats, and hybrid ferrets x Siberian polecats. Intranasal inoculation, which reproduces the natural CDV infection [1], was the most commonly employed route (Figure 3a–e). Although most employed vaccines have demonstrated moderate to high efficacy, the MLV CDV vaccine is the most effective immunogen in dogs, ferrets, and other evaluated species, as shown in Figure 3, which is consistent with the current literature [20,99]. Moreover, other vaccines, such as DNA plasmids and recombinant viruses, could be employed in animal populations. High survival rates after CDV challenge were demonstrated, in contrast to individuals that were employed as negative vaccination controls, which developed CDD and mostly died (Figure 3a–e, right panels). As with MLV CDV vaccines, recombinant vaccines have been used in different animals, such as dogs, ferrets, minks, and red pandas, to control CDV infection and disease [101,102]. However, commercial recombinant vaccines are based on the Onderstepoort lineage, which reduces the neutralization ability of newly emerged CDV lineages [14]. Although MLVs can induce a strong immune response, attenuated viral strains may not adequately cover the spectrum of CDV variants circulating in diverse geographical regions [16].
One crucial approach to address this evolving scenario is the development of a universal CDV vaccine, as has been proposed for other viruses, such as the Alphainfluenzavirus influenzae (Influenza A virus) [103,104], considering the emergence of diverse CDV lineages. The development of a universal vaccine against CDV holds significant importance in mitigating the global impact of this highly contagious and often fatal disease in domestic and wild animals. The current commercially available vaccines offer protection against specific strains of CDV; however, the virus exhibits considerable genetic diversity, leading to vaccine escape and outbreaks among susceptible populations [13,17]. A universal vaccine targeting the conserved regions of the CDV genome could provide broad-spectrum protection against diverse viral strains. Such a vaccine would not only benefit domestic dogs but also contribute to the conservation of endangered wildlife species susceptible to CDV, such as African lions and Amur tigers [18,105].
Through diverse advances in immunology and genomics, researchers are actively exploring novel vaccine candidates capable of eliciting robust and durable immune responses against CDV. The development of a universal vaccine represents a crucial step in safeguarding the health and well-being of canine populations worldwide, as well as protecting vulnerable wildlife species from this devastating viral pathogen [106]. Efficacy assessments have revealed notable variability in vaccine performance across different animal hosts and formulations (Table 4 and Figure 3). Factors such as the vaccine strain, dose, and administration route influence the level of protection conferred against CDV infection [107]. Such variability underscores the complexity of vaccine–host interactions and highlights the need to explore new vaccination approaches. Furthermore, the vaccine efficacy in domestic and wildlife animal species emphasizes the importance of species-specific considerations in vaccine development. Peptide-based vaccines could be a potential alternative [108], because they enable the introduction of multiple immunogenic epitopes. Moreover, their safe profile due to the absence of potential reversion has been considered a notable advantage compared to the inherent risks associated with highly effective live-attenuated vaccine formulations, especially for endangered wildlife animals [109].
This scoping review underscores the imperative need for the design of standardized international trials in prototype species by family or species groups, enabling the validation of the safety and efficacy of the current and prototype vaccine technologies. Emphasis is placed on the necessity of public–private partnerships, as these vaccines have a significantly limited market, rendering them unattractive for investment by commercial enterprises [110]. Additionally, alternative vaccination systems must be developed to reach target species without direct contact with humans, akin to those established for rabies and plague viruses [111,112]; moreover, “transmissible vaccines” have emerged as a novel strategy to augment the reach and protection in wildlife populations [113,114].
Among the limitations of this scoping review were the language restrictions, as the article search was confined to Spanish, English, and Portuguese. Although these languages constitute an essential portion of the scientific production, they do not account for all of it, as studies in Mandarin, German, French, and other languages were excluded. Similarly, the full texts of many articles could not be accessed due to their unavailability. Additionally, the heterogeneity in defining vaccine safety and efficacy and the diversity of CDV vaccine platforms prevented the inclusion of a greater number of articles, since, in some cases, the information could have been confusing. Another important limitation is that the vaccine industry is not required to publish of all its developments and clinical trials in the scientific literature. Therefore, it is plausible that the industrial developmental data of vaccines may not entirely align with the data presented in the current scoping review, despite the existence of animal study guidelines such as GRADE. There are currently no standardized guidelines for the development and evaluation of biological agents, which would allow for the uniformity and systematization of all existing trials across different species. Consequently, significant developments and evaluations in species crucial for conservation efforts could not be included in this scoping review. This limitation arose due to the unavailability of the required information as inclusion criteria in published articles, as exemplified by studies on tigers (Panthera tigris) [27], pandas (Ailuropoda melanoleuca) [21,115], and red pandas (Ailurus fulgens) [101], among others. Moreover, considering that there are no specific repositories in the veterinary sciences for this type of integrative research, this scoping review protocol was not registered. Finally, the survival results combined data from different vaccines, administration routes, and strains used in the immunological challenge. Future studies should conduct meta-analyses of the efficacy considering this variability and heterogeneity.
5. Conclusions
In conclusion, this scoping review provides a comprehensive synthesis of the current literature on the safety and efficacy of CDV vaccines in vivo in domestic and wildlife animal populations. Our analysis underscores the importance of ongoing vaccine efficacy and safety evaluation, particularly in diverse animal populations affected by CDV, with insufficient vaccine alternatives or studies of commercially available vaccines. While CDV vaccines generally exhibit an acceptable safety profile in dogs, there are significant gaps in vaccine research, particularly in the context of wildlife reservoirs, since little information regarding the current vaccines and new alternatives has been described, although vaccines have shown promise as tools for the control of CDV. Addressing these knowledge gaps through targeted research efforts and collaborative initiatives is imperative for the development of effective vaccination strategies to safeguard animal health and mitigate the impact of CDV on global animal populations, especially for endangered species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v16071078/s1; Table S1: PRISMA-ScR checklist.
Author Contributions
Conceptualization, S.R.-M. and J.R.-S.; methodology, S.R.-M., L.F.H.-G. and J.R.-S.; software, S.R.-M. and L.F.H.-G.; validation, L.F.H.-G. and J.R.-S.; formal analysis, S.R.-M.; investigation, S.R.-M. and L.F.H.-G.; resources S.R.-M., L.F.H.-G. and J.R.-S.; data curation, S.R.-M. and J.R.-S.; writing—original S.R.-M.; writing—review and editing, S.R.-M., L.F.H.-G. and J.R.-S.; visualization, S.R.-M. and L.F.H.-G.; supervision, L.F.H.-G. and J.R.-S.; project administration, J.R.-S.; funding acquisition, J.R.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONADI-UCC, grant numbers INV2716 and INV3307 (TTC02), to J.R.-S. This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors. S.R.-M. received a Ph.D. fellowship from MINCIENCIAS. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Data Availability Statement
All data are presented in the paper and in the Supplementary Materials. The scooping review was registered at https://osf.io/n9sed (accessed on 22 May 2024) under the https://doi.org/10.17605/OSF.IO/N9SED.
Acknowledgments
Authors would like to thank Anke L. W. Huckriede, Francisco J. Díaz, Jorge E. Forero and Carolina Quintero for all their suggestions, advise and support in this work as part of the doctorate thesis of Santiago Rendon-Marin in CCBB from Universidad de Antioquia.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Rendon-Marin, S.; da Fontoura Budaszewski, R.; Canal, C.W.; Ruiz-Saenz, J. Tropism and molecular pathogenesis of canine distemper virus. Virol. J. 2019, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, N.; Dubovi, E.; Fenner, F. Paramyxoviridae. In Fenner’s Veterinary Virology; Academic Press: London, UK, 2011; pp. 299–325. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, M.; Ruiz-Saenz, J. Diversity of susceptible hosts in canine distemper virus infection: A systematic review and data synthesis. BMC Vet. Res. 2016, 12, 78. [Google Scholar] [CrossRef]
- Rendon-Marin, S.; Martinez-Gutierrez, M.; Suarez, J.A.; Ruiz-Saenz, J. Canine Distemper Virus (CDV) Transit Through the Americas: Need to Assess the Impact of CDV Infection on Species Conservation. Front. Microbiol. 2020, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Beineke, A.; Baumgartner, W.; Wohlsein, P. Cross-species transmission of canine distemper virus-an update. One Health 2015, 1, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lempp, C.; Spitzbarth, I.; Puff, C.; Cana, A.; Kegler, K.; Techangamsuwan, S.; Baumgartner, W.; Seehusen, F. New aspects of the pathogenesis of canine distemper leukoencephalitis. Viruses 2014, 6, 2571–2601. [Google Scholar] [CrossRef] [PubMed]
- Loots, A.K.; Mitchell, E.; Dalton, D.L.; Kotze, A.; Venter, E.H. Advances in canine distemper virus pathogenesis research: A wildlife perspective. J. Gen. Virol. 2017, 98, 311–321. [Google Scholar] [CrossRef] [PubMed]
- von Messling, V.; Zimmer, G.; Herrler, G.; Haas, L.; Cattaneo, R. The hemagglutinin of canine distemper virus determines tropism and cytopathogenicity. J. Virol. 2001, 75, 6418–6427. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.C.; Tanner, P.; Bauman, J.; Silver, K.; Fischer, L. Immunization of puppies in the presence of maternally derived antibodies against canine distemper virus. J. Comp. Pathol. 2007, 137 (Suppl. 1), S72–S75. [Google Scholar] [CrossRef]
- Zhao, S.; Han, X.; Lang, Y.; Xie, Y.; Yang, Z.; Zhao, Q.; Wen, Y.; Xia, J.; Wu, R.; Huang, X.; et al. Development and efficacy evaluation of remodeled canine parvovirus-like particles displaying major antigenic epitopes of a giant panda derived canine distemper virus. Front. Microbiol. 2023, 14, 1117135. [Google Scholar] [CrossRef]
- Ghosh, S.; Walker, J.; Jackson, D.C. Identification of canine helper T-cell epitopes from the fusion protein of canine distemper virus. Immunology 2001, 104, 58–66. [Google Scholar] [CrossRef]
- Ross, P.; Nemec, P.S.; Kapatos, A.; Miller, K.R.; Holmes, J.C.; Suter, S.E.; Buntzman, A.S.; Soderblom, E.J.; Collins, E.J.; Hess, P.R. The canine MHC class Ia allele DLA-88*508:01 presents diverse self- and canine distemper virus-origin peptides of varying length that have a conserved binding motif. Vet. Immunol. Immunopathol. 2018, 197, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Martella, V.; Cirone, F.; Elia, G.; Lorusso, E.; Decaro, N.; Campolo, M.; Desario, C.; Lucente, M.S.; Bellacicco, A.L.; Blixenkrone-Moller, M.; et al. Heterogeneity within the hemagglutinin genes of canine distemper virus (CDV) strains detected in Italy. Vet. Microbiol. 2006, 116, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Duque-Valencia, J.; Diaz, F.J.; Ruiz-Saenz, J. Phylogenomic Analysis of Two Co-Circulating Canine Distemper Virus Lineages in Colombia. Pathogens 2019, 9, 26. [Google Scholar] [CrossRef]
- Duque-Valencia, J.; Forero-Munoz, N.R.; Diaz, F.J.; Martins, E.; Barato, P.; Ruiz-Saenz, J. Phylogenetic evidence of the intercontinental circulation of a Canine distemper virus lineage in the Americas. Sci. Rep. 2019, 9, 15747. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, R.P. Canine Distemper Virus in Endangered Species: Species Jump, Clinical Variations, and Vaccination. Pathogens 2022, 12, 57. [Google Scholar] [CrossRef]
- Duque-Valencia, J.; Sarute, N.; Olarte-Castillo, X.A.; Ruiz-Saenz, J. Evolution and Interspecies Transmission of Canine Distemper Virus-An Outlook of the Diverse Evolutionary Landscapes of a Multi-Host Virus. Viruses 2019, 11, 582. [Google Scholar] [CrossRef]
- Gilbert, M.; Sulikhan, N.; Uphyrkina, O.; Goncharuk, M.; Kerley, L.; Castro, E.H.; Reeve, R.; Seimon, T.; McAloose, D.; Seryodkin, I.V.; et al. Distemper, extinction, and vaccination of the Amur tiger. Proc. Natl. Acad. Sci. USA 2020, 117, 31954–31962. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Zong, X.; Wang, C.; Zhu, G.; Yang, G.; Jiang, Y.; Yang, W.; Huang, H.; Shi, C.; et al. Immunogenicity and protective efficacy of a novel bacterium-like particle-based vaccine displaying canine distemper virus antigens in mice and dogs. Microbiol. Spectr. 2024, 12, e03477-23. [Google Scholar] [CrossRef]
- Buczkowski, H.; Muniraju, M.; Parida, S.; Banyard, A.C. Morbillivirus vaccines: Recent successes and future hopes. Vaccine 2014, 32, 3155–3161. [Google Scholar] [CrossRef]
- Bronson, E.; Deem, S.L.; Sanchez, C.; Murray, S. Serologic response to a canarypox-vectored canine distemper virus vaccine in the giant panda (Ailuropoda melanoleuca). J. Zoo Wildl. Med. 2007, 38, 363–366. [Google Scholar] [CrossRef]
- Coke, R.L.; Backues, K.A.; Hoover, J.P.; Saliki, J.T.; Ritchey, J.W.; West, G.D. Serologic responses after vaccination of fennec foxes (Vulpes zerda) and meerkats (Suricata suricatta) with a live, canarypox-vectored canine distemper virus vaccine. J. Zoo Wildl. Med. 2005, 36, 326–330. [Google Scholar] [CrossRef]
- Stephensen, C.B.; Welter, J.; Thaker, S.R.; Taylor, J.; Tartaglia, J.; Paoletti, E. Canine distemper virus (CDV) infection of ferrets as a model for testing Morbillivirus vaccine strategies: NYVAC- and ALVAC-based CDV recombinants protect against symptomatic infection. J. Virol. 1997, 71, 1506–1513. [Google Scholar] [CrossRef]
- Wimsatt, J.; Biggins, D.; Innes, K.; Taylor, B.; Garell, D. Evaluation of oral and subcutaneous delivery of an experimental canarypox recombinant canine distemper vaccine in the Siberian polecat (Mustela eversmanni). J. Zoo Wildl. Med. 2003, 34, 25–35. [Google Scholar] [CrossRef]
- Schultz, R.D. Duration of immunity for canine and feline vaccines: A review. Vet. Microbiol. 2006, 117, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhao, Z.; Xue, X.; Zheng, W.; Xu, T.; Liu, L.; Tian, L.; Wang, X.; He, H.; Zheng, X. A Bivalent Human Adenovirus Type 5 Vaccine Expressing the Rabies Virus Glycoprotein and Canine Distemper Virus Hemagglutinin Protein Confers Protective Immunity in Mice and Foxes. Front. Microbiol. 2020, 11, 1070. [Google Scholar] [CrossRef] [PubMed]
- Sadler, R.A.; Ramsay, E.; McAloose, D.; Rush, R.; Wilkes, R.P. Evaluation of Two Canine Distemper Virus Vaccines in Captive Tigers (Panthera tigris). J. Zoo Wildl. Med. 2016, 47, 558–563. [Google Scholar] [CrossRef]
- Pujol, J.; Rousseau, C.; Vergneau-Grosset, C. Serologic Response and Adverse Effects of Recombinant Canine Distemper Vaccination in Three Aquarium-Housed Walruses (Odobenus rosmarus). J. Zoo Wildl. Med. 2023, 54, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chen, T.; Feng, N.; Meng, X.; Sun, W.; Wang, T.; Zhao, Y.; Yang, S.; Song, X.; Li, W.; et al. A highly efficient recombinant canarypox virus-based vaccine against canine distemper virus constructed using the CRISPR/Cas9 gene editing method. Vet. Microbiol. 2020, 251, 108920. [Google Scholar] [CrossRef]
- Rouxel, R.N.; Svitek, N.; von Messling, V. A chimeric measles virus with canine distemper envelope protects ferrets from lethal distemper challenge. Vaccine 2009, 27, 4961–4966. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, Y.; Sui, P.; Pan, H.; Shi, Y.; Chen, J.; Zhang, H.; Wang, X.; Tao, R.; Liu, M.; et al. DNA Vaccine Co-Expressing Hemagglutinin and IFN-gamma Provides Partial Protection to Ferrets against Lethal Challenge with Canine Distemper Virus. Viruses 2023, 15, 1873. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Ludlow, M.; van Amerongen, G.; de Vries, R.D.; Yuksel, S.; Verburgh, R.J.; Osterhaus, A.D.; Duprex, W.P.; de Swart, R.L. Evaluation of synthetic infection-enhancing lipopeptides as adjuvants for a live-attenuated canine distemper virus vaccine administered intra-nasally to ferrets. Vaccine 2012, 30, 5073–5080. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.; Sogaard, M.; Karlskov-Mortensen, P.; Jensen, T.H.; Jensen, T.D.; Aasted, B.; Blixenkrone-Moller, M. Humoral and cell-mediated immune responses in DNA immunized mink challenged with wild-type canine distemper virus. Vaccine 2009, 27, 4791–4797. [Google Scholar] [CrossRef] [PubMed]
- Norrby, E.; Utter, G.; Orvell, C.; Appel, M.J. Protection against canine distemper virus in dogs after immunization with isolated fusion protein. J. Virol. 1986, 58, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Goffin, E.; Gillard, L.; Machiels, B.; Gillet, L. A Single Oral Immunization with Replication-Competent Adenovirus-Vectored Vaccine Induces a Neutralizing Antibody Response in Mice against Canine Distemper Virus. Viruses 2022, 14, 1847. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Burgher, J.A.; Baker, J.A.; Sarkar, S.; Marshall, V.; Gillespie, J.H. Evaluation of a combined vaccine consisting of modified canine distemper virus and modified infectious canine hepatitis virus for simultaneous immunization of dogs. Cornell Vet. 1958, 48, 214–223. [Google Scholar] [PubMed]
- Gillespie, J.H. A Study of Inactivated Distemper Virus in the Dog. Cornell Vet. 1965, 55, 3–8. [Google Scholar]
- Ackermann, O. Comparative Experimental Studies on Vaccines against Distemper and Canine Viral Hepatitis. J. Small Anim. Pract. 1965, 6, 171–184. [Google Scholar] [CrossRef]
- Norrby, E.; Appel, M.J. Humoral immunity to canine distemper after immunization of dogs with inactivated and live measles virus. Arch. Virol. 1980, 66, 169–177. [Google Scholar] [CrossRef]
- Appel, M.J.; Shek, W.R.; Shesberadaran, H.; Norrby, E. Measles virus and inactivated canine distemper virus induce incomplete immunity to canine distemper. Arch. Virol. 1984, 82, 73–82. [Google Scholar] [CrossRef]
- Mastro, J.M.; Axthelm, M.; Mathes, L.E.; Krakowka, S.; Ladiges, W.; Olsen, R.G. Repeated suppression of lymphocyte blastogenesis following vaccinations of CPV-immune dogs with modified-live CPV vaccines. Vet. Microbiol. 1986, 12, 201–211. [Google Scholar] [CrossRef] [PubMed]
- De Vries, P.; Uytdehaag, F.G.; Osterhaus, A.D. Canine distemper virus (CDV) immune-stimulating complexes (Iscoms), but not measles virus iscoms, protect dogs against CDV infection. J. Gen. Virol. 1988, 69 Pt 8, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Pincus, S.; Tartaglia, J.; Richardson, C.; Alkhatib, G.; Briedis, D.; Appel, M.; Norton, E.; Paoletti, E. Vaccinia virus recombinants expressing either the measles virus fusion or hemagglutinin glycoprotein protect dogs against canine distemper virus challenge. J. Virol. 1991, 65, 4263–4274. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Weinberg, R.; Tartaglia, J.; Richardson, C.; Alkhatib, G.; Briedis, D.; Appel, M.; Norton, E.; Paoletti, E. Nonreplicating viral vectors as potential vaccines: Recombinant canarypox virus expressing measles virus fusion (F) and hemagglutinin (HA) glycoproteins. Virology 1992, 187, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Wild, T.F.; Bernard, A.; Spehner, D.; Villeval, D.; Drillien, R. Vaccination of mice against canine distemper virus-induced encephalitis with vaccinia virus recombinants encoding measles or canine distemper virus antigens. Vaccine 1993, 11, 438–444. [Google Scholar] [CrossRef]
- Chalmers, W.S.; Baxendale, W. A comparison of canine distemper vaccine and measles vaccine for the prevention of canine distemper in young puppies. Vet. Rec. 1994, 135, 349–353. [Google Scholar] [CrossRef]
- Goodrich, J.M.; Williams, E.S.; Buskirk, S.W. Effects of a modified-live virus canine distemper vaccine on captive badgers (Taxidea taxus). J. Wildl. Dis. 1994, 30, 492–496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Obeid, O.E.; Partidos, C.D.; Howard, C.R.; Steward, M.W. Protection against morbillivirus-induced encephalitis by immunization with a rationally designed synthetic peptide vaccine containing B- and T-cell epitopes from the fusion protein of measles virus. J. Virol. 1995, 69, 1420–1428. [Google Scholar] [CrossRef]
- Williams, E.S.; Anderson, S.L.; Cavender, J.; Lynn, C.; List, K.; Hearn, C.; Appel, M.J. Vaccination of black-footed ferret (Mustela nigripes) × Siberian polecat (M. eversmanni) hybrids and domestic ferrets (M. putorius furo)against canine distemper. J. Wildl. Dis. 1996, 32, 417–423. [Google Scholar] [CrossRef]
- Pardo, M.C.; Bauman, J.E.; Mackowiak, M. Protection of dogs against canine distemper by vaccination with a canarypox virus recombinant expressing canine distemper virus fusion and hemagglutinin glycoproteins. Am. J. Vet. Res. 1997, 58, 833–836. [Google Scholar] [CrossRef]
- Sixt, N.; Cardoso, A.; Vallier, A.; Fayolle, J.; Buckland, R.; Wild, T.F. Canine distemper virus DNA vaccination induces humoral and cellular immunity and protects against a lethal intracerebral challenge. J. Virol. 1998, 72, 8472–8476. [Google Scholar] [CrossRef] [PubMed]
- Pare, J.A.; Barker, I.K.; Crawshaw, G.J.; McEwen, S.A.; Carman, P.S.; Johnson, R.P. Humoral response and protection from experimental challenge following vaccination of raccoon pups with a modified-live canine distemper virus vaccine. J. Wildl. Dis. 1999, 35, 430–439. [Google Scholar] [CrossRef]
- Welter, J.; Taylor, J.; Tartaglia, J.; Paoletti, E.; Stephensen, C.B. Mucosal vaccination with recombinant poxvirus vaccines protects ferrets against symptomatic CDV infection. Vaccine 1999, 17, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Cherpillod, P.; Tipold, A.; Griot-Wenk, M.; Cardozo, C.; Schmid, I.; Fatzer, R.; Schobesberger, M.; Zurbriggen, R.; Bruckner, L.; Roch, F.; et al. DNA vaccine encoding nucleocapsid and surface proteins of wild type canine distemper virus protects its natural host against distemper. Vaccine 2000, 18, 2927–2936. [Google Scholar] [CrossRef] [PubMed]
- Coyne, M.J. Seroconversion of puppies to canine parvovirus and canine distemper virus: A comparison of two combination vaccines. J. Am. Anim. Hosp. Assoc. 2000, 36, 137–142. [Google Scholar] [CrossRef]
- Welter, J.; Taylor, J.; Tartaglia, J.; Paoletti, E.; Stephensen, C.B. Vaccination against canine distemper virus infection in infant ferrets with and without maternal antibody protection, using recombinant attenuated poxvirus vaccines. J. Virol. 2000, 74, 6358–6367. [Google Scholar] [CrossRef] [PubMed]
- Wimsatt, J.; Jay, M.T.; Innes, K.E.; Jessen, M.; Collins, J.K. Serologic evaluation, efficacy, and safety of a commerical modified-live canine distemper vaccine in domestic ferrets. Am. J. Vet. Res. 2001, 62, 736–740. [Google Scholar] [CrossRef]
- Biazzono, L.; Hagiwara, M.K.; Corrêa, A.R. Evaluation of humoral immune response in puppies immunized to canine distemper with attenuated virus vaccine. J. Vet. Res. Anim. Sci. 2001, 38, 245–250. [Google Scholar] [CrossRef]
- Rikula, U.; Pankala, L.; Jalkanen, L.; Sihvonen, L. Distemper vaccination of farmed fur animals in Finland. Prev. Vet. Med. 2001, 49, 125–133. [Google Scholar] [CrossRef]
- Griot-Wenk, M.E.; Cherpillod, P.; Koch, A.; Zurbriggen, R.; Bruckner, L.; Wittek, R.; Zurbriggen, A. The humoral immune response to recombinant nucleocapsid antigen of canine distemper virus in dogs vaccinated with attenuated distemper virus or DNA encoding the nucleocapsid of wild-type virus. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2001, 48, 295–302. [Google Scholar] [CrossRef]
- van Heerden, J.; Bingham, J.; van Vuuren, M.; Burroughs, R.E.; Stylianides, E. Clinical and serological response of wild dogs (Lycaon pictus) to vaccination against canine distemper, canine parvovirus infection and rabies. J. S. Afr. Vet. Assoc. 2002, 73, 8–12. [Google Scholar] [CrossRef]
- Renddy, G.S.; Srinivasan, V.A. Comparative efficacy of combined vaccine for canines containing canine distemper, hepatitis, parvovirus, Rabies and Leptospira antigens and monovalent vaccines. Indian Vet. J. 2002, 79, 435–439. [Google Scholar]
- Fischer, L.; Tronel, J.P.; Pardo-David, C.; Tanner, P.; Colombet, G.; Minke, J.; Audonnet, J.C. Vaccination of puppies born to immune dams with a canine adenovirus-based vaccine protects against a canine distemper virus challenge. Vaccine 2002, 20, 3485–3497. [Google Scholar] [CrossRef]
- Fischer, L.; Tronel, J.P.; Minke, J.; Barzu, S.; Baudu, P.; Audonnet, J.C. Vaccination of puppies with a lipid-formulated plasmid vaccine protects against a severe canine distemper virus challenge. Vaccine 2003, 21, 1099–1102. [Google Scholar] [CrossRef]
- Dahl, L.; Jensen, T.H.; Gottschalck, E.; Karlskov-Mortensen, P.; Jensen, T.D.; Nielsen, L.; Andersen, M.K.; Buckland, R.; Wild, T.F.; Blixenkrone-Moller, M. Immunization with plasmid DNA encoding the hemagglutinin and the nucleoprotein confers robust protection against a lethal canine distemper virus challenge. Vaccine 2004, 22, 3642–3648. [Google Scholar] [CrossRef]
- Cirone, F.; Elia, G.; Campolo, M.; Friedrich, K.; Martella, V.; Pratelli, A.; Buonavoglia, C. Immunogenicity of an inactivated oil-emulsion canine distemper vaccine in African wild dogs. J. Wildl. Dis. 2004, 40, 343–346. [Google Scholar] [CrossRef]
- Jacobs, A.A.; Bergman, J.G.; Theelen, R.P.; Jaspers, R.; Helps, J.M.; Horspool, L.J.; Paul, G. Compatibility of a bivalent modified-live vaccine against Bordetella bronchiseptica and CPiV, and a trivalent modified-live vaccine against CPV, CDV and CAV-2. Vet. Rec. 2007, 160, 41–45. [Google Scholar] [CrossRef]
- Silin, D.; Lyubomska, O.; Ludlow, M.; Duprex, W.P.; Rima, B.K. Development of a challenge-protective vaccine concept by modification of the viral RNA-dependent RNA polymerase of canine distemper virus. J. Virol. 2007, 81, 13649–13658. [Google Scholar] [CrossRef]
- Pavlačík, L.; Celer, V., Jr.; Kajerová, V.; Jekl, V.; Knotek, Z.; Literák, I. Monitoring of Antibodies Titre Against Canine Distemper Virus in Ferrets Vaccinated with a Live Modified Vaccine. Acta Vet. Brno 2007, 76, 423–429. [Google Scholar] [CrossRef]
- Jessup, D.A.; Murray, M.J.; Casper, D.R.; Brownstein, D.; Kreuder-Johnson, C. Canine distemper vaccination is a safe and useful preventive procedure for southern sea otters (Enhydra lutra nereis). J. Zoo Wildl. Med. 2009, 40, 705–710. [Google Scholar] [CrossRef]
- Jensen, T.H.; Nielsen, L.; Aasted, B.; Blixenkrone-Moller, M. Early life DNA vaccination with the H gene of Canine distemper virus induces robust protection against distemper. Vaccine 2009, 27, 5178–5183. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.; Jensen, T.H.; Kristensen, B.; Jensen, T.D.; Karlskov-Mortensen, P.; Lund, M.; Aasted, B.; Blixenkrone-Moller, M. DNA vaccines encoding proteins from wild-type and attenuated canine distemper virus protect equally well against wild-type virus challenge. Arch. Virol. 2012, 157, 1887–1896. [Google Scholar] [CrossRef]
- Mitchell, S.A.; Zwijnenberg, R.J.; Huang, J.; Hodge, A.; Day, M.J. Duration of serological response to canine parvovirus-type 2, canine distemper virus, canine adenovirus type 1 and canine parainfluenza virus in client-owned dogs in Australia. Aust. Vet. J. 2012, 90, 468–473. [Google Scholar] [CrossRef]
- Connolly, M.; Thomas, P.; Woodroffe, R.; Raphael, B.L. Comparison of oral and intramuscular recombinant canine distemper vaccination in African wild dogs (Lycaon pictus). J. Zoo Wildl. Med. 2013, 44, 882–888. [Google Scholar] [CrossRef]
- Quinley, N.; Mazet, J.A.; Rivera, R.; Schmitt, T.L.; Dold, C.; McBain, J.; Fritsch, V.; Yochem, P.K. Serologic response of harbor seals (Phoca vitulina) to vaccination with a recombinant canine distemper vaccine. J. Wildl. Dis. 2013, 49, 579–586. [Google Scholar] [CrossRef]
- Peper, S.T.; Peper, R.L.; Kollias, G.V.; Brooks, R.P.; Stevens, S.S.; Serfass, T.L. Efficacy of two canine distemper vaccines in wild Nearctic river otters (Lontra canadensis). J. Zoo Wildl. Med. 2014, 45, 520–526. [Google Scholar] [CrossRef]
- Wilson, S.; Siedek, E.; Thomas, A.; King, V.; Stirling, C.; Plevová, E.; Salt, J.; Sture, G. Influence of maternally-derived antibodies in 6-week old dogs for the efficacy of a new vaccine to protect dogs against virulent challenge with canine distemper virus, adenovirus or parvovirus. Trials Vaccinol. 2014, 3, 107–113. [Google Scholar] [CrossRef]
- Wang, F.X.; Zhang, S.Q.; Zhu, H.W.; Yang, Y.; Sun, N.; Tan, B.; Li, Z.G.; Cheng, S.P.; Fu, Z.F.; Wen, Y.J. Recombinant rabies virus expressing the H protein of canine distemper virus protects dogs from the lethal distemper challenge. Vet. Microbiol. 2014, 174, 362–371. [Google Scholar] [CrossRef]
- Wilson, S.; Illambas, J.; Siedek, E.; Thomas, A.; King, V.; Stirling, C.; Plevová, E.; Salt, J.; Sture, G. The administration of a single dose of a multivalent (DHPPiL4R) vaccine prevents clinical signs and mortality following virulent challenge with canine distemper virus, canine adenovirus or canine parvovirus. Trials Vaccinol. 2014, 3, 101–106. [Google Scholar] [CrossRef]
- Ge, J.; Wang, X.; Tian, M.; Gao, Y.; Wen, Z.; Yu, G.; Zhou, W.; Zu, S.; Bu, Z. Recombinant Newcastle disease viral vector expressing hemagglutinin or fusion of canine distemper virus is safe and immunogenic in minks. Vaccine 2015, 33, 2457–2462. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, E.; Sadler, R.; Rush, R.; Seimon, T.; Tomaszewicz, A.; Fleetwood, E.A.; McAloose, D.; Wilkes, R.P. Canine Distemper Virus Antibody Titers in Domestic Cats after Delivery of a Live Attenuated Virus Vaccine. J. Zoo Wildl. Med. 2016, 47, 551–557. [Google Scholar] [CrossRef]
- Sheldon, J.D.; Cushing, A.C.; Wilkes, R.P.; Anis, E.; Dubovi, E.J. Serologic Response to Canine Distemper Vaccination in Captive Linnaeus’s Two-Toed Sloths (Choloepus didactylus) after a Fatal Canine Distemper Virus Outbreak. J. Zoo Wildl. Med. 2017, 48, 1250–1253. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Liu, J.; Ma, J.; Jin, Q.; Yao, H.; Osterrieder, N. The recombinant EHV-1 vector producing CDV hemagglutinin as potential vaccine against canine distemper. Microb. Pathog. 2017, 111, 388–394. [Google Scholar] [CrossRef]
- Perdrizet, J.A.; Shiau, D.S.; Xie, H. The serological response in dogs inoculated with canine distemper virus vaccine at the acupuncture point governing vessel-14: A randomized controlled trial. Vaccine 2019, 37, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, M.; Yan, X.G.; Chen, Y.X.; Cui, M.; Chen, H.C.; Fu, Z.F.; Zhao, L. A recombinant canine distemper virus expressing interleukin-7 enhances humoral immunity. J. Gen. Virol. 2019, 100, 602–615. [Google Scholar] [CrossRef]
- Jiang, Y.; Jia, S.; Zheng, D.; Li, F.; Wang, S.; Wang, L.; Qiao, X.; Cui, W.; Tang, L.; Xu, Y.; et al. Protective Immunity against Canine Distemper Virus in Dogs Induced by Intranasal Immunization with a Recombinant Probiotic Expressing the Viral H Protein. Vaccines 2019, 7, 213. [Google Scholar] [CrossRef]
- Hidalgo-Hermoso, E.; Mathieu-Benson, C.; Celis-Diez, S.; Soto-Guerrero, P.; Carmona-Schmidt, S.; Cabello-Stom, J.; Ortiz-Tacchi, C. Safety and Serological Response to Multivalent Canine Distemper Virus Vaccine in Red Foxes (Vulpes vulpes). J. Zoo Wildl. Med. 2019, 50, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.A.; Joyner, P.H.; Anis, E.; Wilkes, R.P.; Aitken-Palmer, C. Safety of and Humoral Immune Response to the Merial Recombitek Canine Distemper Virus Vaccine in Maned Wolves (Chrysocyon brachyurus). J. Zoo Wildl. Med. 2020, 50, 972–975. [Google Scholar] [CrossRef]
- McEntire, M.; Ramsay, E.C.; Kania, S.; Prestia, P.; Anis, E.; Cushing, A.C.; Wilkes, R.P. Tiger (Panthera tigris) and Domestic Cat (Felis catus) Immune Responses to Canarypox-Vectored Canine Distemper Vaccination. J. Zoo Wildl. Med. 2020, 50, 798–802. [Google Scholar] [CrossRef]
- Mulreany, L.M.; Cushing, A.C.; Ramsay, E.C. Canine Distemper and Parvovirus Vaccination with Recombitek C3 in African Wild Dogs (Lycaon pictus). J. Zoo Wildl. Med. 2021, 52, 1229–1233. [Google Scholar] [CrossRef]
- Thibault, J.C.; Bouvet, J.; Cupillard, L.; Cariou, C.; Oberli, F. Compatibility between a rabies vaccine and two canine combined vaccines against canine distemper, adenovirosis, parvovirosis, parainfluenza virus disease and leptospirosis, with or without canine coronavirus. Comp. Immunol. Microbiol. Infect. Dis. 2022, 86, 101803. [Google Scholar] [CrossRef]
- Pagliusi, S.; Che, Y.; Dong, S. The art of partnerships for vaccines. Vaccine 2019, 37, 5909–5919. [Google Scholar] [CrossRef]
- Francisco, J.S. International Scientific Collaborations: A Key to Scientific Success. Angew. Chem. Int. Ed. Engl. 2015, 54, 14984–14985. [Google Scholar] [CrossRef] [PubMed]
- Bregu, M.; Draper, S.J.; Hill, A.V.; Greenwood, B.M. Accelerating vaccine development and deployment: Report of a Royal Society satellite meeting. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 2841–2849. [Google Scholar] [CrossRef]
- Gilbert, M.; Soutyrina, S.V.; Seryodkin, I.V.; Sulikhan, N.; Uphyrkina, O.V.; Goncharuk, M.; Matthews, L.; Cleaveland, S.; Miquelle, D.G. Canine distemper virus as a threat to wild tigers in Russia and across their range. Integr. Zool. 2015, 10, 329–343. [Google Scholar] [CrossRef]
- van de Bildt, M.W.; Kuiken, T.; Visee, A.M.; Lema, S.; Fitzjohn, T.R.; Osterhaus, A.D. Distemper outbreak and its effect on African wild dog conservation. Emerg. Infect. Dis. 2002, 8, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Anis, E.; Holford, A.L.; Galyon, G.D.; Wilkes, R.P. Antigenic analysis of genetic variants of Canine distemper virus. Vet. Microbiol. 2018, 219, 154–160. [Google Scholar] [CrossRef]
- Woodroffe, R. Modified Live Distemper Vaccines Carry Low Mortality Risk for Captive African Wild Dogs, Lycaon Pictus. J. Zoo Wildl. Med. 2021, 52, 176–184. [Google Scholar] [CrossRef]
- Schmid, E.; Zurbriggen, A.; Gassen, U.; Rima, B.; ter Meulen, V.; Schneider-Schaulies, J. Antibodies to CD9, a tetraspan transmembrane protein, inhibit canine distemper virus-induced cell-cell fusion but not virus-cell fusion. J. Virol. 2000, 74, 7554–7561. [Google Scholar] [CrossRef]
- Ramsay, E.C.; Georoff, T.A.; Burrell, C.; Anis, E.; Wilkes, R.P. Red Pandas’ (Ailurus fulgens) Serological Response to Canarypox-Vectored Canine Distemper Vaccines. J. Zoo Wildl. Med. 2019, 50, 478–481. [Google Scholar] [CrossRef]
- Wang, X.; Feng, N.; Ge, J.; Shuai, L.; Peng, L.; Gao, Y.; Yang, S.; Xia, X.; Bu, Z. Recombinant canine distemper virus serves as bivalent live vaccine against rabies and canine distemper. Vaccine 2012, 30, 5067–5072. [Google Scholar] [CrossRef] [PubMed]
- Stanekova, Z.; Vareckova, E. Conserved epitopes of influenza A virus inducing protective immunity and their prospects for universal vaccine development. Virol. J. 2010, 7, 351. [Google Scholar] [CrossRef]
- Opriessnig, T.; Gauger, P.C.; Filippsen Favaro, P.; Rawal, G.; Magstadt, D.R.; Digard, P.; Lee, H.M.; Halbur, P.G. An experimental universal swine influenza a virus (IAV) vaccine candidate based on the M2 ectodomain (M2e) peptide does not provide protection against H1N1 IAV challenge in pigs. Vaccine 2024, 42, 220–228. [Google Scholar] [CrossRef]
- Weckworth, J.K.; Davis, B.W.; Roelke-Parker, M.E.; Wilkes, R.P.; Packer, C.; Eblate, E.; Schwartz, M.K.; Mills, L.S. Identifying Candidate Genetic Markers of CDV Cross-Species Pathogenicity in African Lions. Pathogens 2020, 9, 872. [Google Scholar] [CrossRef]
- Martella, V.; Elia, G.; Buonavoglia, C. Canine distemper virus. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 787–797. [Google Scholar] [CrossRef]
- Gonzalez, S.E.; Gogal, R.M., Jr.; Meindl, A.G.; Boyer, N.; Nelson, S.; Everett, S.E.; Vetter, C.A.; Gonzalez, J.M. Influence of age and vaccination interval on canine parvovirus, distemper virus, and adenovirus serum antibody titers. Vet. Immunol. Immunopathol. 2023, 262, 110630. [Google Scholar] [CrossRef] [PubMed]
- Tahir Ul Qamar, M.; Rehman, A.; Tusleem, K.; Ashfaq, U.A.; Qasim, M.; Zhu, X.; Fatima, I.; Shahid, F.; Chen, L.L. Designing of a next generation multiepitope based vaccine (MEV) against SARS-CoV-2: Immunoinformatics and in silico approaches. PLoS ONE 2020, 15, e0244176. [Google Scholar] [CrossRef] [PubMed]
- Purcell, A.W.; McCluskey, J.; Rossjohn, J. More than one reason to rethink the use of peptides in vaccine design. Nat. Rev. Drug Discov. 2007, 6, 404–414. [Google Scholar] [CrossRef]
- McDowell, A. Pharmaceutics for free-ranging wildlife: Case studies to illustrate considerations and future prospects. Int. J. Pharm. 2022, 628, 122284. [Google Scholar] [CrossRef]
- Ballesteros, C.; de la Lastra, J.M.; de la Fuente, J. Recent developments in oral bait vaccines for wildlife. Recent. Pat. Drug Deliv. Formul. 2007, 1, 230–235. [Google Scholar] [CrossRef]
- Abbott, R.C.; Osorio, J.E.; Bunck, C.M.; Rocke, T.E. Sylvatic plague vaccine: A new tool for conservation of threatened and endangered species? Ecohealth 2012, 9, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Smithson, M.W.; Basinki, A.J.; Nuismer, S.L.; Bull, J.J. Transmissible vaccines whose dissemination rates vary through time, with applications to wildlife. Vaccine 2019, 37, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Streicker, D.G.; Griffiths, M.E.; Antia, R.; Bergner, L.; Bowman, P.; dos Santos de Moraes, M.V.; Esvelt, K.; Famulare, M.; Gilbert, A.; He, B.; et al. Developing transmissible vaccines for animal infections. Science 2024, 348, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Shen, F.; Wu, W.; Zhang, L.; Luo, L.; Fan, Z.; Hou, R.; Yue, B.; Zhang, X. First demonstration of giant panda’s immune response to canine distemper vaccine. Dev. Comp. Immunol. 2020, 102, 103489. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).