Rotavirus Sickness Symptoms: Manifestations of Defensive Responses from the Brain
Abstract
1. Introduction
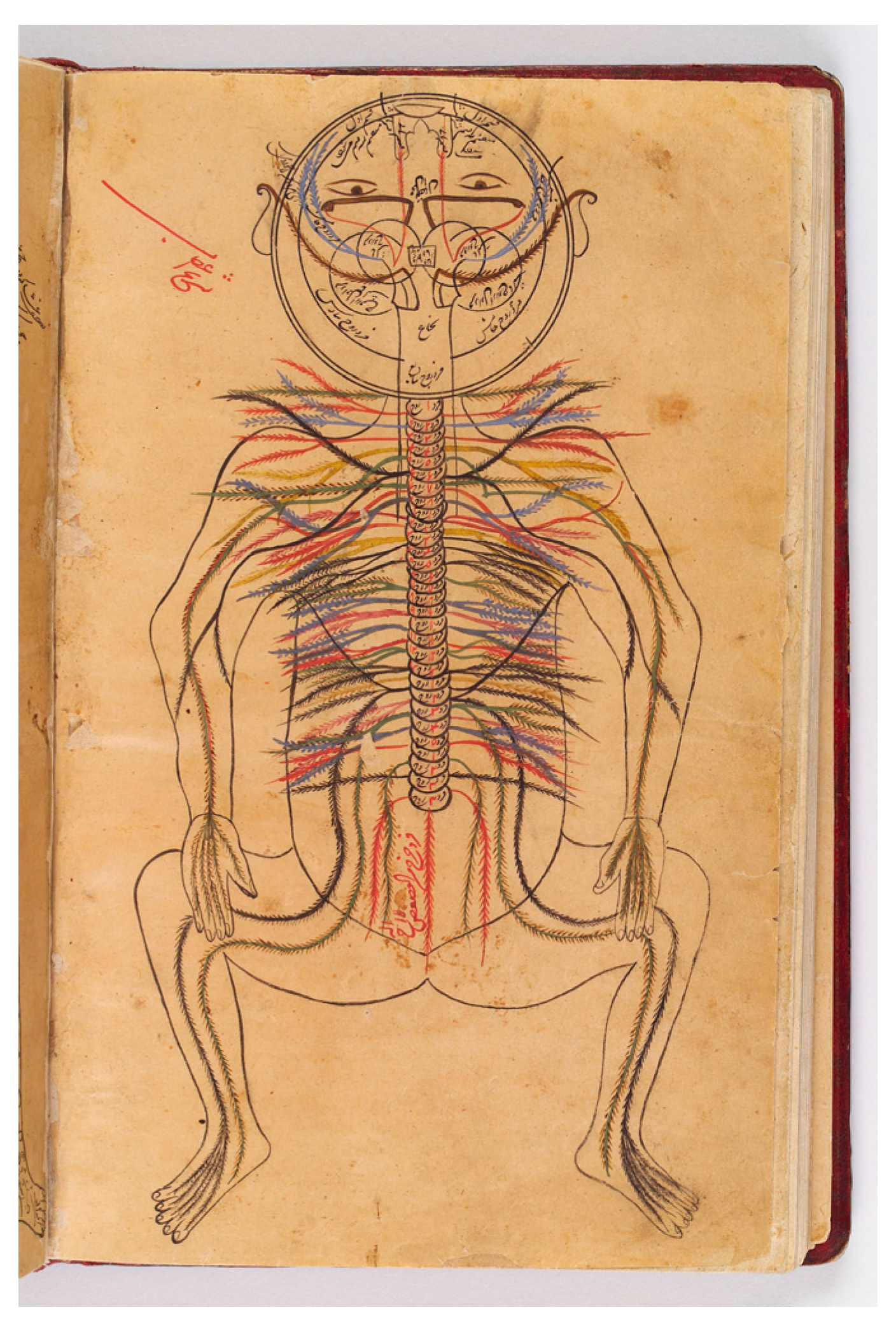
2. Gut–Brain Crosstalk
2.1. Ascending Pathways
2.1.1. Nervous Signaling from the Gastrointestinal Tract
2.1.2. Direct Invasion of the Brain
2.1.3. Chemical Signaling
2.2. Descending Pathways
3. Central Coordination of Defense
4. Sickness Symptoms
4.1. Diarrhea
4.2. Vomiting
4.3. Fever
4.4. Fatigue and Sleepiness
4.5. Stress
4.6. Loss of Appetite
5. Evolution of Defense Strategies
6. Conclusions
Funding
Conflicts of Interest
References
- Grimwood, K.; Lambert, S.B. Rotavirus vaccines: Opportunities and challenges. Hum. Vaccines 2009, 5, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Weber, S.G. Rotavirus infection in adults. Lancet Infect. Dis. 2004, 4, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.N.; Husberg, M.; Bennet, R.; Brytting, M.; Carlsson, P.; Eriksson, M.; Storsaeter, J.; Osterlin, B.; Johansen, K. Impact on affected families and society of severe rotavirus infections in Swedish children assessed in a prospective cohort study. Infect. Dis. 2018, 50, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Chia, G.; Ho, H.J.; Ng, C.G.; Neo, F.J.; Win, M.K.; Cui, L.; Leo, Y.S.; Chow, A. An unusual outbreak of rotavirus G8P[8] gastroenteritis in adults in an urban community, Singapore, 2016. J. Clin. Virol. 2018, 105, 57–63. [Google Scholar] [CrossRef]
- Niendorf, S.; Ebner, W.; Marques, A.M.; Bierbaum, S.; Babikir, R.; Huzly, D.; Maassen, S.; Grundmann, H.; Panning, M. Rotavirus outbreak among adults in a university hospital in Germany. J. Clin. Virol. 2020, 129, 104532. [Google Scholar] [CrossRef] [PubMed]
- Pacilli, M.; Cortese, M.M.; Smith, S.; Siston, A.; Samala, U.; Bowen, M.D.; Parada, J.P.; Tam, K.I.; Rungsrisuriyachai, K.; Roy, S.; et al. Outbreak of Gastroenteritis in Adults Due to Rotavirus Genotype G12P[8]. Clin. Infect. Dis. 2015, 61, e20–e25. [Google Scholar] [CrossRef]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Steele, A.D.; Duque, J.; Parashar, U.D.; MBBS the WHO-Coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 136–141. [Google Scholar] [CrossRef]
- Hellysaz, A.; Neijd, M.; Vesikari, T.; Svensson, L.; Hagbom, M. Viral gastroenteritis: Sickness symptoms and behavioral responses. mBio 2023, 14, e0356722. [Google Scholar] [CrossRef]
- Edwards, N.; Abasszade, J.H.; Nan, K.; Abrahams, T.; La, P.B.D.; Tinson, A.J. Severe adult rotavirus gastroenteritis: A rare case with multi-organ failure and critical management. Am. J. Case Rep. 2023, 24, e940967. [Google Scholar] [CrossRef]
- Ramig, R.F. Pathogenesis of intestinal and systemic rotavirus infection. J. Virol. 2004, 78, 10213–10220. [Google Scholar] [CrossRef]
- Estes, M.K.; Kang, G.; Zeng, C.Q.; Crawford, S.E.; Ciarlet, M. Pathogenesis of rotavirus gastroenteritis. Novartis Found Symp. 2001, 238, 82–96; discussion 96–100. [Google Scholar]
- Li, N.; Wang, Z.Y. Viremia and extraintestinal infections in infants with rotavirus diarrhea. Di Yi Jun Yi Da Xue Xue Bao 2003, 23, 643–648. [Google Scholar] [PubMed]
- Gilger, M.A.; Matson, D.O.; Conner, M.E.; Rosenblatt, H.M.; Finegold, M.J.; Estes, M.K. Extraintestinal rotavirus infections in children with immunodeficiency. J. Pediatr. 1992, 120, 912–917. [Google Scholar] [CrossRef]
- Paul, S.P.; Candy, D.C. Extra-intestinal manifestation of rotavirus infection....Beyond the gut. Indian J. Pediatr. 2014, 81, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, O.; Svensson, L. Pathogenesis of rotavirus diarrhea. Microbes Infect. 2001, 3, 1145–1156. [Google Scholar] [CrossRef]
- Morris, A.P.; Estes, M.K. Microbes and microbial toxins: Paradigms for microbial-mucosal interactions VIII. Pathological consequences of rotavirus infection and its enterotoxin. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G303–G310. [Google Scholar] [PubMed]
- Greenberg, H.B.; Estes, M.K. Rotaviruses: From pathogenesis to vaccination. Gastroenterology 2009, 136, 1939–1951. [Google Scholar] [CrossRef]
- Lundgren, O.; Peregrin, A.T.; Persson, K.; Kordasti, S.; Uhnoo, I.; Svensson, L. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science 2000, 287, 491–495. [Google Scholar] [CrossRef]
- Hellysaz, A.; Hagbom, M. Understanding the central nervous system symptoms of rotavirus: A qualitative review. Viruses 2021, 13, 658. [Google Scholar] [CrossRef] [PubMed]
- Hagbom, M.; Istrate, C.; Engblom, D.; Karlsson, T.; Rodriguez-Diaz, J.; Buesa, J.; Taylor, J.A.; Loitto, V.M.; Magnusson, K.E.; Ahlman, H.; et al. Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog. 2011, 7, e1002115. [Google Scholar] [CrossRef] [PubMed]
- Hellysaz, A.; Svensson, L.; Hagbom, M. Rotavirus downregulates tyrosine hydroxylase in the noradrenergic sympathetic nervous system in ileum, early in infection and simultaneously with increased intestinal transit and altered brain activities. mBio 2022, 13, e0138722. [Google Scholar] [CrossRef]
- Dadmehr, M.; Amini-Behbahani, F.; Eftekhar, B.; Minaei, B.; Bahrami, M. Peritoneum as an origin of epilepsy from the viewpoint of Avicenna. Neurol. Sci. 2018, 39, 1121–1124. [Google Scholar] [CrossRef]
- Bahrami, M.; Shokri, S.; Mastery Farahani, R.; Dadmehr, M. A brief historical overview of the anatomy of fascia in medieval Persian medicine. J. Med. Ethics Hist. Med. 2020, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Gorji, A.; Khaleghi Ghadiri, M. History of headache in medieval Persian medicine. Lancet Neurol. 2002, 1, 510–515. [Google Scholar] [CrossRef]
- Shoja, M.M.; Tubbs, R.S. The history of anatomy in Persia. J. Anat. 2007, 210, 359–378. [Google Scholar] [CrossRef]
- Dadmehr, M.; Seif, F.; Bahrami, M.; Amini-Behbahni, F.; Minaii Zangi, B.; Tavakol, C. A Historical overview of the neurological disorders associated with gastrointestinal ailments from the viewpoint of Avicenna. Acta Med. Hist. Adriat. 2024, 21, 307–319. [Google Scholar] [PubMed]
- Mazengenya, P.; Bhikha, R. Revisiting Avicenna’s (980–1037 AD) anatomy of the abdominal viscera from the Canon of Medicine. Morphologie 2018, 102, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Li, S.; Liu, M.; Cao, S.; Liu, B.; Li, D.; Wang, Z.; Sun, H.; Cui, Y.; Shi, Y. The mechanism of the gut-brain axis in regulating food intake. Nutrients 2023, 15, 3728. [Google Scholar] [CrossRef]
- Bialowas, S.; Hagbom, M.; Nordgren, J.; Karlsson, T.; Sharma, S.; Magnusson, K.E.; Svensson, L. Rotavirus and serotonin cross-talk in Diarrhoea. PLoS ONE 2016, 11, e0159660. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Primers 2017, 3, 17083. [Google Scholar] [CrossRef] [PubMed]
- Kordasti, S.; Sjovall, H.; Lundgren, O.; Svensson, L. Serotonin and vasoactive intestinal peptide antagonists attenuate rotavirus diarrhoea. Gut 2004, 53, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Menetrey, D.; De Pommery, J. Origins of spinal ascending pathways that reach central areas involved in visceroception and visceronociception in the rat. Eur. J. Neurosci. 1991, 3, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Aburto, M.R.; Cryan, J.F. Gastrointestinal and brain barriers: Unlocking gates of communication across the microbiota-gut-brain axis. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 222–247. [Google Scholar] [CrossRef]
- Ahmed, H.; Leyrolle, Q.; Koistinen, V.; Karkkainen, O.; Laye, S.; Delzenne, N.; Hanhineva, K. Microbiota-derived metabolites as drivers of gut-brain communication. Gut Microbes. 2022, 14, 2102878. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Fowler, A.; Ygberg, S.; Svensson, E.; Bergman, K.; Cooray, G.; Wickstrom, R. Prospective evaluation of childhood encephalitis: Predisposing factors, prevention and outcome. Pediatr. Infect. Dis. J. 2020, 39, e417–e422. [Google Scholar] [CrossRef]
- Nakagomi, T.; Nakagomi, O. Rotavirus antigenemia in children with encephalopathy accompanied by rotavirus gastroenteritis. Arch. Virol. 2005, 150, 1927–1931. [Google Scholar] [CrossRef]
- Takanashi, J.; Miyamoto, T.; Ando, N.; Kubota, T.; Oka, M.; Kato, Z.; Hamano, S.; Hirabayashi, S.; Kikuchi, M.; Barkovich, A.J. Clinical and radiological features of rotavirus cerebellitis. Am. J. Neuroradiol. 2010, 31, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y. Rotavirus infection-associated central nervous system complications: Clinicoradiological features and potential mechanisms. Clin. Exp. Pediatr. 2022, 65, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Hellysaz, A.; Nordgren, J.; Neijd, M.; Marti, M.; Svensson, L.; Hagbom, M. Microbiota do not restrict rotavirus infection of colon. J. Virol. 2023, 97, e0152623. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.R.; Kraft, L.M. Epizootic diarrhea of infant mice: Indentification of the etiologic agent. Science 1963, 141, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Kraft, L.M. Observations on the control and natural history of epidemic diarrhea of infant mice (EDIM). Yale J. Biol. Med. 1958, 31, 121–137. [Google Scholar] [PubMed]
- Kraft, L.M. The Problems of Laboratory Animal Disease; Harris, R.J.C., Ed.; Academic Press: New York, NY, USA, 1962. [Google Scholar]
- Kraft, L.M. The Mouse in Biomedical Research; Foster, H.L., Fox, J.G., Small, D.J., Eds.; Academic Press: New York, NY, USA, 1982; Volume 2, pp. 159–191. [Google Scholar]
- Krapic, M.; Kavazovic, I.; Wensveen, F.M. Immunological mechanisms of sickness behavior in viral infection. Viruses 2021, 13, 2245. [Google Scholar] [CrossRef] [PubMed]
- Browning, K.N.; Travagli, R.A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol. 2014, 4, 1339–1368. [Google Scholar]
- Gibbons, C.H. Basics of autonomic nervous system function. Handb. Clin. Neurol. 2019, 160, 407–418. [Google Scholar] [PubMed]
- Cook, T.M.; Mansuy-Aubert, V. Communication between the gut microbiota and peripheral nervous system in health and chronic disease. Gut Microbes 2022, 14, 2068365. [Google Scholar] [CrossRef]
- Flament-Durand, J. The hypothalamus: Anatomy and functions. Acta Psychiatr. Belg. 1980, 80, 364–375. [Google Scholar] [PubMed]
- Peruzzotti-Jametti, L.; Donega, M.; Giusto, E.; Mallucci, G.; Marchetti, B.; Pluchino, S. The role of the immune system in central nervous system plasticity after acute injury. Neuroscience 2014, 283, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.M.; Guillery, R.W. Functional organization of thalamocortical relays. J. Neurophysiol. 1996, 76, 1367–1395. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, M.R.; Sharma, S. Physiology, Chemoreceptor Trigger Zone. In Disclosure Sandeep Sharma Declares No Relevant Financial Relationships with Ineligible Companies; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Forsythe, P.; Sudo, N.; Dinan, T.; Taylor, V.H.; Bienenstock, J. Mood and gut feelings. Brain Behav. Immun. 2010, 24, 9–16. [Google Scholar] [CrossRef]
- Prather, A.A. Sickness Behavior. In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Hart, B.L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988, 12, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Kapikian, A.Z.; Wyatt, R.G.; Levine, M.M.; Black, R.E.; Greenberg, H.B.; Flores, J.; Kalica, A.R.; Hoshino, Y.; Chanock, R.M. Studies in volunteers with human rotaviruses. Dev. Biol. Stand. 1983, 53, 209–218. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention. Foodborne outbreak of group a rotavirus gastroenteritis among college students—District of Columbia, March–April 2000. MMWR Morb. Mortal Wkly. Rep. 2000, 49, 1131–1133. [Google Scholar]
- Williams, G.N.R. Why We Get Sick: The New Science of Darwinian Medicine; Vintage Books: New York, NY, USA, 1996. [Google Scholar]
- Tsai, P.Y.; Zhang, B.; He, W.Q.; Zha, J.M.; Odenwald, M.A.; Singh, G.; Tamura, A.; Shen, L.; Sailer, A.; Yeruva, S.; et al. IL-22 upregulates epithelial Claudin-2 to drive diarrhea and enteric pathogen clearance. Cell Host Microbe 2017, 21, 671–681.e4. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, V.; Nista, E.C.; Rosa, T.; Brigida, M.; Franceschi, F. Clinical management of infectious Diarrhea. Rev. Recent Clin. Trials 2020, 15, 298–308. [Google Scholar] [CrossRef]
- Uhnoo, I.; Olding-Stenkvist, E.; Kreuger, A. Clinical features of acute gastroenteritis associated with rotavirus, enteric adenoviruses, and bacteria. Arch. Dis. Child. 1986, 61, 732–738. [Google Scholar] [CrossRef]
- Diarrhoea and Vomiting Caused by Gastroenteritis: Diagnosis, Assessment and Management in Children Younger than 5 Years. In National Collaborating Centre for Women’s and Children’s Health (UK); RCOG Press at the Royal College of Obstetricians and Gynaecologists: London, UK, 2009.
- Graham, D.Y.; Estes, M.K. Pathogenesis and treatment of rotavirus diarrhea. Gastroenterology 1991, 101, 1140–1141. [Google Scholar] [CrossRef]
- Graham, D.Y.; Sackman, J.W.; Estes, M.K. Pathogenesis of rotavirus-induced diarrhea. Preliminary studies in miniature swine piglet. Dig. Dis. Sci. 1984, 29, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.P.; Scott, J.K.; Ball, J.M.; Zeng, C.Q.; O’Neal, W.K.; Estes, M.K. NSP4 elicits age-dependent diarrhea and Ca(2+)mediated I(-) influx into intestinal crypts of CF mice. Am. J. Physiol. 1999, 277, G431–G444. [Google Scholar]
- Lundgren, O.; Svensson, L. The enteric nervous system and infectious diarrhea. In Viral Gastroenteritis; Desselberger, U., Gray, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 9, pp. 51–67. [Google Scholar]
- Istrate, C.; Hagbom, M.; Vikstrom, E.; Magnusson, K.E.; Svensson, L. Rotavirus infection increases intestinal motility but not permeability at the onset of diarrhea. J. Virol. 2014, 88, 3161–3169. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar] [PubMed]
- Horn, C.C. Why is the neurobiology of nausea and vomiting so important? Appetite 2008, 50, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Nesse, R.M.; Williams, G.C. Evolution and the origins of disease. Sci. Am. 1998, 279, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Parashar, U.D.; Nelson, E.A.; Kang, G. Diagnosis, management, and prevention of rotavirus gastroenteritis in children. BMJ 2013, 347, f7204. [Google Scholar] [CrossRef] [PubMed]
- Uhnoo, I.; Svensson, L. Clinical and epidemiological features of acute infantile gastroenteritis associated with human rotavirus subgroups 1 and 2. J. Clin. Microbiol. 1986, 23, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Kapikian, A.Z.; Shope, R.E. Rotaviruses, Reoviruses, Coltiviruses, and Orbiviruses. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Hagbom, M.; Novak, D.; Ekstrom, M.; Khalid, Y.; Andersson, M.; Lindh, M.; Nordgren, J.; Svensson, L. Ondansetron treatment reduces rotavirus symptoms-A randomized double-blinded placebo-controlled trial. PLoS ONE 2017, 12, e0186824. [Google Scholar] [CrossRef]
- Bonvanie, I.J.; Weghorst, A.A.; Holtman, G.A.; Russchen, H.A.; Fickweiler, F.; Verkade, H.J.; Kollen, B.J.; Berger, M.Y. Oral ondansetron for paediatric gastroenteritis in primary care: A randomised controlled trial. Br. J. Gen. Pract. 2021, 71, e728–e735. [Google Scholar] [CrossRef]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Kutukculer, N.; Caglayan, S. Tumor necrosis factor-alpha and interleukin-6 in stools of children with bacterial and viral gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 1997, 25, 556–557. [Google Scholar]
- Jiang, B.; Snipes-Magaldi, L.; Dennehy, P.; Keyserling, H.; Holman, R.C.; Bresee, J.; Gentsch, J.; Glass, R.I. Cytokines as mediators for or effectors against rotavirus disease in children. Clin. Diagn. Lab. Immunol. 2003, 10, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Biddle, C. The neurobiology of the human febrile response. AANA J. 2006, 74, 145–150. [Google Scholar] [PubMed]
- The Pituitary, 3rd ed.; Academic Press: London, UK, 2011.
- Morrison, S.F. Central control of body temperature. F1000Research 2016, 5, 880. [Google Scholar] [CrossRef] [PubMed]
- Clinical Manual of Fever in Children; Springer: Berlin/Heidelberg, Germany, 2009.
- Harden, L.M.; Kent, S.; Pittman, Q.J.; Roth, J. Fever and sickness behavior: Friend or foe? Brain Behav. Immun. 2015, 50, 322–333. [Google Scholar] [CrossRef]
- Szelenyi, Z.; Szekely, M. Sickness behavior in fever and hypothermia. Front. Biosci. 2004, 9, 2447–2456. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silverman, M.N.; Pearce, B.D.; Biron, C.A.; Miller, A.H. Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol 2005, 18, 41–78. [Google Scholar] [CrossRef] [PubMed]
- Shattuck, E.C.; Muehlenbein, M.P. Towards an integrative picture of human sickness behavior. Brain Behav. Immun. 2016, 57, 255–262. [Google Scholar] [CrossRef]
- Hossain, J.L.; Ahmad, P.; Reinish, L.W.; Kayumov, L.; Hossain, N.K.; Shapiro, C.M. Subjective fatigue and subjective sleepiness: Two independent consequences of sleep disorders? J. Sleep Res. 2005, 14, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Imeri, L.; Opp, M.R. How (and why) the immune system makes us sleep. Nat. Rev. Neurosci. 2009, 10, 199–210. [Google Scholar] [CrossRef]
- Rezai, M.; Fullwood, C.; Hird, B.; Chawla, M.; Tetlow, L.; Banerjee, I.; Patel, L. Cortisol levels during acute illnesses in children and adolescents: A systematic review. JAMA Netw. Open 2022, 5, e2217812. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.J.; Powell, M.L.; Meitin, C.; Small, P.A., Jr. Virus infection as a stressor: Influenza virus elevates plasma concentrations of corticosterone, and brain concentrations of MHPG and tryptophan. Physiol. Behav. 1989, 45, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Cristiano, C.; Luckman, S.M.; D’Agostino, G. Brain control of appetite during sickness. Br. J. Pharmacol. 2021, 178, 2096–2110. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Peripheral mechanisms in appetite regulation. Gastroenterology 2015, 148, 1219–1233. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Huen, S.C.; Luan, H.H.; Yu, S.; Zhang, C.; Gallezot, J.D.; Booth, C.J.; Medzhitov, R. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell 2016, 166, 1512–1525.e12. [Google Scholar] [CrossRef] [PubMed]
- Verkerke, H.; Sobuz, S.; Ma, J.Z.; Petri, S.E.; Reichman, D.; Qadri, F.; Rahman, M.; Haque, R.; Petri, W.A., Jr. Malnutrition is associated with protection from rotavirus diarrhea: Evidence from a longitudinal birth cohort study in Bangladesh. J. Clin. Microbiol. 2016, 54, 2568–2574. [Google Scholar] [CrossRef]
- Burnett, E.; Parashar, U.D.; Tate, J.E. Rotavirus Infection, Illness, and vaccine performance in malnourished children: A review of the literature. Pediatr. Infect Dis. J. 2021, 40, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Mannion, M.; O’Reilly, P.; Crushell, E.; Hughes, J.; Knerr, I.; Gavin, P.; Monavari, A. Rotavirus gastroenteritis is associated with increased morbidity and mortality in children with inherited metabolic disorders. Ir. Med. J. 2017, 110, 546. [Google Scholar]
- Broecker, F.; Moelling, K. What viruses tell us about evolution and immunity: Beyond Darwin? Ann. N. Y. Acad. Sci. 2019, 1447, 53–68. [Google Scholar] [CrossRef]
- Hu, D.L.; Nakane, A. Mechanisms of staphylococcal enterotoxin-induced emesis. Eur. J. Pharmacol. 2014, 722, 95–107. [Google Scholar] [CrossRef]
- Stuempfig, N.D.; Seroy, J. Viral Gastroenteritis. In Disclosure: Justin Seroy Declares No Relevant Financial Relationships with Ineligible Companies; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellysaz, A.; Hagbom, M. Rotavirus Sickness Symptoms: Manifestations of Defensive Responses from the Brain. Viruses 2024, 16, 1086. https://doi.org/10.3390/v16071086
Hellysaz A, Hagbom M. Rotavirus Sickness Symptoms: Manifestations of Defensive Responses from the Brain. Viruses. 2024; 16(7):1086. https://doi.org/10.3390/v16071086
Chicago/Turabian StyleHellysaz, Arash, and Marie Hagbom. 2024. "Rotavirus Sickness Symptoms: Manifestations of Defensive Responses from the Brain" Viruses 16, no. 7: 1086. https://doi.org/10.3390/v16071086
APA StyleHellysaz, A., & Hagbom, M. (2024). Rotavirus Sickness Symptoms: Manifestations of Defensive Responses from the Brain. Viruses, 16(7), 1086. https://doi.org/10.3390/v16071086







