Viral Vector Based Immunotherapy for Peanut Allergy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of Plasmids
2.2. AAV Production
2.3. Experimental Mice
2.4. Peanut Extract Protocol
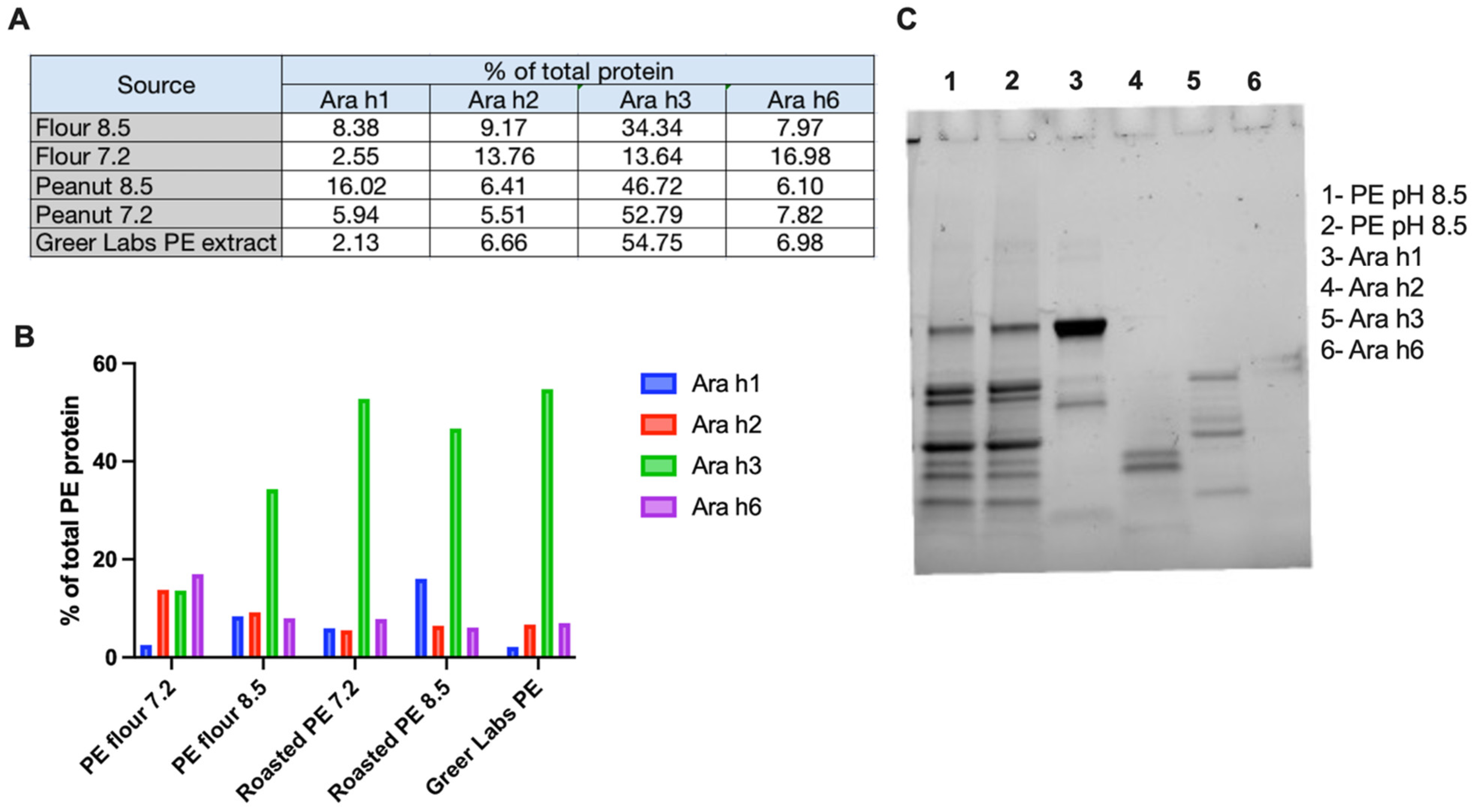
2.5. Qualitative and Quantitative Comparison of Peanut Extracts
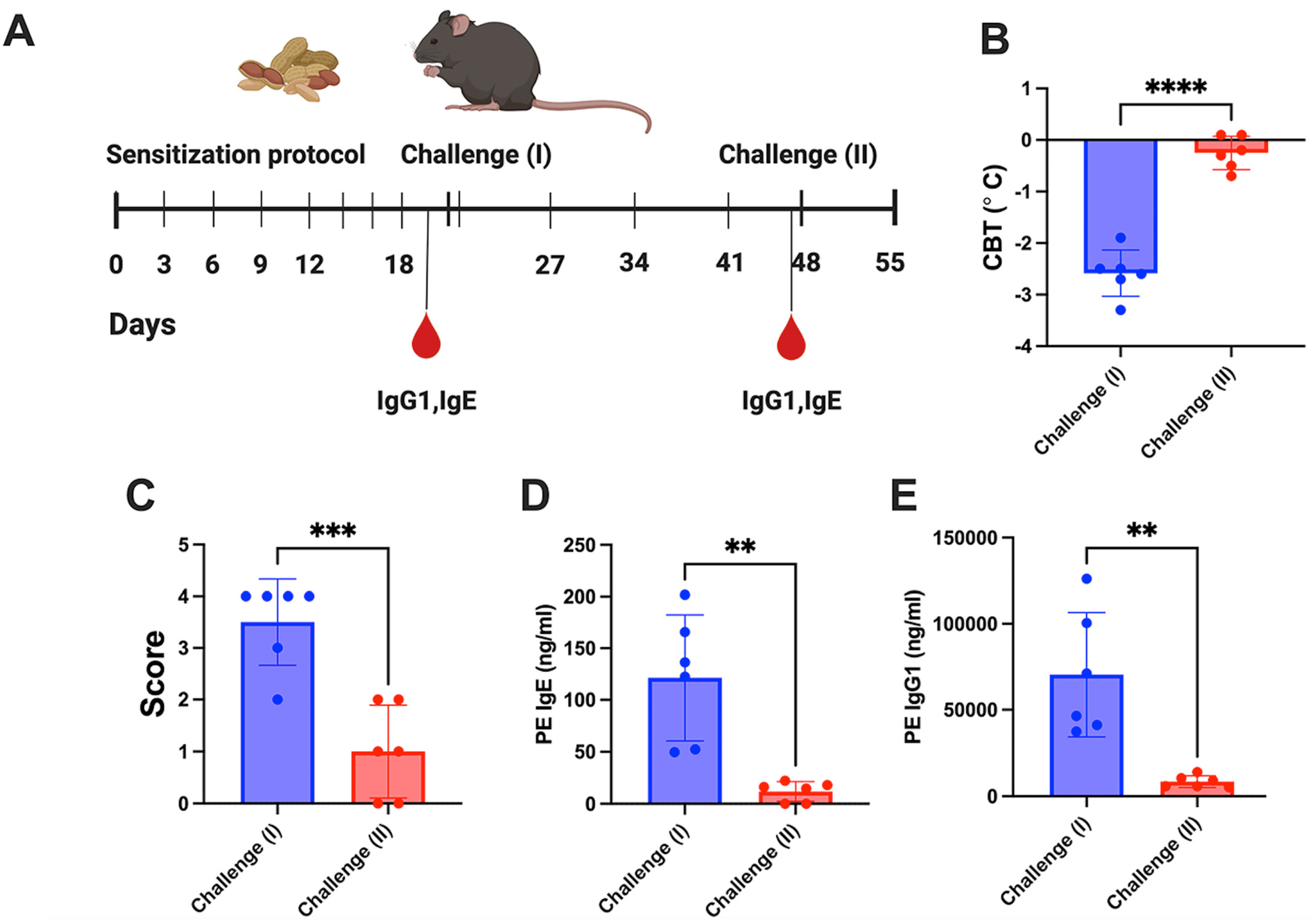
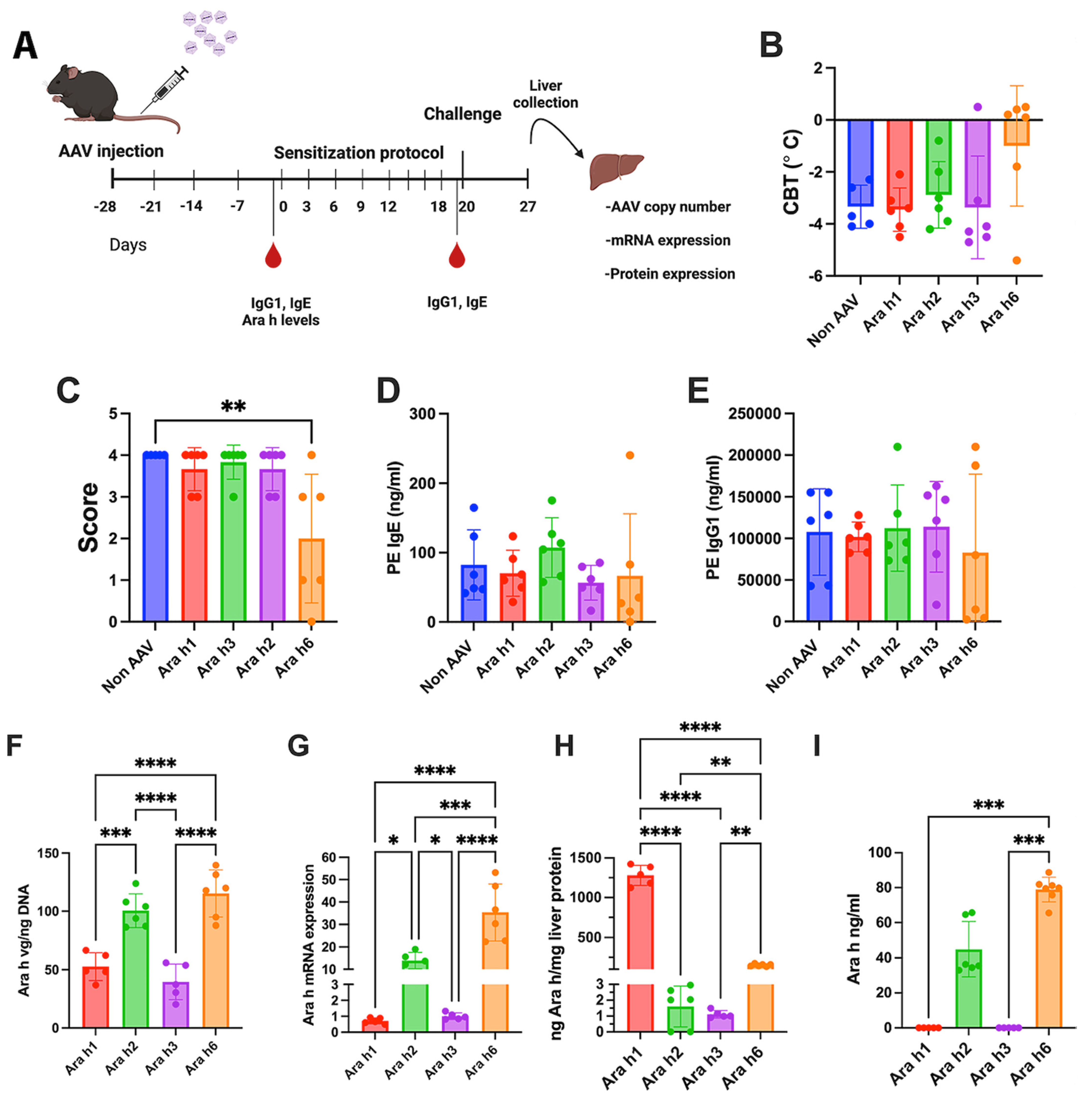
2.6. Animal Treatment
2.7. Challenge Procedure
2.8. Vector Copy Numbers and mRNA Levels in Liver Tissue
2.9. Liver Lysates Preparation
2.10. Analysis of Plasma Samples
3. Results
3.1. Characterization of Peanut Extract
3.2. Hepatic Gene Transfer with a Cocktail of Vectors Expressing 4 Allergens Protects from Peanut Allergy while Single Vectors Are Ineffective
3.3. Increased Vector Dose Results in Efficacy for Ara h 3 but Not Ara h1 Expression from Single Vector
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mingozzi, F.; Liu, Y.L.; Dobrzynski, E.; Kaufhold, A.; Liu, J.H.; Wang, Y.; Arruda, V.R.; High, K.A.; Herzog, R.W. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J. Clin. Investig. 2003, 111, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Mount, J.D.; Herzog, R.W.; Tillson, D.M.; Goodman, S.A.; Robinson, N.; McCleland, M.L.; Bellinger, D.; Nichols, T.C.; Arruda, V.R.; Lothrop, C.D., Jr.; et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood 2002, 99, 2670–2676. [Google Scholar] [CrossRef] [PubMed]
- Keeler, G.D.; Markusic, D.M.; Hoffman, B.E. Liver induced transgene tolerance with AAV vectors. Cell Immunol. 2019, 342, 103728. [Google Scholar] [CrossRef] [PubMed]
- Akbarpour, M.; Goudy, K.S.; Cantore, A.; Russo, F.; Sanvito, F.; Naldini, L.; Annoni, A.; Roncarolo, M.G. Insulin B chain 9-23 gene transfer to hepatocytes protects from type 1 diabetes by inducing Ag-specific FoxP3+ Tregs. Sci. Transl. Med. 2015, 7, 289ra81. [Google Scholar] [CrossRef] [PubMed]
- Annoni, A.; Cantore, A.; Della Valle, P.; Goudy, K.; Akbarpour, M.; Russo, F.; Bartolaccini, S.; D’Angelo, A.; Roncarolo, M.G.; Naldini, L. Liver gene therapy by lentiviral vectors reverses anti-factor IX pre-existing immunity in haemophilic mice. EMBO Mol. Med. 2013, 5, 1684–1697. [Google Scholar] [CrossRef] [PubMed]
- Merlin, S.; Cannizzo, E.S.; Borroni, E.; Bruscaggin, V.; Schinco, P.; Tulalamba, W.; Chuah, M.K.; Arruda, V.R.; VandenDriessche, T.; Prat, M.; et al. A Novel Platform for Immune Tolerance Induction in Hemophilia A Mice. Mol. Ther. 2017, 25, 1815–1830. [Google Scholar] [CrossRef] [PubMed]
- Markusic, D.M.; Herzog, R.W. Liver-Directed Adeno-Associated Viral Gene Therapy for Hemophilia. J. Genet. Syndr. Gene Ther. 2012, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, B.; Osada, T.; Rodriguiz, R.; Yang, X.Y.; Luo, X.; Kemper, A.R.; Clay, T.M.; Koeberl, D.D. Immunodominant liver-specific expression suppresses transgene-directed immune responses in murine pompe disease. Hum. Gene Ther. 2012, 23, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Finn, J.D.; Ozelo, M.C.; Sabatino, D.E.; Franck, H.W.; Merricks, E.P.; Crudele, J.M.; Zhou, S.; Kazazian, H.H.; Lillicrap, D.; Nichols, T.C.; et al. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood 2010, 116, 5842–5848. [Google Scholar] [CrossRef] [PubMed]
- Markusic, D.M.; Hoffman, B.E.; Perrin, G.Q.; Nayak, S.; Wang, X.; LoDuca, P.A.; High, K.A.; Herzog, R.W. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol. Med. 2013, 5, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, R.; Samelson-Jones, B.J.; Herzog, R.W. Immune tolerance induction by hepatic gene transfer: First-in-human evidence. Mol. Ther. 2024, 32, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.C.; Lai, C.W.; Wu, C.J.; Chen, L.C.; Tao, M.H.; Kuo, M.L. Liver-Specific Allergen Gene Transfer by Adeno-Associated Virus Suppresses Allergic Airway Inflammation in Mice. Hum. Gene Ther. 2016, 27, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Visiedo, M.; Li, X.; Munoz-Melero, M.; Kulis, M.D.; Daniell, H.; Markusic, D.M. Single-dose AAV vector gene immunotherapy to treat food allergy. Mol. Ther. Methods Clin. Dev. 2022, 26, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Keeler, G.D.; Gaddie, C.D.; Sagadevan, A.S.; Senior, K.G.; Cote, I.; Rechdan, M.; Min, D.; Mahan, D.; Poma, B.; Hoffman, B.E. Induction of antigen-specific tolerance by hepatic AAV immunotherapy regardless of T cell epitope usage or mouse strain background. Mol. Ther. Methods Clin. Dev. 2023, 28, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Keeler, G.D.; Kumar, S.; Palaschak, B.; Silverberg, E.L.; Markusic, D.M.; Jones, N.T.; Hoffman, B.E. Gene Therapy-Induced Antigen-Specific Tregs Inhibit Neuro-inflammation and Reverse Disease in a Mouse Model of Multiple Sclerosis. Mol. Ther. 2018, 26, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.W.; Knolle, P.A. Antigen-presenting cell function in the tolerogenic liver environment. Nat. Rev. Immunol. 2010, 10, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Perrin, G.Q.; Zolotukhin, I.; Sherman, A.; Biswas, M.; de Jong, Y.P.; Terhorst, C.; Davidoff, A.M.; Herzog, R.W. Dynamics of antigen presentation to transgene product-specific CD4(+) T cells and of Treg induction upon hepatic AAV gene transfer. Mol. Ther. Methods Clin. Dev. 2016, 3, 16083. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, B.E.; Martino, A.T.; Sack, B.K.; Cao, O.; Liao, G.; Terhorst, C.; Herzog, R.W. Nonredundant roles of IL-10 and TGF-beta in suppression of immune responses to hepatic AAV-factor IX gene transfer. Mol. Ther. 2011, 19, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, T.B.; Biswas, M.; Terhorst, C.; Daniell, H.; Herzog, R.W.; Pineros, A.R. Role of orally induced regulatory T cells in immunotherapy and tolerance. Cell Immunol. 2021, 359, 104251. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; So, K.; Bertolini, T.B.; Krishnan, P.; Rana, J.; Munoz-Melero, M.; Syed, F.; Kumar, S.R.P.; Gao, H.; Xuei, X.; et al. Distinct functions and transcriptional signatures in orally induced regulatory T cell populations. Front. Immunol. 2023, 14, 1278184. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, C.; Weiner, H.L. Immunology. How does the immune system tolerate food? Science 2016, 351, 810–811. [Google Scholar] [CrossRef] [PubMed]
- McGowan, E.C.; Wood, R.A. Sublingual (SLIT) versus oral immunotherapy (OIT) for food allergy. Curr. Allergy Asthma Rep. 2014, 14, 486. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Kulis, M.; Herzog, R.W. Plant cell-made protein antigens for induction of Oral tolerance. Biotechnol. Adv. 2019, 37, 107413. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, W.; Berin, M.C. Oral tolerance and oral immunotherapy for food allergy: Evidence for common mechanisms? Cell Immunol. 2023, 383, 104650. [Google Scholar] [CrossRef] [PubMed]
- Martino, A.T.; Markusic, D.M. Immune Response Mechanisms against AAV Vectors in Animal Models. Mol. Ther. Methods Clin. Dev. 2020, 17, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Verdera, H.C.; Kuranda, K.; Mingozzi, F. AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Mol. Ther. 2020, 28, 723–746. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chawla, K.; Jarvinen, K.M.; Nowak-Wegrzyn, A. Anaphylaxis in a New York City pediatric emergency department: Triggers, treatments, and outcomes. J. Allergy Clin. Immunol. 2012, 129, 162–168.e1–3. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.A.; Munoz-Furlong, A.; Sampson, H.A. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J. Allergy Clin. Immunol. 2007, 119, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.A.; Munoz-Furlong, A.; Sampson, H.A. Fatalities due to anaphylactic reactions to foods. J. Allergy Clin. Immunol. 2001, 107, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Skolnick, H.S.; Conover-Walker, M.K.; Koerner, C.B.; Sampson, H.A.; Burks, W.; Wood, R.A. The natural history of peanut allergy. J. Allergy Clin. Immunol. 2001, 107, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, D.M. The natural history of peanut and tree nut allergy. Curr. Allergy Asthma Rep. 2007, 7, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Ortiz, M.; Turner, P.J. Improving the safety of oral immunotherapy for food allergy. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2016, 27, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.S.; Rachid, R. The Heterogeneity of Oral Immunotherapy Clinical Trials: Implications and Future Directions. Curr. Allergy Asthma Rep. 2016, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Wood, R.A.; French, S.; Fiocchi, A.; Jordana, M.; Waserman, S.; Brozek, J.L.; Schunemann, H.J. Oral immunotherapy for peanut allergy (PACE): A systematic review and meta-analysis of efficacy and safety. Lancet 2019, 393, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Sayre, P.H.; Roberts, G.; Sever, M.L.; Lawson, K.; Bahnson, H.T.; Brough, H.A.; Santos, A.F.; Harris, K.M.; Radulovic, S.; et al. Effect of Avoidance on Peanut Allergy after Early Peanut Consumption. N. Engl. J. Med. 2016, 374, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Ballmer-Weber, B.K.; Lidholm, J.; Fernandez-Rivas, M.; Seneviratne, S.; Hanschmann, K.M.; Vogel, L.; Bures, P.; Fritsche, P.; Summers, C.; Knulst, A.C.; et al. IgE recognition patterns in peanut allergy are age dependent: Perspectives of the EuroPrevall study. Allergy 2015, 70, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Datema, M.R.; Lyons, S.A.; Fernandez-Rivas, M.; Ballmer-Weber, B.; Knulst, A.C.; Asero, R.; Barreales, L.; Belohlavkova, S.; de Blay, F.; Clausen, M.; et al. Estimating the Risk of Severe Peanut Allergy Using Clinical Background and IgE Sensitization Profiles. Front. Allergy 2021, 2, 670789. [Google Scholar] [CrossRef] [PubMed]
- Sack, B.K.; Merchant, S.; Markusic, D.M.; Nathwani, A.C.; Davidoff, A.M.; Byrne, B.J.; Herzog, R.W. Transient B cell depletion or improved transgene expression by codon optimization promote tolerance to factor VIII in gene therapy. PLoS ONE 2012, 7, e37671. [Google Scholar] [CrossRef] [PubMed]
- Crosson, S.M.; Dib, P.; Smith, J.K.; Zolotukhin, S. Helper-free Production of Laboratory Grade AAV and Purification by Iodixanol Density Gradient Centrifugation. Mol. Ther. Methods Clin. Dev. 2018, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Saunders, S.P.; Goh, C.S.; Brown, S.J.; Palmer, C.N.; Porter, R.M.; Cole, C.; Campbell, L.E.; Gierlinski, M.; Barton, G.J.; Schneider, G.; et al. Tmem79/Matt is the matted mouse gene and is a predisposing gene for atopic dermatitis in human subjects. J. Allergy Clin. Immunol. 2013, 132, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- van den Oord, R.A.; Sheikh, A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: Systematic review and meta-analysis. BMJ 2009, 339, b2433. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.T.; Green, J.E.; Ferrie, R.P.; Queener, A.M.; Kaplan, M.H.; Cook-Mills, J.M. Mechanism for initiation of food allergy: Dependence on skin barrier mutations and environmental allergen costimulation. J. Allergy Clin. Immunol. 2018, 141, 1711–1725.e9. [Google Scholar] [CrossRef] [PubMed]
- Orgel, K.; Smeekens, J.M.; Ye, P.; Fotsch, L.; Guo, R.; Miller, D.R.; Pardo-Manuel de Villena, F.; Burks, A.W.; Ferris, M.T.; Kulis, M.D. Genetic diversity between mouse strains allows identification of the CC027/GeniUnc strain as an orally reactive model of peanut allergy. J. Allergy Clin. Immunol. 2019, 143, 1027–1037.e7. [Google Scholar] [CrossRef] [PubMed]
- Poms, R.E.; Capelletti, C.; Anklam, E. Effect of roasting history and buffer composition on peanut protein extraction efficiency. Mol. Nutr. Food Res. 2004, 48, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, J.S.S.; Yamada, K.; Bertolini, T.B.; Syed, F.; Kumar, S.R.P.; Li, X.; Arisa, S.; Pineros, A.R.; Tapia, A.; Rogers, C.A.; et al. IL-15 blockade and rapamycin rescue multifactorial loss of factor VIII from AAV-transduced hepatocytes in hemophilia A mice. Mol. Ther. 2022, 30, 3552–3569. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Dreskin, S.C. Redefining the major peanut allergens. Immunol. Res. 2013, 55, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Koppelman, S.J.; Wensing, M.; Ertmann, M.; Knulst, A.C.; Knol, E.F. Relevance of Ara h1, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clin. Exp. Allergy 2004, 34, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Flinterman, A.E.; van Hoffen, E.; den Hartog Jager, C.F.; Koppelman, S.; Pasmans, S.G.; Hoekstra, M.O.; Bruijnzeel-Koomen, C.A.; Knulst, A.C.; Knol, E.F. Children with peanut allergy recognize predominantly Ara h2 and Ara h6, which remains stable over time. Clin. Exp. Allergy 2007, 37, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.A.; Maleki, S.J.; Pedersen, L.C. The molecular basis of peanut allergy. Curr. Allergy Asthma Rep. 2014, 14, 429. [Google Scholar] [CrossRef] [PubMed]
- Orgel, K.; Kulis, M. A Mouse Model of Peanut Allergy Induced by Sensitization Through the Gastrointestinal Tract. Methods Mol. Biol. 2018, 1799, 39–47. [Google Scholar] [PubMed]
- Codreanu, F.; Collignon, O.; Roitel, O.; Thouvenot, B.; Sauvage, C.; Vilain, A.C.; Cousin, M.O.; Decoster, A.; Renaudin, J.M.; Astier, C.; et al. A novel immunoassay using recombinant allergens simplifies peanut allergy diagnosis. Int. Arch. Allergy Immunol. 2011, 154, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.H.; Ohashi, K.; Patijn, G.A.; Meuse, L.; Ye, X.; Thompson, A.R.; Kay, M.A. Inclusion of the hepatic locus control region, an intron, and untranslated region increases and stabilizes hepatic factor IX gene expression in vivo but not in vitro. Mol. Ther. 2000, 1, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, A.C.; Gray, J.T.; Ng, C.Y.; Zhou, J.; Spence, Y.; Waddington, S.N.; Tuddenham, E.G.; Kemball-Cook, G.; McIntosh, J.; Boon-Spijker, M.; et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood 2006, 107, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.; Nayak, S.; Hoffman, B.E.; Terhorst, C.; Cao, O.; Herzog, R.W. Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Hum. Gene Ther. 2009, 20, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Markusic, D.M.; Herzog, R.W.; Aslanidi, G.V.; Hoffman, B.E.; Li, B.; Li, M.; Jayandharan, G.R.; Ling, C.; Zolotukhin, I.; Ma, W.; et al. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol. Ther. 2010, 18, 2048–2056. [Google Scholar] [CrossRef] [PubMed]
- Koid, A.E.; Chapman, M.D.; Hamilton, R.G.; van Ree, R.; Versteeg, S.A.; Dreskin, S.C.; Koppelman, S.J.; Wunschmann, S. Ara h 6 complements Ara h 2 as an important marker for IgE reactivity to peanut. J. Agric. Food Chem. 2014, 62, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, M.; Warrier, I. Anaphylaxis in patients with hemophilia. Semin. Thromb. Hemost. 2000, 26, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Recht, M.; Pollmann, H.; Tagliaferri, A.; Musso, R.; Janco, R.; Neuman, W.R. A retrospective study to describe the incidence of moderate to severe allergic reactions to factor IX in subjects with haemophilia B. Haemophilia 2011, 17, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Moghimi, B.; LoDuca, P.A.; Singh, H.D.; Hoffman, B.E.; Herzog, R.W.; Daniell, H. Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc. Natl. Acad. Sci. USA 2010, 107, 7101–7106. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, G.P.; Herzog, R.W.; Mount, J.; Arruda, V.R.; Tillson, D.M.; Hathcock, J.; van Ginkel, F.W.; High, K.A.; Lothrop, C.D., Jr. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood 2009, 113, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Crudele, J.M.; Finn, J.D.; Siner, J.I.; Martin, N.B.; Niemeyer, G.P.; Zhou, S.; Mingozzi, F.; Lothrop, C.D., Jr.; Arruda, V.R. AAV liver expression of FIX-Padua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia B dogs and mice. Blood 2015, 125, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mei, M.; Haskins, M.E.; Nichols, T.C.; O’Donnell, P.; Cullen, K.; Dillow, A.; Bellinger, D.; Ponder, K.P. Immune response after neonatal transfer of a human factor IX-expressing retroviral vector in dogs, cats, and mice. Thromb. Res. 2007, 120, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, J.S.S.; Li, X.; Arisa, S.; Kwon, K.C.; Daniell, H.; Herzog, R.W. Potential role for oral tolerance in gene therapy. Cell Immunol. 2023, 391–392, 104742. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Kwon, K.C.; Hoffman, B.E.; Kamesh, A.; Jones, N.T.; Herzog, R.W.; Daniell, H. Low cost delivery of proteins bioencapsulated in plant cells to human non-immune or immune modulatory cells. Biomaterials 2016, 80, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Kulchar, R.J.; Herzog, R.W.; Kulis, M.; Leong, K.W. Plant cell-based drug delivery enhances affordability of biologics. Nat. Biotechnol. 2023, 41, 1186–1187. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.P.; Biswas, M.; Cao, D.; Arisa, S.; Munoz-Melero, M.; Lam, A.K.; Pineros, A.R.; Kapur, R.; Kaisho, T.; Kaufman, R.J.; et al. TLR9-independent CD8(+) T cell responses in hepatic AAV gene transfer through IL-1R1-MyD88 signaling. Mol. Ther. 2024, 32, 325–339. [Google Scholar] [CrossRef] [PubMed]


| Score | Symptom |
|---|---|
| 0 | No symptoms |
| 1 | Scratching and rubbing around the nose and head |
| 2 | Puffiness around the eyes and mouth, diarrhea, piloerection, reduced activity, and/or decreased activity with increased respiratory rate |
| 3 | Wheezing, labored respiration, and cyanosis around the mouth and the tail |
| 4 | No activity after prodding, or tremor and convulsion |
| 5 | Death |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Visiedo, M.; Herzog, R.W.; Munoz-Melero, M.; Blessinger, S.A.; Cook-Mills, J.M.; Daniell, H.; Markusic, D.M. Viral Vector Based Immunotherapy for Peanut Allergy. Viruses 2024, 16, 1125. https://doi.org/10.3390/v16071125
Gonzalez-Visiedo M, Herzog RW, Munoz-Melero M, Blessinger SA, Cook-Mills JM, Daniell H, Markusic DM. Viral Vector Based Immunotherapy for Peanut Allergy. Viruses. 2024; 16(7):1125. https://doi.org/10.3390/v16071125
Chicago/Turabian StyleGonzalez-Visiedo, Miguel, Roland W. Herzog, Maite Munoz-Melero, Sophia A. Blessinger, Joan M. Cook-Mills, Henry Daniell, and David M. Markusic. 2024. "Viral Vector Based Immunotherapy for Peanut Allergy" Viruses 16, no. 7: 1125. https://doi.org/10.3390/v16071125
APA StyleGonzalez-Visiedo, M., Herzog, R. W., Munoz-Melero, M., Blessinger, S. A., Cook-Mills, J. M., Daniell, H., & Markusic, D. M. (2024). Viral Vector Based Immunotherapy for Peanut Allergy. Viruses, 16(7), 1125. https://doi.org/10.3390/v16071125






