Deisolation in the Healthcare Setting Following Recent COVID-19 Infection
Abstract
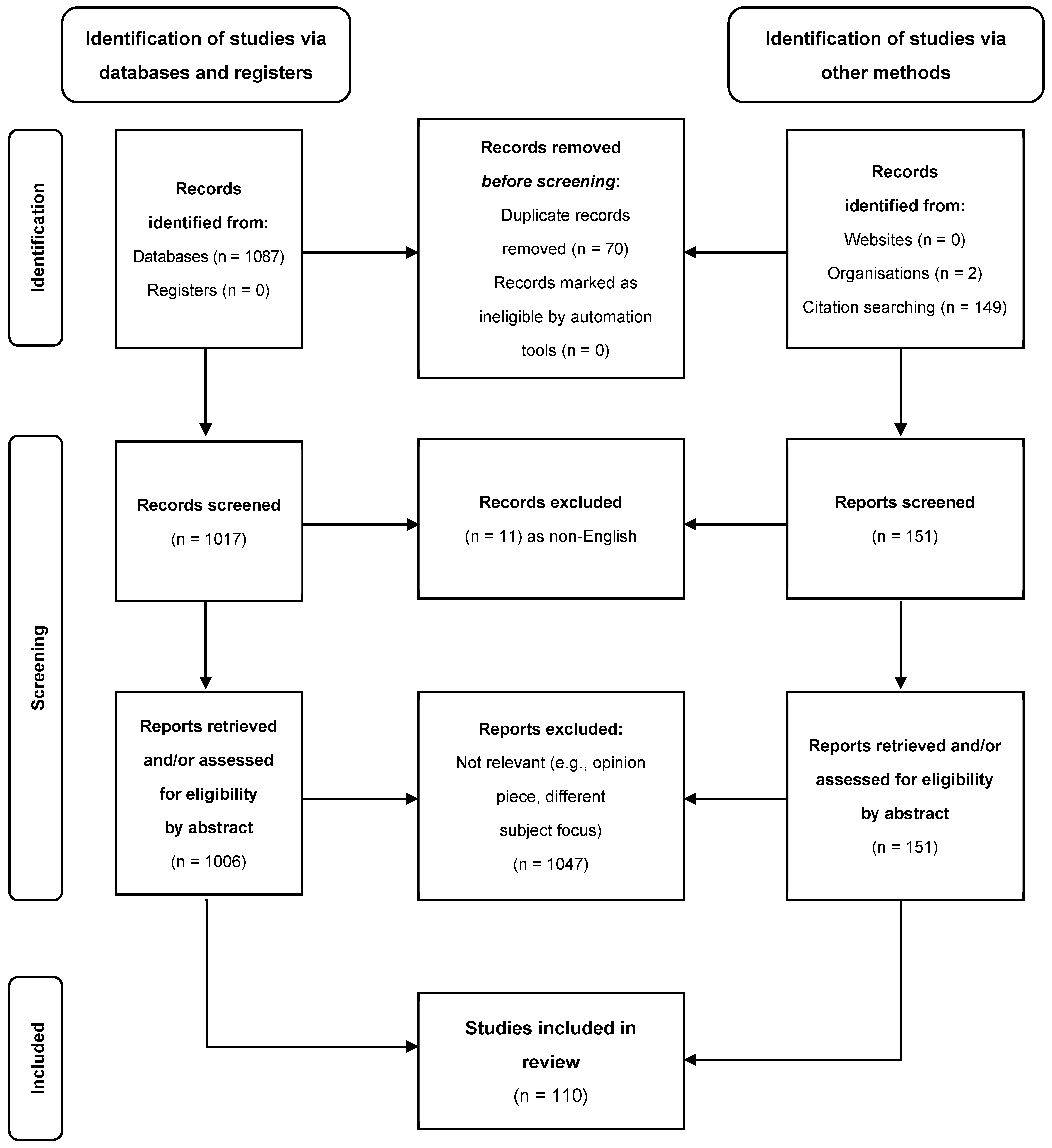
:1. Introduction
2. Objectives and Methods
3. A Brief Summary of SARS-CoV-2 Transmission
4. Laboratory Testing to Guide Inpatient Deisolation
5. SARS-CoV-2-Specific Immunity
6. Evidence Base for Deisolating Inpatients within Healthcare Facilities and Recommendations
7. Evidence Base for Deisolating Healthcare Workers and Recommendations
8. Limitations
9. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Luker, S.; Laver, K.; Lane, R.; Potter, E.; Harrod, A.; Bierer, P.; Adey-Wakeling, Z.; Karnon, J.; Cameron, I.D.; Crotty, M. “Put in a Room and Left”: A Qualitative Study Exploring the Lived Experiences of COVID-19 Isolation and Quarantine among Rehabilitation Inpatients. Ann. Med. 2023, 55, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.-J.; Rabheru, K.; Peisah, C.; Reichman, W.; Ikeda, M. Loneliness and Social Isolation during the COVID-19 Pandemic. Int. Psychogeriatr. 2020, 32, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The Psychological Impact of Quarantine and How to Reduce It: Rapid Review of the Evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhang, W.-B.; Wu, X.; Ruktanonchai, C.W.; Liu, H.; Wang, J.; Song, Y.; Liu, M.; Yan, W.; Yang, J.; et al. Untangling the Changing Impact of Non-Pharmaceutical Interventions and Vaccination on European COVID-19 Trajectories. Nat. Commun. 2022, 13, 3106. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-P.; Han, J.-Y.; Zhou, S.-X.; Yu, L.-J.; Lu, Q.-B.; Zhang, X.-A.; Zhang, H.-Y.; Ren, X.; Zhang, C.-H.; Wang, Y.-F.; et al. The Changing Pattern of Enteric Pathogen Infections in China during the COVID-19 Pandemic: A Nation-Wide Observational Study. Lancet Reg. Health West. Pac. 2021, 16, 100268. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.S.; Wood, T.; Jelley, L.; Jennings, T.; Jefferies, S.; Daniells, K.; Nesdale, A.; Dowell, T.; Turner, N.; Campbell-Stokes, P.; et al. Impact of the COVID-19 Nonpharmaceutical Interventions on Influenza and Other Respiratory Viral Infections in New Zealand. Nat. Commun. 2021, 12, 1001. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, H.; Chen, J.; Jia, C.; Siddique, A.; Wu, B.; Wang, H.; Tang, B.; He, F.; Zhao, G.; et al. Impact of COVID-19-Related Nonpharmaceutical Interventions on Diarrheal Diseases and Zoonotic Salmonella. hLife 2024, 2, 246–256. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Ending Isolation. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html (accessed on 12 September 2023).
- European Centre for Diseases Prevention and Control. Guidance on Ending the Isolation Period for People with COVID-19, Third Update; ECDC: Stockholm, Sweden, 2022.
- World Health Organisation Standard Precautions for the Prevention and Control of Infections: Aide-Memoire. Available online: https://www.who.int/publications/i/item/WHO-UHL-IHS-IPC-2022.1 (accessed on 16 June 2024).
- Greenhalgh, T.; MacIntyre, C.R.; Baker, M.G.; Bhattacharjee, S.; Chughtai, A.A.; Fisman, D.; Kunasekaran, M.; Kvalsvig, A.; Lupton, D.; Oliver, M.; et al. Masks and Respirators for Prevention of Respiratory Infections: A State of the Science Review. Clin. Microbiol. Rev. 2024, 37, e0012423. [Google Scholar] [CrossRef]
- Global Technical Consultation Report on Proposed Terminology for Pathogens That Transmit through the Air; World Health Organisation: Geneva, Switzerland, 2024.
- Jimenez, J.L.; Marr, L.C.; Randall, K.; Ewing, E.T.; Tufekci, Z.; Greenhalgh, T.; Tellier, R.; Tang, J.W.; Li, Y.; Morawska, L.; et al. What Were the Historical Reasons for the Resistance to Recognizing Airborne Transmission during the COVID-19 Pandemic? Indoor Air 2022, 32, e13070. [Google Scholar] [CrossRef]
- Loeb, M.; Bartholomew, A.; Hashmi, M.; Tarhuni, W.; Hassany, M.; Youngster, I.; Somayaji, R.; Larios, O.; Kim, J.; Missaghi, B.; et al. Medical Masks Versus N95 Respirators for Preventing COVID-19 Among Health Care Workers. Ann. Intern. Med. 2022, 175, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health Clinical Spectrum of SARS-CoV-2 Infection. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 12 September 2023).
- World Health Organization Clinical Management of COVID-19: Interim Guidance, 27 May 2020; World Health Organization: Geneva, Switzerland, 2020.
- Dadras, O.; Afsahi, A.M.; Pashaei, Z.; Mojdeganlou, H.; Karimi, A.; Habibi, P.; Barzegary, A.; Fakhfouri, A.; Mirzapour, P.; Janfaza, N.; et al. The Relationship between COVID-19 Viral Load and Disease Severity: A Systematic Review. Immun. Inflamm. Dis. 2022, 10, e580. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jin, C.; Zhang, L.; Kong, D.; Hu, K.; Xuan, M.; Liu, Q.; Li, S.; Zhang, K.; Xue, Y. Clinical Characteristics and Risk Factors Analysis of Viral Shedding Time in Mildly Symptomatic and Asymptomatic Patients with SARS-CoV-2 Omicron Variant Infection in Shanghai. Front. Public Health 2023, 10, 1073387. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.A.; Spillane, S.; Comber, L.; Cardwell, K.; Harrington, P.; Connell, J.; Teljeur, C.; Broderick, N.; de Gascun, C.F.; Smith, S.M.; et al. The Duration of Infectiousness of Individuals Infected with SARS-CoV-2. J. Infect. 2020, 81, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Puhach, O.; Meyer, B.; Eckerle, I. SARS-CoV-2 Viral Load and Shedding Kinetics. Nat. Rev. Microbiol. 2023, 21, 147–161. [Google Scholar] [CrossRef]
- Qutub, M.; Aldabbagh, Y.; Mehdawi, F.; Alraddadi, A.; Alhomsy, M.; Alnahdi, A.; Fakeeh, M.; Maghrabi, A.; Alwagdani, M.; Bahabri, N. Duration of Viable SARS-CoV-2 Shedding from Respiratory Tract in Different Human Hosts and Its Impact on Isolation Discontinuation Polices Revision; a Narrative Review. Clin. Infect. Pract. 2022, 13, 100140. [Google Scholar] [CrossRef]
- Lopera, T.J.; Alzate-Ángel, J.C.; Díaz, F.J.; Rugeles, M.T.; Aguilar-Jiménez, W. The Usefulness of Antigen Testing in Predicting Contagiousness in COVID-19. Microbiol. Spectr. 2022, 10, e0196221. [Google Scholar] [CrossRef] [PubMed]
- Bullard, J.; Dust, K.; Funk, D.; Strong, J.E.; Alexander, D.; Garnett, L.; Boodman, C.; Bello, A.; Hedley, A.; Schiffman, Z.; et al. Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples. Clin. Infect. Dis. 2020, 71, 2663–2666. [Google Scholar] [CrossRef]
- Dimcheff, D.E.; Valesano, A.L.; Rumfelt, K.E.; Fitzsimmons, W.J.; Blair, C.; Mirabelli, C.; Petrie, J.G.; Martin, E.T.; Bhambhani, C.; Tewari, M.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Total and Subgenomic RNA Viral Load in Hospitalized Patients. J. Infect. Dis. 2021, 224, 1287–1293. [Google Scholar] [CrossRef]
- Alexandersen, S.; Chamings, A.; Bhatta, T.R. SARS-CoV-2 Genomic and Subgenomic RNAs in Diagnostic Samples Are Not an Indicator of Active Replication. Nat. Commun. 2020, 11, 6059. [Google Scholar] [CrossRef]
- Kirby, J.E.; Riedel, S.; Dutta, S.; Arnaout, R.; Cheng, A.; Ditelberg, S.; Hamel, D.J.; Chang, C.A.; Kanki, P.J. Sars-Cov-2 Antigen Tests Predict Infectivity Based on Viral Culture: Comparison of Antigen, PCR Viral Load, and Viral Culture Testing on a Large Sample Cohort. Clin. Microbiol. Infect. 2023, 29, 94–100. [Google Scholar] [CrossRef]
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV Viral Load Dynamics, Duration of Viral Shedding, and Infectiousness: A Systematic Review and Meta-Analysis. Lancet Microbe 2021, 2, e13–e22. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, H.; Recoing, A.; Bridier-Nahmias, A.; Rahi, M.; Lambert, C.; Martres, P.; Lucet, J.-C.; Rioux, C.; Bouzid, D.; Lebourgeois, S.; et al. Long-Term Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infectiousness Among Three Immunocompromised Patients: From Prolonged Viral Shedding to SARS-CoV-2 Superinfection. J. Infect. Dis. 2021, 223, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Raglow, Z.; Surie, D.; Chappell, J.D.; Zhu, Y.; Martin, E.T.; Kwon, J.H.; Frosch, A.E.; Mohamed, A.; Gilbert, J.; Bendall, E.E.; et al. SARS-CoV-2 Shedding and Evolution in Patients Who Were Immunocompromised during the Omicron Period: A Multicentre, Prospective Analysis. Lancet Microbe 2024, 5, e235–e246. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Knight, M.; Anglin, K.; Tassetto, M.; Lu, S.; Zhang, A.; Goldberg, S.A.; Catching, A.; Davidson, M.C.; Shak, J.R.; Romero, M.; et al. Infectious Viral Shedding of SARS-CoV-2 Delta Following Vaccination: A Longitudinal Cohort Study. PLoS Pathog. 2022, 18, e1010802. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, Z.; Yuan, J.; Cao, G.; Wang, Y.; Gao, P.; Liu, J.; Liu, M. Duration of Viable Virus Shedding and Polymerase Chain Reaction Positivity of the SARS-CoV-2 Omicron Variant in the Upper Respiratory Tract: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2023, 129, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Cento, V.; Colagrossi, L.; Nava, A.; Lamberti, A.; Senatore, S.; Travi, G.; Rossotti, R.; Vecchi, M.; Casati, O.; Matarazzo, E.; et al. Persistent Positivity and Fluctuations of SARS-CoV-2 RNA in Clinically-Recovered COVID-19 Patients. J. Infect. 2020, 81, e90–e92. [Google Scholar] [CrossRef] [PubMed]
- van Kampen, J.J.A.; van de Vijver, D.A.M.C.; Fraaij, P.L.A.; Haagmans, B.L.; Lamers, M.M.; Okba, N.; van den Akker, J.P.C.; Endeman, H.; Gommers, D.A.M.P.J.; Cornelissen, J.J.; et al. Duration and Key Determinants of Infectious Virus Shedding in Hospitalized Patients with Coronavirus Disease-2019 (COVID-19). Nat. Commun. 2021, 12, 267. [Google Scholar] [CrossRef] [PubMed]
- La Scola, B.; Le Bideau, M.; Andreani, J.; Hoang, V.T.; Grimaldier, C.; Colson, P.; Gautret, P.; Raoult, D. Viral RNA Load as Determined by Cell Culture as a Management Tool for Discharge of SARS-CoV-2 Patients from Infectious Disease Wards. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1059–1061. [Google Scholar] [CrossRef]
- Basile, K.; McPhie, K.; Carter, I.; Alderson, S.; Rahman, H.; Donovan, L.; Kumar, S.; Tran, T.; Ko, D.; Sivaruban, T.; et al. Cell-Based Culture of SARS-CoV-2 Informs Infectivity and Safe de-Isolation Assessments during COVID-19. Clin. Infect. Dis. 2021, 73, e2952–e2959. [Google Scholar] [CrossRef]
- Dinnes, J.; Sharma, P.; Berhane, S.; van Wyk, S.S.; Nyaaba, N.; Domen, J.; Taylor, M.; Cunningham, J.; Davenport, C.; Dittrich, S.; et al. Rapid, Point-of-Care Antigen Tests for Diagnosis of SARS-CoV-2 Infection. Cochrane Database Syst. Rev. 2022, 7, CD013705. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Sakai-Tagawa, Y.; Koga, M.; Akasaka, O.; Nakachi, I.; Koh, H.; Maeda, K.; Adachi, E.; Saito, M.; Nagai, H.; et al. Comparison of Rapid Antigen Tests for COVID-19. Viruses 2020, 12, 1420. [Google Scholar] [CrossRef]
- Alshukairi, A.N.; Al-Omari, A.; Al Hroub, M.K.; Al-Tawfiq, J.A.; Qutub, M.; Shaikh, S.; Allali, K.; Saeedi, M.F.; Alosaimi, R.S.; Alamoudi, E.; et al. De-Isolation of Vaccinated COVID-19 Health Care Workers Using Rapid Antigen Detection Test. J. Infect. Public Health 2022, 15, 902–905. [Google Scholar] [CrossRef]
- Currie, D.W.; Shah, M.M.; Salvatore, P.P.; Ford, L.; Whaley, M.J.; Meece, J.; Ivacic, L.; Thornburg, N.J.; Tamin, A.; Harcourt, J.L.; et al. Relationship of SARS-CoV-2 Antigen and Reverse Transcription PCR Positivity for Viral Cultures. Emerg. Infect. Dis. 2022, 28, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Glans, H.; Gredmark-Russ, S.; Olausson, M.; Falck-Jones, S.; Varnaite, R.; Christ, W.; Maleki, K.T.; Karlberg, M.L.; Broddesson, S.; Falck-Jones, R.; et al. Shedding of Infectious SARS-CoV-2 by Hospitalized COVID-19 Patients in Relation to Serum Antibody Responses. BMC Infect. Dis. 2021, 21, 494. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Liu, J.; Liu, Q.; Kang, L.; Liu, R.; Jing, W.; Wu, Y.; Liu, M. Global Percentage of Asymptomatic SARS-CoV-2 Infections Among the Tested Population and Individuals with Confirmed COVID-19 Diagnosis: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e2137257. [Google Scholar] [CrossRef]
- Bartsch, Y.C.; Tong, X.; Kang, J.; Avendaño, M.J.; Serrano, E.F.; García-Salum, T.; Pardo-Roa, C.; Riquelme, A.; Cai, Y.; Renzi, I.; et al. Omicron Variant Spike-Specific Antibody Binding and Fc Activity Are Preserved in Recipients of mRNA or Inactivated COVID-19 Vaccines. Sci. Transl. Med. 2022, 14, eabn9243. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Norddahl, G.L.; Melsted, P.; Gunnarsdottir, K.; Holm, H.; Eythorsson, E.; Arnthorsson, A.O.; Helgason, D.; Bjarnadottir, K.; Ingvarsson, R.F.; et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020, 383, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Vanshylla, K.; Di Cristanziano, V.; Kleipass, F.; Dewald, F.; Schommers, P.; Gieselmann, L.; Gruell, H.; Schlotz, M.; Ercanoglu, M.S.; Stumpf, R.; et al. Kinetics and Correlates of the Neutralizing Antibody Response to SARS-CoV-2 Infection in Humans. Cell Host Microbe 2021, 29, 917–929.e4. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-Neutralizing Antibodies Predict Disease Severity and Survival. Cell 2021, 184, 476–488.e11. [Google Scholar] [CrossRef]
- Shrock, E.; Fujimura, E.; Kula, T.; Timms, R.T.; Lee, I.-H.; Leng, Y.; Robinson, M.L.; Sie, B.M.; Li, M.Z.; Chen, Y.; et al. Viral Epitope Profiling of COVID-19 Patients Reveals Cross-Reactivity and Correlates of Severity. Science 2020, 370, eabd4250. [Google Scholar] [CrossRef]
- Cervia, C.; Nilsson, J.; Zurbuchen, Y.; Valaperti, A.; Schreiner, J.; Wolfensberger, A.; Raeber, M.E.; Adamo, S.; Weigang, S.; Emmenegger, M.; et al. Systemic and Mucosal Antibody Responses Specific to SARS-CoV-2 during Mild versus Severe COVID-19. J. Allergy Clin. Immunol. 2021, 147, 545–557.e9. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Connors, T.J.; Zhu, Y.; Baldwin, M.R.; Lin, W.-H.; Wontakal, S.; Szabo, P.A.; Wells, S.B.; Dogra, P.; Gray, J.; et al. Distinct Antibody Responses to SARS-CoV-2 in Children and Adults across the COVID-19 Clinical Spectrum. Nat. Immunol. 2021, 22, 25–31. [Google Scholar] [CrossRef]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and Immunological Assessment of Asymptomatic SARS-CoV-2 Infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive Immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H. COVID-19 Vaccines—Immunity, Variants, Boosters. N. Engl. J. Med. 2022, 387, 1011–1020. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; COVID-19 Genomics UK Consortium; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 Variant Biology: Immune Escape, Transmission and Fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Regev-Yochay, G.; Lustig, Y.; Joseph, G.; Gilboa, M.; Barda, N.; Gens, I.; Indenbaum, V.; Halpern, O.; Katz-Likvornik, S.; Levin, T.; et al. Correlates of Protection against COVID-19 Infection and Intensity of Symptomatic Disease in Vaccinated Individuals Exposed to SARS-CoV-2 in Households in Israel (ICoFS): A Prospective Cohort Study. Lancet Microbe 2023, 4, e309–e318. [Google Scholar] [CrossRef] [PubMed]
- Rapaka, R.R.; Hammershaimb, E.A.; Neuzil, K.M. Are Some COVID-19 Vaccines Better Than Others? Interpreting and Comparing Estimates of Efficacy in Vaccine Trials. Clin. Infect. Dis. 2022, 74, 352–358. [Google Scholar] [CrossRef]
- Castro Dopico, X.; Ols, S.; Loré, K.; Karlsson Hedestam, G.B. Immunity to SARS-CoV-2 Induced by Infection or Vaccination. J. Intern. Med. 2022, 291, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.-Y.; Gu, Y.; Wheeler, B.; Young, H.; Holloway, S.; Sunny, S.-K.; Moore, Z.; Zeng, D. Effectiveness of COVID-19 Vaccines over a 9-Month Period in North Carolina. N. Engl. J. Med. 2022, 386, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Stenehjem, E.; Grannis, S.; Ball, S.W.; Naleway, A.L.; Ong, T.C.; DeSilva, M.B.; Natarajan, K.; Bozio, C.H.; Lewis, N.; et al. Effectiveness of COVID-19 Vaccines in Ambulatory and Inpatient Care Settings. N. Engl. J. Med. 2021, 385, 1355–1371. [Google Scholar] [CrossRef]
- Danese, E.; Montagnana, M.; Salvagno, G.L.; Peserico, D.; Pighi, L.; De Nitto, S.; Henry, B.M.; Porru, S.; Lippi, G. Comprehensive Assessment of Humoral Response after Pfizer BNT162b2 mRNA COVID-19 Vaccination: A Three-Case Series. Clin. Chem. Lab. Med. 2021, 59, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Frenck, R.W.; Walsh, E.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Bailey, R.; Swanson, K.A.; Xu, X.; et al. SARS-CoV-2 Neutralization with BNT162b2 Vaccine Dose 3. N. Engl. J. Med. 2021, 385, 1627–1629. [Google Scholar] [CrossRef] [PubMed]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for COVID-19. N. Engl. J. Med. 2021, 384, 2259–2261. [Google Scholar] [CrossRef]
- Alter, G.; Yu, J.; Liu, J.; Chandrashekar, A.; Borducchi, E.N.; Tostanoski, L.H.; McMahan, K.; Jacob-Dolan, C.; Martinez, D.R.; Chang, A.; et al. Immunogenicity of Ad26.COV2.S Vaccine against SARS-CoV-2 Variants in Humans. Nature 2021, 596, 268–272. [Google Scholar] [CrossRef]
- Stephenson, K.E.; Le Gars, M.; Sadoff, J.; de Groot, A.M.; Heerwegh, D.; Truyers, C.; Atyeo, C.; Loos, C.; Chandrashekar, A.; McMahan, K.; et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA 2021, 325, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Stephenson, K.E.; Sadoff, J.; Yu, J.; Chang, A.; Gebre, M.; McMahan, K.; Liu, J.; Chandrashekar, A.; Patel, S.; et al. Durable Humoral and Cellular Immune Responses 8 Months after Ad26.COV2.S Vaccination. N. Engl. J. Med. 2021, 385, 951–953. [Google Scholar] [CrossRef]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 mRNA Vaccines Induce Persistent Human Germinal Centre Responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous COVID-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron Extensively but Incompletely Escapes Pfizer BNT162b2 Neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Amir, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. N. Engl. J. Med. 2022, 386, 1712–1720. [Google Scholar] [CrossRef]
- Liu, J.; Chandrashekar, A.; Sellers, D.; Barrett, J.; Jacob-Dolan, C.; Lifton, M.; McMahan, K.; Sciacca, M.; VanWyk, H.; Wu, C.; et al. Vaccines Elicit Highly Conserved Cellular Immunity to SARS-CoV-2 Omicron. Nature 2022, 603, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Lipsitch, M.; Krammer, F.; Regev-Yochay, G.; Lustig, Y.; Balicer, R.D. SARS-CoV-2 Breakthrough Infections in Vaccinated Individuals: Measurement, Causes and Impact. Nat. Rev. Immunol. 2022, 22, 57–65. [Google Scholar] [CrossRef]
- Gray, G.; Collie, S.; Goga, A.; Garrett, N.; Champion, J.; Seocharan, I.; Bamford, L.; Moultrie, H.; Bekker, L.-G. Effectiveness of Ad26.COV2.S and BNT162b2 Vaccines against Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Yaseen, F.S.; Saide, K.; Kim, S.-H.; Monshi, M.; Tailor, A.; Wood, S.; Meng, X.; Jenkins, R.; Faulkner, L.; Daly, A.K.; et al. Promiscuous T-Cell Responses to Drugs and Drug-Haptens. J. Allergy Clin. Immunol. 2015, 136, 474–476.e8. [Google Scholar] [CrossRef]
- Flacco, M.E.; Acuti Martellucci, C.; Baccolini, V.; De Vito, C.; Renzi, E.; Villari, P.; Manzoli, L. Risk of Reinfection and Disease after SARS-CoV-2 Primary Infection: Meta-Analysis. Eur. J. Clin. Investig. 2022, 52, e13845. [Google Scholar] [CrossRef]
- Chenchula, S.; Karunakaran, P.; Sharma, S.; Chavan, M. Current Evidence on Efficacy of COVID-19 Booster Dose Vaccination against the Omicron Variant: A Systematic Review. J. Med. Virol. 2022, 94, 2969–2976. [Google Scholar] [CrossRef]
- Wang, Z.; Muecksch, F.; Schaefer-Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.-H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally Enhanced Neutralizing Breadth against SARS-CoV-2 One Year after Infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef]
- Collier, A.-R.Y.; Brown, C.M.; McMahan, K.A.; Yu, J.; Liu, J.; Jacob-Dolan, C.; Chandrashekar, A.; Tierney, D.; Ansel, J.L.; Rowe, M.; et al. Characterization of Immune Responses in Fully Vaccinated Individuals after Breakthrough Infection with the SARS-CoV-2 Delta Variant. Sci. Transl. Med. 2022, 14, eabn6150. [Google Scholar] [CrossRef]
- Akerman, A.; Fitcher, C.; Milogiannakis, V.; Esneu, C.; Ruiz Silva, M.; Ison, T.; Lopez, J.A.; Naing, Z.; Caguicla, J.; Amatayakul-Chantler, S.; et al. Cross-Sectional and Longitudinal Genotype to Phenotype Surveillance of SARS-CoV-2 Variants Over the First Four Years of the COVID-19 Pandemic 2024. medRxiv 2024. [Google Scholar] [CrossRef]
- Communicable Diseases Network Australia, Australian Government Department of Health and Aged Care. Influenza Infection (Flu)—CDNA National Guidelines for Public Health Units. Available online: https://www.health.gov.au/resources/publications/influenza-infection-flu-cdna-national-guidelines-for-public-health-units?language=en (accessed on 19 June 2024).
- Centers for Disease Control and Prevention Prevention Strategies for Seasonal Influenza in Healthcare Settings|CDC. Available online: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm (accessed on 19 June 2024).
- Clinical Excellence Commission. Infection Prevention and Control Manual COVID-19 and Other Acute Respiratory Infections for Acute and Non-Acute Healthcare Settings, v4.2; Clinical Excellence Commission: Sydney, Australia, 2023.
- Jafari, Y.; Yin, M.; Lim, C.; Pople, D.; Evans, S.; Stimson, J.; Pham, T.M.; Read, J.M.; Robotham, J.V.; Cooper, B.S.; et al. Effectiveness of Infection Prevention and Control Interventions, Excluding Personal Protective Equipment, to Prevent Nosocomial Transmission of SARS-CoV-2: A Systematic Review and Call for Action. Infect. Prev. Pract. 2021, 4, 100192. [Google Scholar] [CrossRef]
- Khan, U.I.; Mahmood, S.F.; Khan, S.; Hasan, Z.; Cheema, A.; Hakim, A.; Ali, S.I. Using Rapid Antigen Testing for Early, Safe Return-to-Work for Healthcare Personnel after SARS-CoV-2 Infection in a Healthcare System in Pakistan: A Retrospective Cross-Sectional Study. PLoS Glob. Public Health 2023, 3, e0001746. [Google Scholar] [CrossRef]
- Raza, M.; Giri, P.; Basu, S. Surveillance and Return to Work of Healthcare Workers Following SARS-CoV-2 Omicron Variant Infection, Sheffield, England, 17 January to 7 February 2022. Eurosurveillance 2022, 27, 2200164. [Google Scholar] [CrossRef]
- Bouton, T.C.; Atarere, J.; Turcinovic, J.; Seitz, S.; Sher-Jan, C.; Gilbert, M.; White, L.; Zhou, Z.; Hossain, M.M.; Overbeck, V.; et al. Viral Dynamics of Omicron and Delta SARS-CoV-2 Variants with Implications for Timing of Release from Isolation: A Longitudinal Cohort Study. Clin. Infect. Dis. 2023, 76, e227–e233. [Google Scholar] [CrossRef]
- Bays, D.; Whiteley, T.; Pindar, M.; Taylor, J.; Walker, B.; Williams, H.; Finnie, T.J.R.; Gent, N. Mitigating Isolation: The Use of Rapid Antigen Testing to Reduce the Impact of Self-Isolation Periods 2021. medRxiv 2021. [Google Scholar] [CrossRef]
- Mowrer, C.T.; Creager, H.; Cawcutt, K.; Birge, J.; Lyden, E.; Schooneveld, T.C.V.; Rupp, M.E.; Hewlett, A. Evaluation of Cycle Threshold Values at Deisolation. Infect. Control Hosp. Epidemiol. 2022, 43, 794–796. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Chen, Y.; Zheng, X.; Gong, F.; Liu, W.; Lin, J.; Zheng, R.; Yang, Z.; Bi, Y.; Chen, E. Comorbidities Prolonged Viral Shedding of Patients Infected with SARS-CoV-2 Omicron Variant in Shanghai: A Multi-Center, Retrospective, Observational Study. J. Infect. Public Health 2023, 16, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, X.; Sun, T.; Jin, X.; Tian, Z.; Xue, M.; Kang, J.; Gao, B.; Xu, A.; Chen, Y.; et al. Chronological Changes of Viral Shedding in Adult Inpatients with Omicron Infection in Shanghai, China. Front. Immunol. 2023, 14, 1090498. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Zhang, X.; Chen, C.; Jiang, D.; Liu, X.; Zhou, Y.; Huang, C.; Zhou, Y.; Guan, Z.; Ding, C.; et al. Characteristics of Viral Shedding Time in SARS-CoV-2 Infections: A Systematic Review and Meta-Analysis. Front. Public Health 2021, 9, 652842. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Ishikane, M.; Ujiie, M.; Iwamoto, N.; Okumura, N.; Sato, T.; Nagashima, M.; Moriya, A.; Suzuki, M.; Hojo, M.; et al. Duration of Infectious Virus Shedding by SARS-CoV-2 Omicron Variant–Infected Vaccinees. Emerg. Infect. Dis. 2022, 28, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Kanjilal, S.; Baker, M.; Klompas, M. Duration of SARS-CoV-2 Infectivity: When Is It Safe to Discontinue Isolation? Clin. Infect. Dis. 2020, 72, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Singanayagam, A.; Patel, M.; Charlett, A.; Bernal, J.L.; Saliba, V.; Ellis, J.; Ladhani, S.; Zambon, M.; Gopal, R. Duration of Infectiousness and Correlation with RT-PCR Cycle Threshold Values in Cases of COVID-19, England, January to May 2020. Eurosurveillance 2020, 25, 2001483. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-W.; Kim, J.Y.; Park, H.; Lim, S.Y.; Kim, J.; Chang, E.; Bae, S.; Jung, J.; Kim, M.J.; Chong, Y.P.; et al. Comparison of Secondary Attack Rate and Viable Virus Shedding between Patients with SARS-CoV-2 Delta and Omicron Variants: A Prospective Cohort Study. J. Med. Virol. 2023, 95, e28369. [Google Scholar] [CrossRef]
- Choi, G.; Lim, A.-Y.; Choi, S.; Park, K.; Lee, S.Y.; Kim, J.-H. Viral Shedding Patterns of Symptomatic SARS-CoV-2 Infections by Periods of Variant Predominance and Vaccination Status in Gyeonggi Province, Korea. Epidemiol. Health 2023, 45, e2023008. [Google Scholar] [CrossRef]
- Maya, S.; Kahn, J.G. COVID-19 Testing Protocols to Guide Duration of Isolation: A Cost-Effectiveness Analysis. BMC Public Health 2023, 23, 864. [Google Scholar] [CrossRef]
- Pekosz, A.; Parvu, V.; Li, M.; Andrews, J.C.; Manabe, Y.C.; Kodsi, S.; Gary, D.S.; Roger-Dalbert, C.; Leitch, J.; Cooper, C.K. Antigen-Based Testing but Not Real-Time Polymerase Chain Reaction Correlates with Severe Acute Respiratory Syndrome Coronavirus 2 Viral Culture. Clin. Infect. Dis. 2021, 73, e2861–e2866. [Google Scholar] [CrossRef]
- Mitchell, S.L.; Loeffelholz, M.J. Considerations Regarding Interpretation of Positive SARS-CoV-2 Molecular Results with Late Cycle Threshold Values. J. Clin. Microbiol. 2022, 60, e00501-22. [Google Scholar] [CrossRef]
- Han, M.S.; Byun, J.-H.; Cho, Y.; Rim, J.H. RT-PCR for SARS-CoV-2: Quantitative versus Qualitative. Lancet Infect. Dis. 2021, 21, 165. [Google Scholar] [CrossRef]
- Nasa, P.; Azoulay, E.; Chakrabarti, A.; Divatia, J.V.; Jain, R.; Rodrigues, C.; Rosenthal, V.D.; Alhazzani, W.; Arabi, Y.M.; Bakker, J.; et al. Infection Control in the Intensive Care Unit: Expert Consensus Statements for SARS-CoV-2 Using a Delphi Method. Lancet Infect. Dis. 2022, 22, e74–e87. [Google Scholar] [CrossRef]
- Alfano, G.; Fontana, F.; Ferrari, A.; Morisi, N.; Gregorini, M.; Cappelli, G.; Magistroni, R.; Guaraldi, G.; Donati, G. Which Criteria Should We Use to End Isolation in Hemodialysis Patients with COVID-19? Clin. Kidney J. 2022, 15, 1450–1454. [Google Scholar] [CrossRef]
- Kim, C.S.; Kim, U.J.; Lee, Y.; Lee, U.; Choi, O.; Kim, S.-H.; Lee, K.; Chung, Y.-S.; Choi, H.S.; Bae, E.H.; et al. Nosocomial Outbreak of COVID-19 from a Kidney Transplant Patient: Necessity of a Longer Isolation Period in Immunocompromised Patients. Infect. Chemother. 2023, 55, 42–49. [Google Scholar] [CrossRef]
- Wong, S.-C.; Au, A.K.-W.; Lo, J.Y.-C.; Ho, P.-L.; Hung, I.F.-N.; To, K.K.-W.; Yuen, K.-Y.; Cheng, V.C.-C. Evolution and Control of COVID-19 Epidemic in Hong Kong. Viruses 2022, 14, 2519. [Google Scholar] [CrossRef]
- Liu, Y.; Rocklöv, J. The Effective Reproductive Number of the Omicron Variant of SARS-CoV-2 Is Several Times Relative to Delta. J. Travel Med. 2022, 29, taac037. [Google Scholar] [CrossRef]
- Theaux, C.; Martin, Y.; Montoto Piazza, L.; Wenk, G.; Notaristefano, G.; Miño, L.; Sevilla, M.E.; Aprea, V.; Claps, A.; Nabaes Jodar, M.; et al. Persistence of SARS-CoV-2 RNA Shedding and Infectivity in Immunized Population: Prospective Study along Different Epidemiological Periods in Argentina. PLoS ONE 2023, 18, e0285704. [Google Scholar] [CrossRef]
- Jung, J.; Kang, S.W.; Lee, S.; Park, H.; Kim, J.Y.; Kim, S.-K.; Park, S.; Lim, Y.-J.; Kim, E.O.; Lim, S.Y.; et al. Risk of Transmission of COVID-19 from Healthcare Workers Returning to Work after a 5-Day Isolation, and Kinetics of Shedding of Viable SARS-CoV-2 Variant B.1.1.529 (Omicron). J. Hosp. Infect. 2023, 131, 228–233. [Google Scholar] [CrossRef]
- Luna-Muschi, A.; Noguera, S.V.; Borges, I.C.; De Paula, A.V.; Côrtes, M.F.; Larocca, C.; Mari, J.F.; Guimarães, L.S.P.; Torres, P.M.; Scaccia, N.; et al. Characterization of Severe Acute Respiratory Syndrome Coronavirus 2 Omicron Variant Shedding and Predictors of Viral Culture Positivity on Vaccinated Healthcare Workers with Mild Coronavirus Disease 2019. J. Infect. Dis. 2022, 226, 1726–1730. [Google Scholar] [CrossRef] [PubMed]
| Mild | Moderate | Severe | |
|---|---|---|---|
| Respiratory symptoms | Symptomatic, no lower respiratory tract symptoms | Lower respiratory tract symptoms present | Lower respiratory tract symptoms present; often tachypneic > 30 breaths per minute |
| Oxygen saturation | Normal | Normal | <94% |
| Chest imaging | Normal | Abnormal | Abnormal, usually lung infiltrates > 50% |
| Advantages for Use in Deisolation Protocols | Disadvantages for Use in Deisolation Protocols | |
|---|---|---|
| Real-time polymerase chain reaction (RT-PCR) | Fast turnaround time, multiple targets, and likely to be adaptable to future variants | Unable to distinguish between non-viable or non-replicating virus; prolonged period of positivity especially if immunocompromised |
| Rapid antigen detection (RAT) | Especially rapid, inexpensive, point-of-care test. Reasonable specificity for transmissible virus | Analytical performance with future variants unknown; utility in cases of acute COVID-19 that are RT-PCR-positive but not RAT-positive is unclear |
| Viral culture | Gold standard. Especially helpful for immunocompromised patients | Labour- and time-intensive, generally only available in reference laboratories |
| Paper | Population Assessed | Testing Method | Positivity (%) and Day (Post-Symptom Onset) | Comments |
|---|---|---|---|---|
| Khan et al. [86] | HCWs (n = 1216) | RAT | 22% at day five 6% at day seven | Contributed 3799 additional days of work compared to 10-day isolation |
| Raza, Giri, and Basu [87] | HCWs (n = 240) | RAT | 36% at day seven | HCWs required two negative RATs, 24 h apart, prior to returning to work |
| Alshukairi et al. [39] | HCWs (n = 480) | RAT | 33% at day seven | All HCWs had received ≥2 doses of vaccination |
| Bouton et al. [88] | Patients (n = 92 for viral culture, n = 12 for RAT) | RAT Viral culture | RAT—75% at day five Culture—71% positive at day six | |
| Bays et al. [89] | HCWs (mathematical modelling of n = 500,000) | RAT | 21% at day seven | Mathematical modelling |
| Mowrer et al. [90] | Hospitalised patients (severe or immunocompromised, n = 23) | RT-PCR | Ct value ≥ 30 for 84% between days 14–21 | Percentages for Ct values ≥ 30; all patients PCR-positive at 21 days |
| Ct value ≥ 30 for 100% by day 21 | ||||
| La Scola et al. [35] | Hospitalised patients (n = 183) | Viral culture | Reportedly none positive after day eight | Culture negativity associated with RT-PCR Ct values ≥ 34 |
| Van Kampen et al. [34] | Hospitalised patients (n = 129) | Viral culture | Mean duration of positivity is eight days (IQR 5–11 days) |
| COVID-19-Infected Individual | Immune Status | Disease Severity | Symptom Criteria | Testing Criteria | Release from Isolation or Return to Work | Comments |
|---|---|---|---|---|---|---|
| Patient | Immunocompetent | Asymptomatic, mild, or moderate | Asymptomatic by day five. | Negative RAT or RT-PCR test on day six or seven | Same day as negative RAT or RT-PCR test OR after day 10 without testing. | |
| Patient | Immunocompetent | Severe or critical disease | Resolution of fever and significant respiratory symptoms. Cough and lethargy may persist. | RAT or RT-PCR test on day 10 | Following negative test on day 10 OR after day 20 without testing. | |
| Patient | Immunocompromised | Any | Resolution of fever and significant respiratory symptoms. Cough and lethargy may persist. | Testing to start on day 14 post-symptom onset; two negative RATs or RT-PCR tests, taken 24 h apart. | Following second negative RAT or RT-PCR test | Masks should be worn until at least day 20 post-symptom onset. |
| Healthcare worker | Immunocompetent | N/A | Resolution of fever and significant respiratory symptoms. Dry cough and lethargy should not preclude return to work. | Negative RAT (or RT-PCR test) on day six | Day seven | Wear surgical masks in patient-facing settings until day 10. |
| Healthcare worker | Immunocompromised | N/A | Resolution of fever and significant respiratory symptoms. | Two negative RATs (or RT-PCR tests) taken 24 h apart, first when clinically improved | Following second negative test | Consider non-patient facing alternatives to work if well but ongoing shedding. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumgart, S.W.L.; McLachlan, A.; Kenny, H.; McKew, G.; Maddocks, S.; Chen, S.C.-A.; Kok, J. Deisolation in the Healthcare Setting Following Recent COVID-19 Infection. Viruses 2024, 16, 1131. https://doi.org/10.3390/v16071131
Baumgart SWL, McLachlan A, Kenny H, McKew G, Maddocks S, Chen SC-A, Kok J. Deisolation in the Healthcare Setting Following Recent COVID-19 Infection. Viruses. 2024; 16(7):1131. https://doi.org/10.3390/v16071131
Chicago/Turabian StyleBaumgart, Samuel W. L., Aidan McLachlan, Hayden Kenny, Genevieve McKew, Susan Maddocks, Sharon C.-A. Chen, and Jen Kok. 2024. "Deisolation in the Healthcare Setting Following Recent COVID-19 Infection" Viruses 16, no. 7: 1131. https://doi.org/10.3390/v16071131
APA StyleBaumgart, S. W. L., McLachlan, A., Kenny, H., McKew, G., Maddocks, S., Chen, S. C.-A., & Kok, J. (2024). Deisolation in the Healthcare Setting Following Recent COVID-19 Infection. Viruses, 16(7), 1131. https://doi.org/10.3390/v16071131







