Host–Virus Cophylogenetic Trajectories: Investigating Molecular Relationships between Coronaviruses and Bat Hosts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phylogenetic Analysis
2.2. Cophylogeny Analysis between Host and Coronaviruses
2.3. Comparison of Host and Coronavirus Phylogenies with Sliding Windows
2.4. Similarity Analysis
2.5. –Test Recombination Analysis
2.6. Horizontal Gene Transfer Detection Analysis
3. Results
3.1. Phylogenetic Analysis
3.2. Cophylogenetic Analysis between Hosts and CoVs
3.3. Comparison of Host and CoV Phylogenies with Sliding Windows
3.4. Detecting the Presence of Recombination
3.5. Horizontal Gene Transfer Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aa | Amino acid |
| bp | Base pair |
| CoVs | Coronaviruses |
| cytb | Cytochrome b |
| DNA | DeoxyriboNucleic acid |
| HGT | Horizontal gene transfer |
| NCBI | National Center for Biotechnology Information |
| MAFFT | Multiple Alignment using Fast Fourier Transform |
| ML | Maximum likelihood |
| MSA | Multiple sequence alignment |
| MUSCLE | MUltiple Sequence Comparison by Log–Expectation |
| ORF1ab | Open Reading Frames 1a and b |
| RAxML–NG | Randomized Axelerated Maximum Likelihood Next Generation |
| RF | Robinson–Foulds distance |
| RNA | RiboNucleic acid |
References
- Brook, C.E.; Dobson, A.P. Bats as ‘special’reservoirs for emerging zoonotic pathogens. Trends Microbiol. 2015, 23, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Nabi, G.; Wang, Y.; Lü, L.; Jiang, C.; Ahmad, S.; Wu, Y.; Li, D. Bats and birds as viral reservoirs: A physiological and ecological perspective. Sci. Total Environ. 2021, 754, 142372. [Google Scholar] [CrossRef] [PubMed]
- Calistri, P.; Decaro, N.; Lorusso, A. SARS-CoV-2 pandemic: Not the first, not the last. Microorganisms 2021, 9, 433. [Google Scholar] [CrossRef] [PubMed]
- Turmelle, A.S.; Olival, K.J. Correlates of viral richness in bats (order Chiroptera). EcoHealth 2009, 6, 522–539. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiu, Y.; Ge, X. The taxonomy, host range and pathogenicity of coronaviruses and other viruses in the Nidovirales order. Anim. Dis. 2021, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.; Shackelton, L.A.; Holmes, E.C. Rates of evolutionary change in viruses: Patterns and determinants. Nat. Rev. Genet. 2008, 9, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.M.; Rambaut, A.; Pybus, O.G.; Holmes, E.C. Rates of molecular evolution in RNA viruses: A quantitative phylogenetic analysis. J. Mol. Evol. 2002, 54, 156–165. [Google Scholar] [CrossRef]
- Gallaher, W.R. A palindromic RNA sequence as a common breakpoint contributor to copy–choice recombination in SARS-CoV-2. Arch. Virol. 2020, 165, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, A.O.; Spaan, W.J.; Snijder, E.J. Nidovirus transcription: How to make sense…? J. Gen. Virol. 2006, 87, 1403–1421. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Yuen, K.y. Infectious diseases emerging from Chinese wet–markets: Zoonotic origins of severe respiratory viral infections. Curr. Opin. Infect. Dis. 2006, 19, 401–407. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Huang, Y.; Yuen, K.Y. Coronavirus diversity, phylogeny and interspecies jumping. Exp. Biol. Med. 2009, 234, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, L.; Ren, X.; He, G.; Zhang, J.; Yang, J.; Qian, Z.; Dong, J.; Sun, L.; Zhu, Y.; et al. Deciphering the bat virome catalog to better understand the ecological diversity of bat viruses and the bat origin of emerging infectious diseases. ISME J. 2016, 10, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Han, N.; Streicker, D.; Li, G.; Tang, X.; Shi, Z.; Hu, Z.; Zhao, G.; Fontanet, A.; Guan, Y.; et al. Evolutionary relationships between bat coronaviruses and their hosts. Emerg. Infect. Dis. 2007, 13, 1526. [Google Scholar] [CrossRef] [PubMed]
- Feijó, A.; Wang, Y.; Sun, J.; Li, F.; Wen, Z.; Ge, D.; Xia, L.; Yang, Q. Research trends on bats in China: A twenty–first century review. Mamm. Biol. 2019, 98, 163–172. [Google Scholar] [CrossRef]
- Latinne, A.; Hu, B.; Olival, K.J.; Zhu, G.; Zhang, L.; Li, H.; Chmura, A.A.; Field, H.E.; Zambrana-Torrelio, C.; Epstein, J.H.; et al. Origin and cross–species transmission of bat coronaviruses in China. Nat. Commun. 2020, 11, 4235. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; He, C.; Shen, C.; Jiang, T.; Shi, L.; Sun, K.; Berquist, S.; Feng, J. Phylogeography and population genetic structure of the great leaf–nosed bat (Hipposideros armiger) in China. J. Hered. 2010, 101, 562–572. [Google Scholar] [CrossRef]
- Agnarsson, I.; Zambrana-Torrelio, C.M.; Flores-Saldana, N.P.; May-Collado, L.J. A time–calibrated species–level phylogeny of bats (Chiroptera, Mammalia). PLoS Curr. 2011, 3, RRN1212. [Google Scholar] [CrossRef]
- Bradley, R.D.; Baker, R.J. A test of the genetic species concept: Cytochrome–b sequences and mammals. J. Mammal. 2001, 82, 960–973. [Google Scholar] [CrossRef]
- Kocher, T.D.; Thomas, W.K.; Meyer, A.; Edwards, S.V.; Pääbo, S.; Villablanca, F.X.; Wilson, A.C. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 1989, 86, 6196–6200. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.i.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with improved accuracy and speed. In Proceedings of the 2004 IEEE Computational Systems Bioinformatics Conference, 2004. CSB 2004, Stanford, CA, USA, 19 August 2004; IEEE: Piscataway, NJ, USA, 2004; pp. 728–729. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef] [PubMed]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny. fr: Robust phylogenetic analysis for the non–specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef]
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny. fr: New generation phylogenetic services for non–specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML–VI–HPC: Maximum likelihood–based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Desdevises, Y. Cophylogeny: Insights from fish–parasite systems. Parassitologia 2007, 49, 125. [Google Scholar]
- Legendre, P.; Desdevises, Y.; Bazin, E. A statistical test for host–parasite Robinson–Fouldsolution. Syst. Biol. 2002, 51, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Fourment, M.; Gibbs, M.J. PATRISTIC: A program for calculating patristic distances and graphically comparing the components of genetic change. BMC Evol. Biol. 2006, 6, 1. [Google Scholar] [CrossRef]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- Balbuena, J.A.; Míguez-Lozano, R.; Blasco-Costa, I. PACo: A novel procrustes application to cophylogenetic analysis. PLoS ONE 2013, 8, e61048. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The vegan package. COmmunity Ecol. Package 2007, 10, 719. [Google Scholar]
- Hutchinson, M.C.; Cagua, E.F.; Balbuena, J.A.; Stouffer, D.B.; Poisot, T. paco: Implementing Procrustean Approach to Cophylogeny in R. Methods Ecol. Evol. 2017, 8, 932–940. [Google Scholar] [CrossRef]
- Tahiri, N. Un Nouvel Algorithme Pour Retrouver les Relations Phylogénétiques Entre la Distribution Géographique des Espèces et Leurs Compositions Génétiques. Master’s Thesis, University of Quebec at Montreal, Montreal, QC, Canada, 2012. Available online: https://archipel.uqam.ca/5389/ (accessed on 10 July 2024).
- Li, W. New Algorithm to Assess the Environmental Influence of Coronavirus through Phylogeographic Analysis. Master’s Thesis, University of Sherbrooke, Sherbrooke, QC, Canada, 2023. Available online: https://savoirs.usherbrooke.ca/handle/11143/21322 (accessed on 10 July 2024).
- Robinson, D.F.; Foulds, L.R. Comparison of phylogenetic trees. Math. Biosci. 1981, 53, 131–147. [Google Scholar] [CrossRef]
- Köster, J.; Rahmann, S. Snakemake—A scalable bioinformatics workflow engine. Bioinformatics 2012, 28, 2520–2522. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high–quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML–NG: A fast, scalable and user–friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tahiri, N. aPhyloGeo–Covid: A Web Interface for Reproducible Phylogeographic Analysis of SARS-CoV-2 Variation using Neo4j and Snakemake. In Proceedings of the 22nd Python in Science Conference, Austin, TX, USA, 10–16 July 2023; pp. 114–123. [Google Scholar] [CrossRef]
- Cock, P.J.; Antao, T.; Chang, J.T.; Chapman, B.A.; Cox, C.J.; Dalke, A.; Friedberg, I.; Hamelryck, T.; Kauff, F.; Wilczynski, B.; et al. Biopython: Freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 2009, 25, 1422. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Cepas, J.; Serra, F.; Bork, P. ETE 3: Reconstruction, analysis, and visualization of phylogenomic data. Mol. Biol. Evol. 2016, 33, 1635–1638. [Google Scholar] [CrossRef]
- Van Der Walt, S.; Colbert, S.C.; Varoquaux, G. The NumPy array: A structure for efficient numerical computation. Comput. Sci. Eng. 2011, 13, 22–30. [Google Scholar] [CrossRef]
- McKinney, W. pandas: A foundational Python library for data analysis and statistics. Python High Perform. Sci. Comput. 2011, 14, 1–9. [Google Scholar]
- Samson, S.; Lord, É.; Makarenkov, V. SimPlot++: A Python application for representing sequence similarity and detecting recombination. Bioinformatics 2022, 38, 3118–3120. [Google Scholar] [CrossRef]
- Bruen, T.; Bruen, T. PhiPack: PHI Test and Other Tests of Recombination; McGill University: Montreal, QC, USA, 2005; pp. 1–8. [Google Scholar]
- Ewens, W.J.; Grant, G. Evolutionary Models. In Statistical Methods in Bioinformatics: An Introduction; Springer: New York, NY, USA, 2005; pp. 475–496. [Google Scholar]
- Bruen, T.C.; Philippe, H.; Bryant, D. A simple and robust statistical test for detecting the presence of recombination. Genetics 2006, 172, 2665–2681. [Google Scholar] [CrossRef]
- Boc, A.; Diallo, A.B.; Makarenkov, V. T–REX: A web server for inferring, validating and visualizing phylogenetic trees and networks. Nucleic Acids Res. 2012, 40, W573–W579. [Google Scholar] [CrossRef]
- Boc, A.; Philippe, H.; Makarenkov, V. Inferring and validating horizontal gene transfer events using bipartition dissimilarity. Syst. Biol. 2010, 59, 195–211. [Google Scholar] [CrossRef]
- Makarenkov, V.; Mazoure, B.; Rabusseau, G.; Legendre, P. Horizontal gene transfer and recombination analysis of SARS-CoV-2 genes helps discover its close relatives and shed light on its origin. BMC Ecol. Evol. 2021, 21, 5. [Google Scholar] [CrossRef]
- Woo, P.C.; Huang, Y.; Lau, S.K.; Yuen, K.Y. Coronavirus genomics and bioinformatics analysis. Viruses 2010, 2, 1804–1820. [Google Scholar] [CrossRef]
- Graham, R.L.; Sparks, J.S.; Eckerle, L.D.; Sims, A.C.; Denison, M.R. SARS coronavirus replicase proteins in pathogenesis. Virus Res. 2008, 133, 88–100. [Google Scholar] [CrossRef]
- Li, F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef]
- Hulswit, R.; De Haan, C.; Bosch, B.J. Coronavirus spike protein and tropism changes. Adv. Virus Res. 2016, 96, 29–57. [Google Scholar]
- Hofmann, H.; Hattermann, K.; Marzi, A.; Gramberg, T.; Geier, M.; Krumbiegel, M.; Kuate, S.; Überla, K.; Niedrig, M.; Pöhlmann, S. S protein of severe acute respiratory syndrome–associated coronavirus mediates entry into hepatoma cell lines and is targeted by neutralizing antibodies in infected patients. J. Virol. 2004, 78, 6134–6142. [Google Scholar] [CrossRef]
- Page, R.D.; Charleston, M.A. Trees within trees: Phylogeny and historical associations. Trends Ecol. Evol. 1998, 13, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Losada, M.; Arenas, M.; Galán, J.C.; Palero, F.; González-Candelas, F. Recombination in viruses: Mechanisms, methods of study, and evolutionary consequences. Infect. Genet. Evol. 2015, 30, 296–307. [Google Scholar] [CrossRef]
- Worobey, M.; Holmes, E.C. Evolutionary aspects of recombination in RNA viruses. J. Gen. Virol. 1999, 80, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.W.; To, K.K.W.; Tse, H.; Jin, D.Y.; Yuen, K.Y. Interspecies transmission and emergence of novel viruses: Lessons from bats and birds. Trends Microbiol. 2013, 21, 544–555. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Lam, C.S.; Lau, C.C.; Tsang, A.K.; Lau, J.H.; Bai, R.; Teng, J.L.; Tsang, C.C.; Wang, M.; et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus Deltacoronavirus supports bat coronaviruses as the gene source of Alphacoronavirus and Betacoronavirus and avian coronaviruses as the gene source of Gammacoronavirus and Deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar]
- Weaver, S. Evolutionary influences in arboviral disease. In Quasispecies: Concept and Implications for Virology; Springer Science & Business Media: Berlin, Germany, 2006; pp. 285–314. [Google Scholar]
- Jackwood, M.W.; Boynton, T.O.; Hilt, D.A.; McKinley, E.T.; Kissinger, J.C.; Paterson, A.H.; Robertson, J.; Lemke, C.; McCall, A.W.; Williams, S.M.; et al. Emergence of a group 3 coronavirus through recombination. Virology 2010, 398, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.P.; Biagini, P.; Lefeuvre, P.; Golden, M.; Roumagnac, P.; Varsani, A. Recombination in eukaryotic single stranded DNA viruses. Viruses 2011, 3, 1699–1738. [Google Scholar] [CrossRef]
- Simon-Loriere, E.; Holmes, E.C. Why do RNA viruses recombine? Nat. Rev. Microbiol. 2011, 9, 617–626. [Google Scholar] [CrossRef]
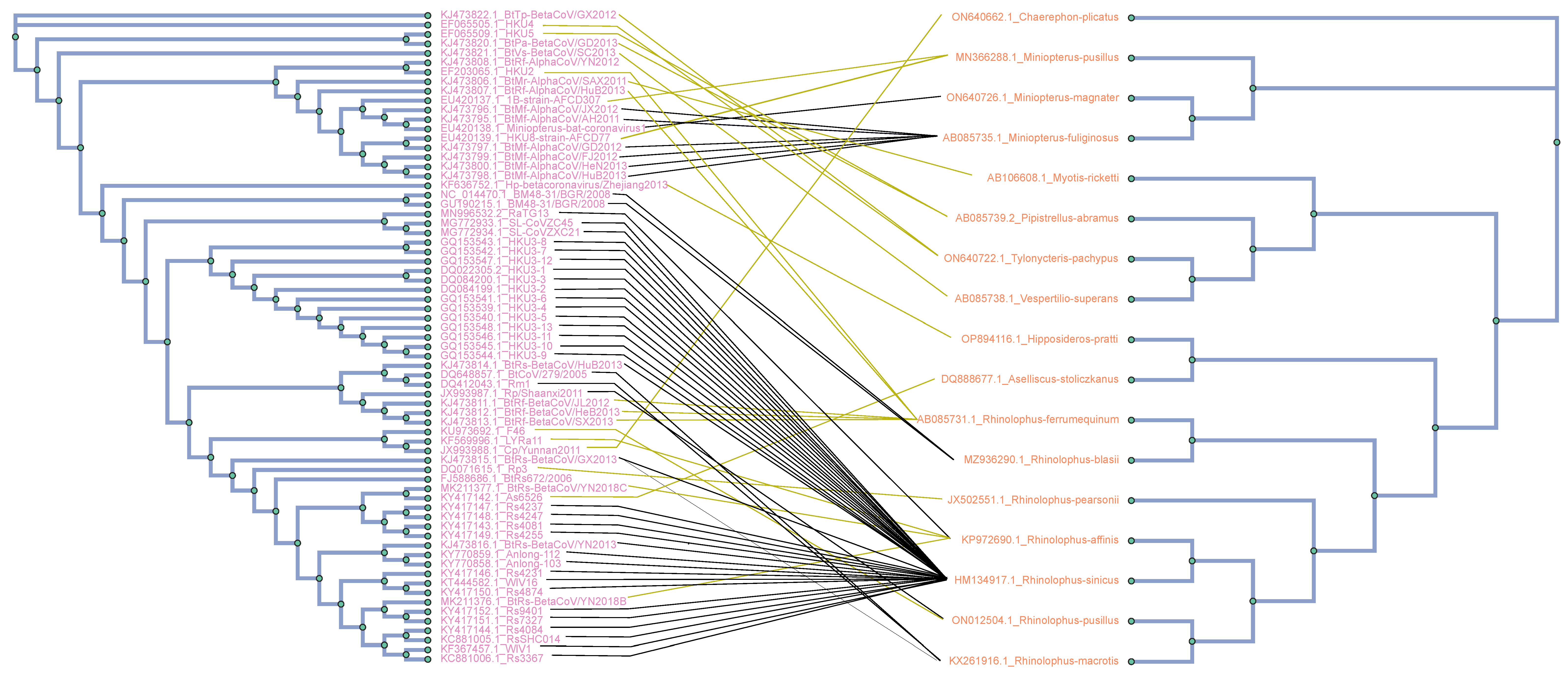
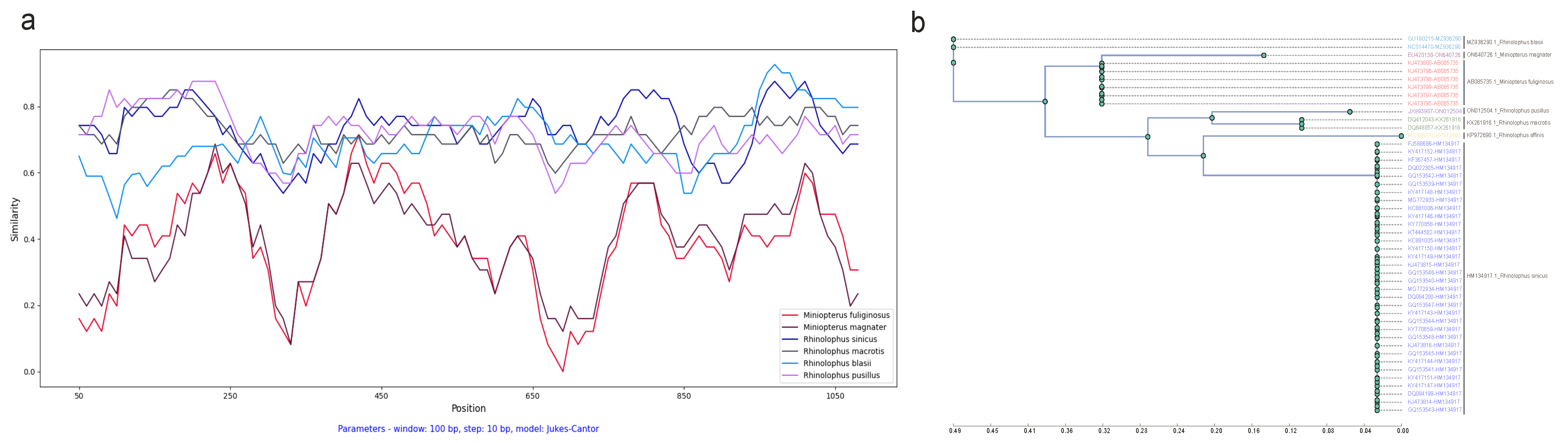
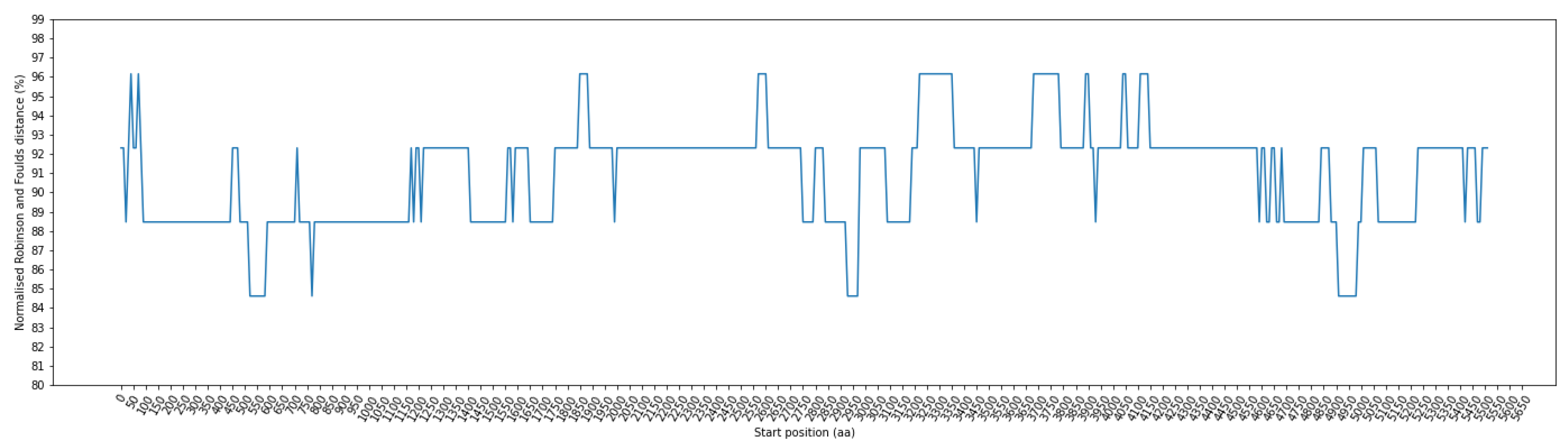
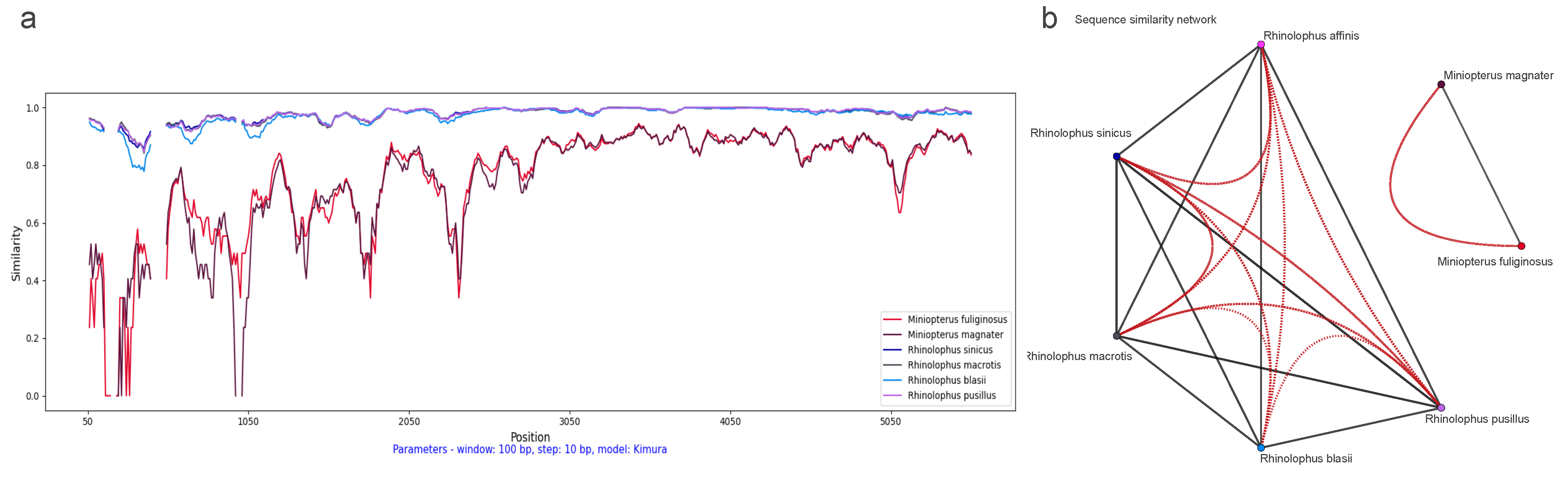


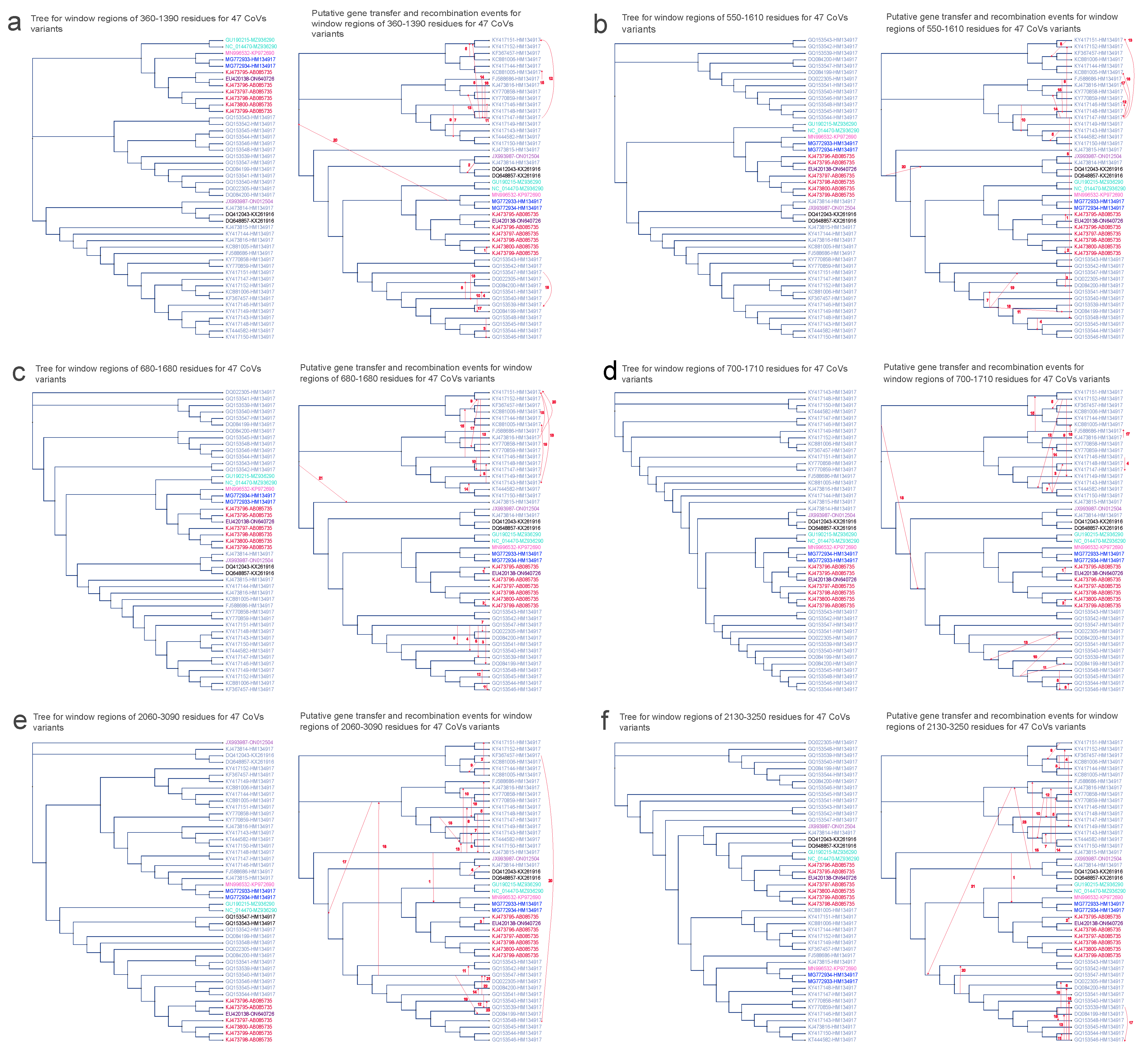
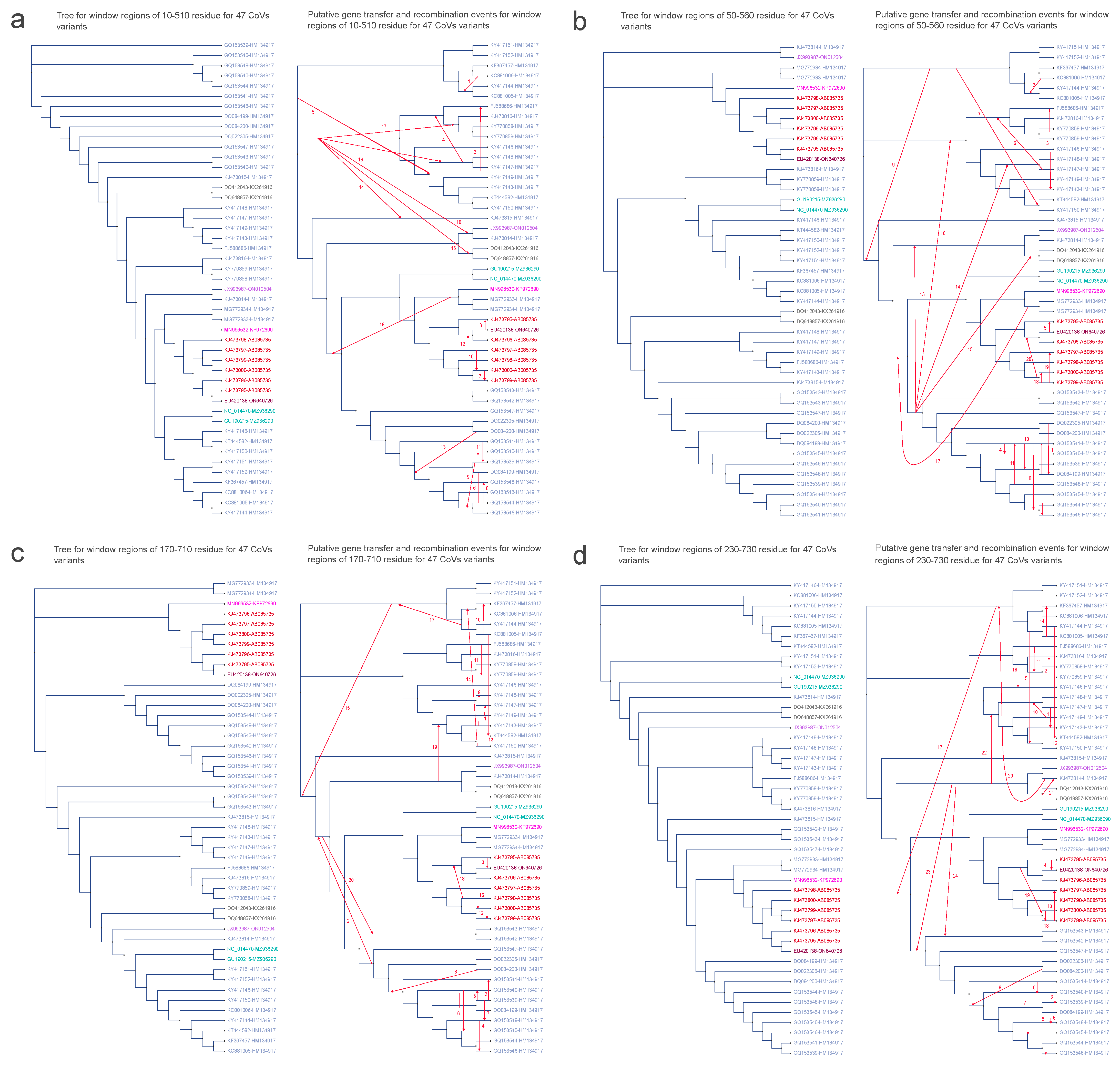
| Start Position of Window (aa) | End Position of Window (aa) | RF Distance (%) | Recombination Detected (Yes/No) |
|---|---|---|---|
| 520 | 680 | 84.62 | yes |
| 770 | 870 | 84.62 | yes |
| 2930 | 3070 | 84.62 | yes |
| 4910 | 5080 | 84.62 | no |
| Start Position of Window (aa) | End Position of Window (aa) | RF Distance (%) | Recombination Detected (Yes/No) |
|---|---|---|---|
| 0 | 140 | 88.46 | yes |
| 60 | 180 | 88.46 | yes |
| 100 | 410 | 88.46 | yes |
| 360 | 550 | 88.46 | yes |
| 630 | 730 | 88.46 | yes |
| Number of Region | Start Position of Break Window (aa) | End Position of Break Window (aa) | –Test Result (p-Value) | Window Size |
|---|---|---|---|---|
| 1 | 360 | 1360 | 150 | |
| 370 | 1370 | 150 | ||
| 380 | 1380 | 150 | ||
| 390 | 1390 | 450 | ||
| 2 | 550 | 1550 | 350 | |
| 560 | 1560 | 300 | ||
| 570 | 1570 | 300 | ||
| 580 | 1580 | 300 | ||
| 590 | 1590 | 300 | ||
| 600 | 1600 | 300 | ||
| 610 | 1610 | 300 | ||
| 3 | 680 | 1680 | 250 | |
| 4 | 700 | 1700 | 250 | |
| 710 | 1710 | 250 | ||
| 5 | 2060 | 3060 | 300 | |
| 2070 | 3070 | 300 | ||
| 2080 | 3080 | 300 | ||
| 2090 | 3090 | 300 | ||
| 6 | 2130 | 3130 | 400 | |
| 2140 | 3140 | 200 | ||
| 2150 | 3150 | 200 | ||
| 2160 | 3160 | 200 | ||
| 2170 | 3170 | 150 | ||
| 2180 | 3180 | 100 | ||
| 2190 | 3190 | 100 | ||
| 2200 | 3200 | 100 | ||
| 2210 | 3210 | 100 | ||
| 2220 | 3220 | 100 | ||
| 2230 | 3230 | 100 | ||
| 2240 | 3240 | 100 | ||
| 2250 | 3250 | 100 |
| Number of Region | Start Position of Break Window (aa) | End Position of Break Window (aa) | –Test Result (p-Value) | Window Size |
|---|---|---|---|---|
| 1 | 10 | 510 | 50 | |
| 2 | 50 | 550 | 50 | |
| 60 | 560 | 50 | ||
| 3 | 170 | 670 | 50 | |
| 180 | 680 | 50 | ||
| 190 | 690 | 50 | ||
| 200 | 700 | 50 | ||
| 210 | 710 | 150 | ||
| 4 | 230 | 730 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Tahiri, N. Host–Virus Cophylogenetic Trajectories: Investigating Molecular Relationships between Coronaviruses and Bat Hosts. Viruses 2024, 16, 1133. https://doi.org/10.3390/v16071133
Li W, Tahiri N. Host–Virus Cophylogenetic Trajectories: Investigating Molecular Relationships between Coronaviruses and Bat Hosts. Viruses. 2024; 16(7):1133. https://doi.org/10.3390/v16071133
Chicago/Turabian StyleLi, Wanlin, and Nadia Tahiri. 2024. "Host–Virus Cophylogenetic Trajectories: Investigating Molecular Relationships between Coronaviruses and Bat Hosts" Viruses 16, no. 7: 1133. https://doi.org/10.3390/v16071133





