Immunogenicity of a Rotavirus VP8* Multivalent Subunit Vaccine in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expression and Purification of Rotavirus VP8* Proteins
2.2. Immunizations
2.3. Immunization Follow Up
2.4. Titration of Serums
2.5. Statistical Analysis
3. Results
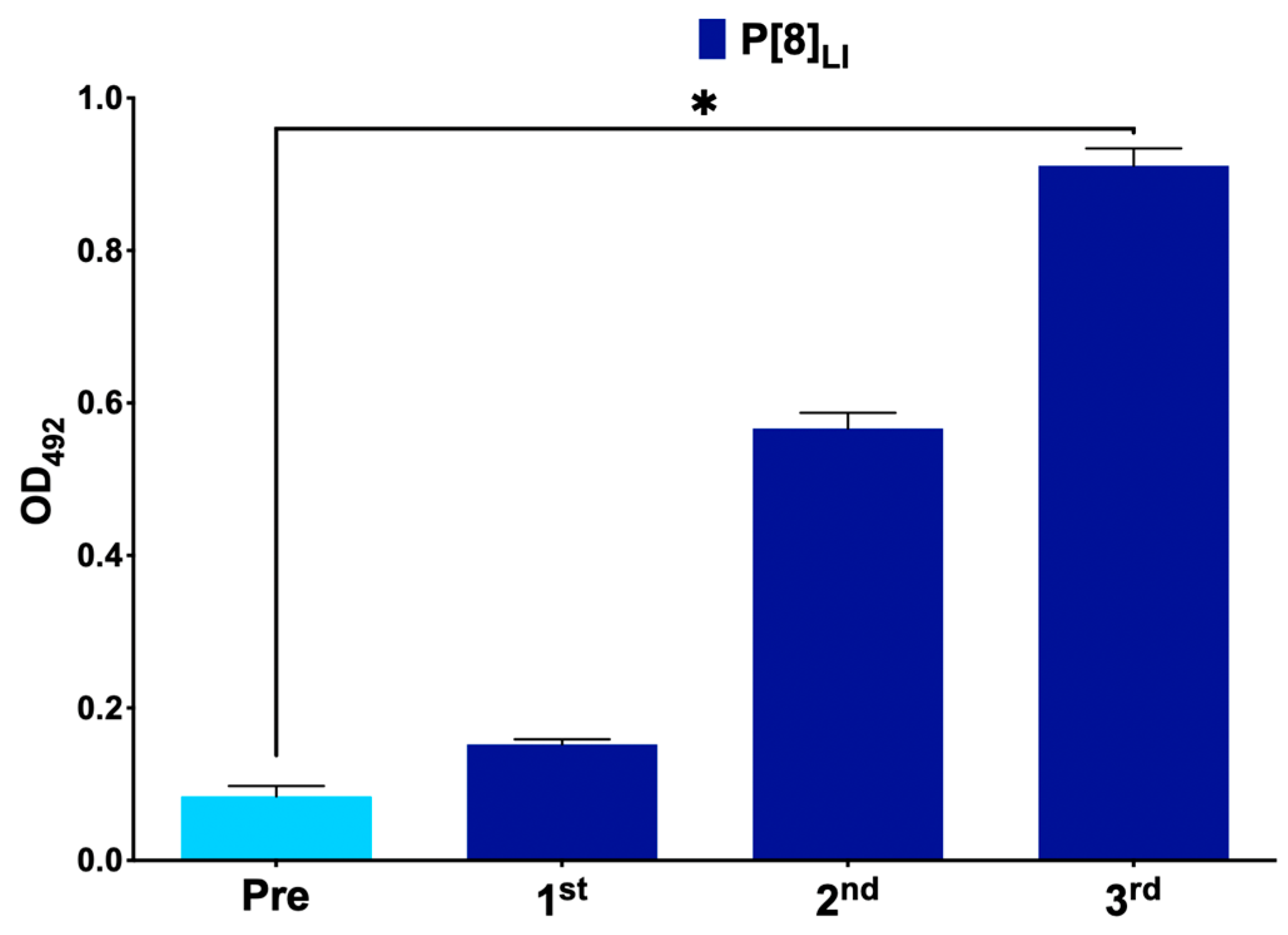
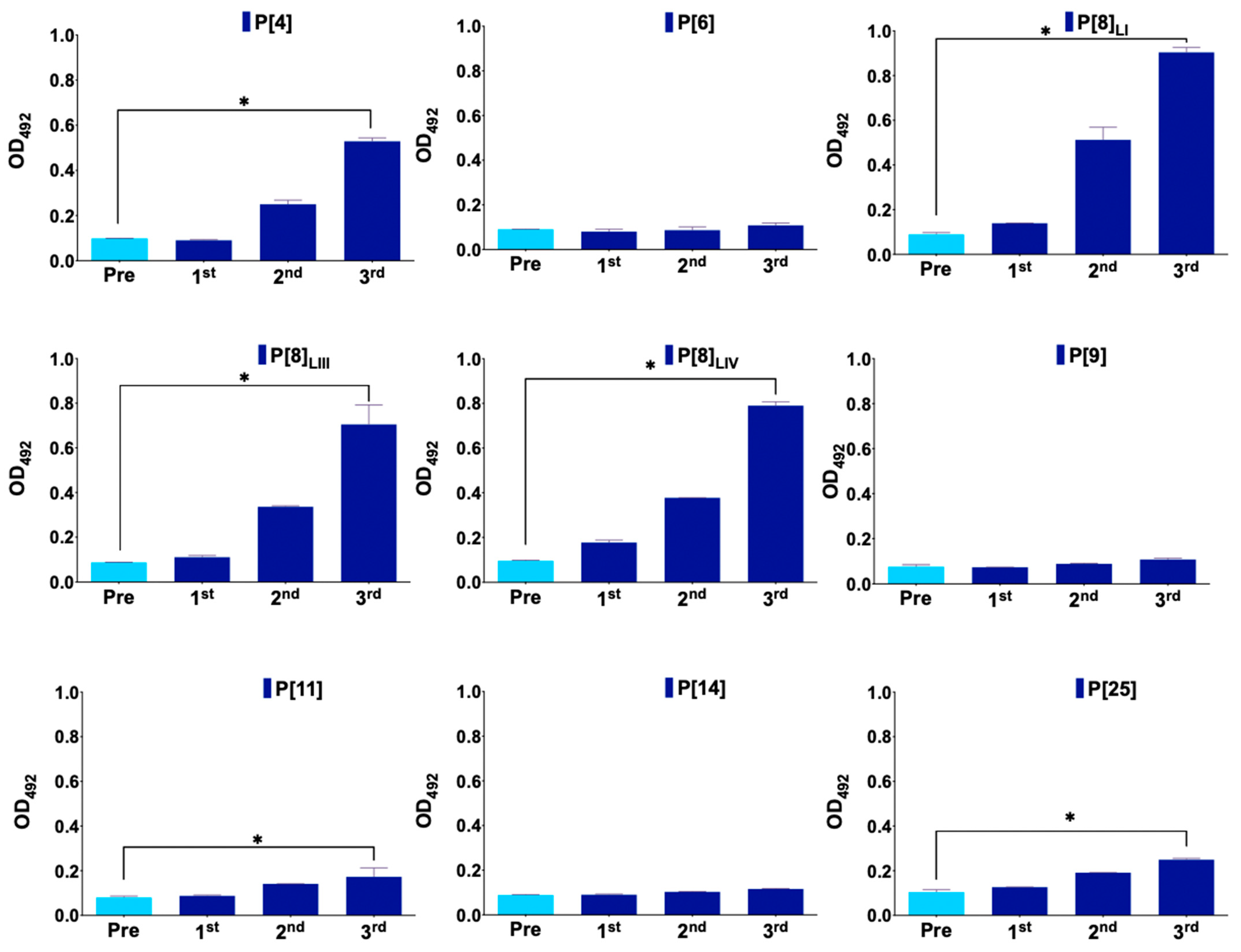
3.1. Immunization Follow Up
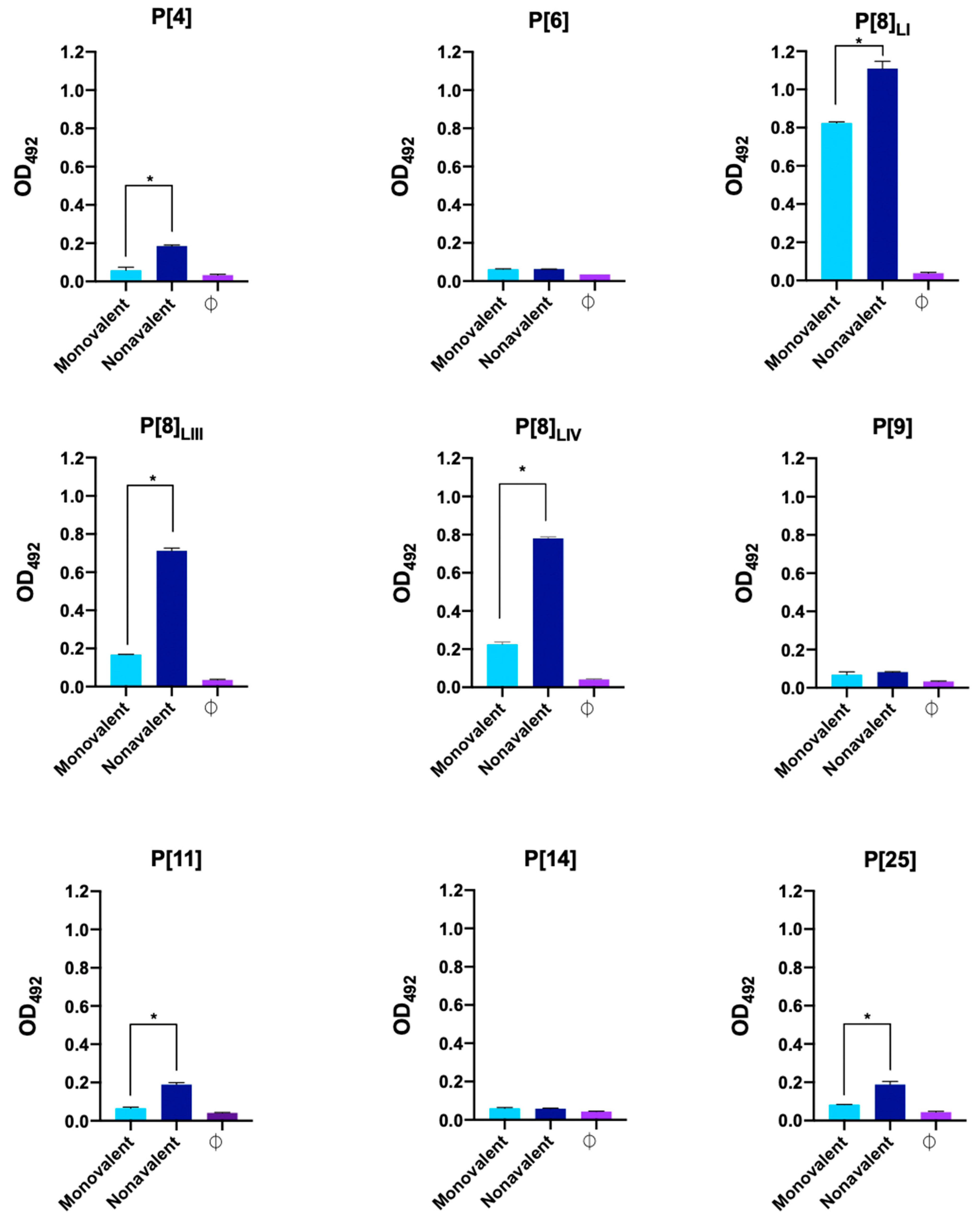
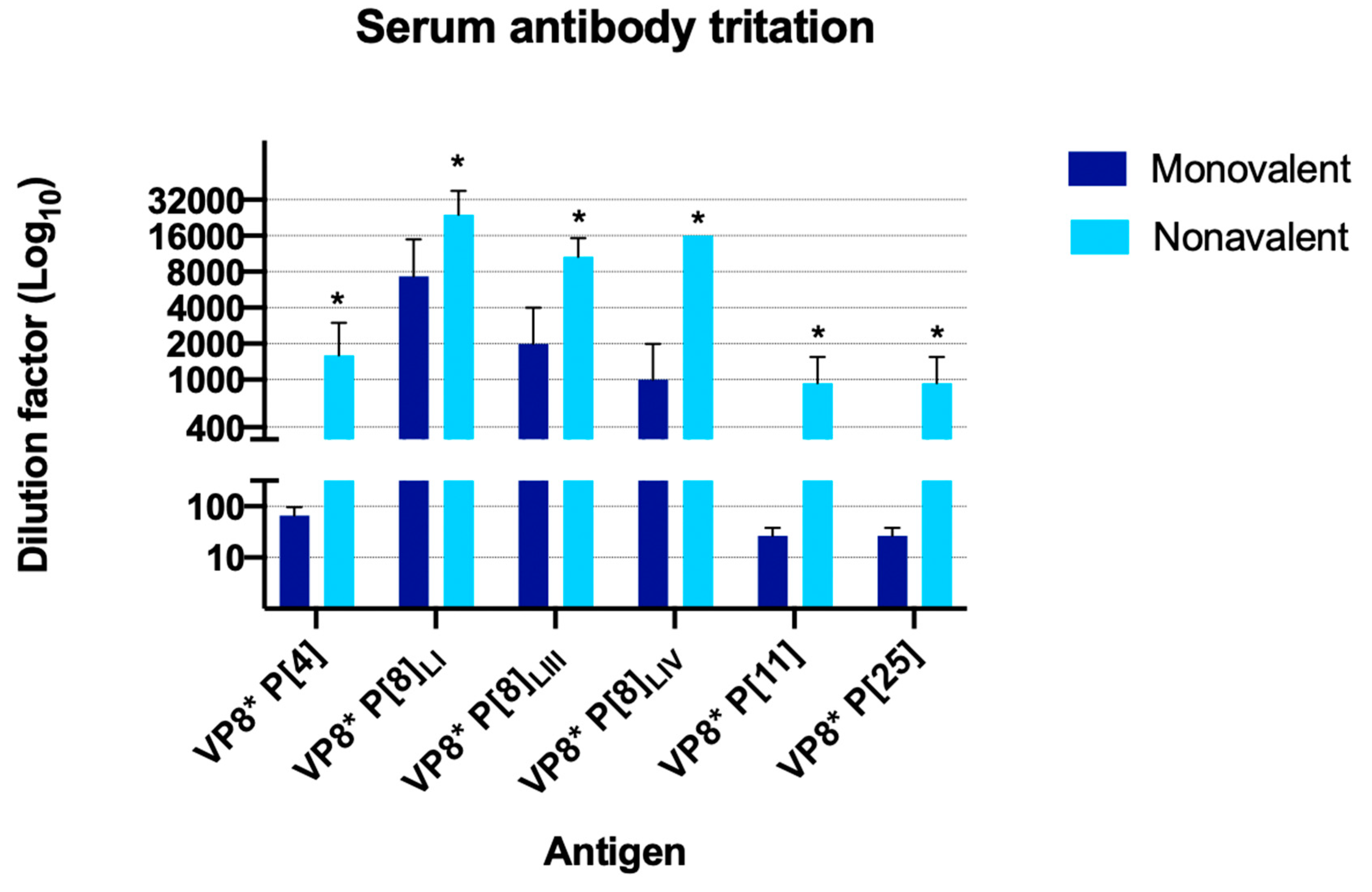
3.2. Serum Titration
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Buesa, J.; Rodriguez-Díaz, J. The Molecular Virology of Enteric Viruses. Viruses Foods 2016, 59–130. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ciarlet, M.; Rahman, M.; Attoui, H.; Bányai, K.; Estes, M.K.; Gentsch, J.R.; Iturriza-Gómara, M.; Kirkwood, C.D.; Martella, V.; et al. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch. Virol. 2008, 153, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Johne, R.; Schilling-Loeffler, K.; Ulrich, R.G.; Tausch, S.H. Whole Genome Sequence Analysis of a Prototype Strain of the Novel Putative Rotavirus Species L. Viruses 2022, 14, 462. [Google Scholar] [CrossRef] [PubMed]
- RCWG. Rotavirus Classification Working Group. Available online: https://rega.kuleuven.be/cev/viralmetagenomics/virus-classification/rcwg (accessed on 15 May 2024).
- Gozalbo-Rovira, R.; Ciges-Tomas, J.R.; Vila-Vicent, S.; Buesa, J.; Santiso-Bellón, C.; Monedero, V.; Yebra, M.J.; Marina, A.; Rodríguez-Díaz, J. Unraveling the role of the secretor antigen in human rotavirus attachment to histo-blood group antigens. PLoS Pathog. 2019, 15, e1007865. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, N.A.; Gondwe, J.S.; Graham, S.M.; Thindwa, B.D.M.; Dove, W.; Broadhead, R.L.; Molyneux, M.E.; Hart, C.A. Rotavirus Strain Diversity in Blantyre, Malawi, from 1997 to 1999. Society 2001, 39, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Arista, S.; Giammanco, G.M.; De Grazia, S.; Ramirez, S.; Lo Biundo, C.; Colomba, C.; Cascio, A.; Martella, V. Heterogeneity and Temporal Dynamics of Evolution of G1 Human Rotaviruses in a Settled Population. J. Virol. 2006, 80, 10724–10733. [Google Scholar] [CrossRef]
- Rahman, M.; De Leener, K.; Goegebuer, T.; Wollants, E.; Van der Donck, I.; Van Hoovels, L.; Van Ranst, M. Genetic characterization of a novel, naturally occurring recombinant human G6P[6] rotavirus. J. Clin. Microbiol. 2003, 41, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Cárcamo-Calvo, R.; Muñoz, C.; Buesa, J.; Rodríguez-Díaz, J.; Gozalbo-Rovira, R. The rotavirus vaccine landscape, an update. Pathogens 2021, 10, 520. [Google Scholar] [CrossRef]
- Omatola, C.; Olaniran, A. Genetic heterogeneity of group A rotaviruses: A review of the evolutionary dynamics and implication on vaccination. Expert. Rev. Anti. Infect. Ther. 2022, 20, 1587–1602. [Google Scholar] [CrossRef]
- Gentsch, J.; Laird, A.R.; Bielfelt, B.; Griffin, D.; Bányai, K.; Ramachandran, M.; Jain, V.; Cunliffe, N.A.; Nakagomi, O.; Kirkwood, C.D.; et al. Serotype diversity and reassortment between human and animal rotavirus strains: Implications for rotavirus vaccine programs. J. Infect. Dis. 2005, 192, S146–S159. [Google Scholar] [CrossRef] [PubMed]
- Ogden, K.M.; Tan, Y.; Akopov, A.; Stewart, L.S.; Mchenry, R.; Fonnesbeck, C.; Piya, B.; Carter, M.H.; Fedorova, N.B.; Halpin, R.A.; et al. Multiple Introductions and Antigenic Mismatch with Vaccines May Contribute to Increased Predominance of G12P[8] Rotaviruses in the United States. J. Virol. 2018, 93, e01476-18. [Google Scholar] [CrossRef] [PubMed]
- Roczo-Farkas, S.; Kirkwood, C.; Cowley, D.; Barnes, G.; Bishop, R.; Bogdanovic-Sakran, N.; Boniface, K.; Donato, C.M.; Bines, J.E. The Impact of Rotavirus Vaccines on Genotype Diversity: A Comprehensive Analysis of 2 Decades of Australian Surveillance Data. J. Infect. Dis. 2018, 218, 546. [Google Scholar] [CrossRef]
- Hoque, S.A.; Khandoker, N.; Thongprachum, A.; Khamrin, P.; Takanashi, S.; Okitsu, S.; Nishimura, S.; Kikuta, H.; Yamamoto, A.; Sugita, K.; et al. Distribution of rotavirus genotypes in Japan from 2015 to 2018: Diversity in genotypes before and after introduction of rotavirus vaccines. Vaccine 2020, 38, 3980–3986. [Google Scholar] [CrossRef] [PubMed]
- Dulgheroff, A.; Figueiredo, E.F.; Moreira, L.; Moreira, K.C.; Moura, L.S.; Gouvea, V.; Domingues, A. Distribution of rotavirus genotypes after vaccine introduction in the Triângulo Mineiro region of Brazil: 4-Year follow-up study. J. Clin. Virol. 2012, 55, 67–71. [Google Scholar] [CrossRef]
- Luchs, A.; Cilli, A.; Morillo, S.G.; Gregório, D.D.S.; Souza, K.A.F.; de Vieira, H.R.; Fernandes, A.D.M.; Carmona, R.d.C.C.; Timenetsky, M.D.C.S.T. Detection of the emerging rotavirus G12P[8] genotype at high frequency in brazil in 2014: Successive replacement of predominant strains after vaccine introduction. Acta Trop. 2016, 156, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Motamedi-Rad, M.; Farahmand, M.; Arashkia, A.; Jalilvand, S.; Shoja, Z. VP7 and VP4 genotypes of rotaviruses cocirculating in Iran, 2015 to 2017: Comparison with cogent sequences of Rotarix and RotaTeq vaccine strains before their use for universal mass vaccination. J. Med. Virol. 2019, 92, 1110–1123. [Google Scholar] [CrossRef] [PubMed]
- Kiulia, N.; Nyaga, M.M.; Seheri, M.; Wolfaardt, M.; van Zyl, W.B.; Esona, M.; Irimu, G.; Inoti, M.; Gatinu, B.W.; Njenga, P.K.; et al. Rotavirus G and P Types Circulating in the Eastern Region of Kenya: Predominance of G9 and Emergence of G12 Genotypes. Pediatr. Infect. Dis. J. 2014, 33 (Suppl. 1), S85–S88. [Google Scholar] [CrossRef] [PubMed]
- Mhango, C.; Mandolo, J.; Chinyama, E.; Wachepa, R.; Kanjerwa, O.; Malamba-Banda, C.; Matambo, P.B.; Barnes, K.G.; Chaguza, C.; Shawa, I.T.; et al. Rotavirus Genotypes in Hospitalized Children With Acute Gastroenteritis Before and After Rotavirus Vaccine Introduction in Blantyre, Malawi, 1997–2019. J. Infect. Dis. 2020, 225, 2127–2136. [Google Scholar] [CrossRef]
- Gómez, M.M.; Mendonça, M.C.L.; de Volotão, E.M.; Tort, L.; Silva, M.F.M.; da Cristina, J.; Cristina, J.; Leite, J.P.G. Rotavirus A genotype P[4]G2: Genetic diversity and reassortment events among strains circulating in Brazil between 2005 and 2009. J. Med. Virol. 2011, 83, 1093–1106. [Google Scholar] [CrossRef]
- Rodriguez-Diaz, J.; Garcia-Mantrana, I.; Vila-Vicent, S.; Gozalbo-Rovira, R.; Buesa, J.; Monedero, V.; Collado, M.C. Relevance of secretor status genotype and microbiota composition in susceptibility to rotavirus and norovirus infections in humans. Sci. Rep. 2017, 7, 45559. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.; Ali, A.; Fuentes, S.; Korpela, K.; Kazi, M.; Tate, J.; Parashar, U.; Wiersinga, W.J.; Giaquinto, C.; de Weerth, C.; et al. Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes 2018, 9, 93–101. [Google Scholar] [CrossRef]
- Parker, E.P.K.; Praharaj, I.; Zekavati, A.; Lazarus, R.P.; Giri, S.; Operario, D.J.; Liu, J.; Houpt, E.; Iturriza-Gómara, M.; Kampmann, B.; et al. Influence of the intestinal microbiota on the immunogenicity of oral rotavirus vaccine given to infants in south India. Vaccine 2018, 36, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Bucardo, F.; Nordgren, J.; Reyes, Y.; Gonzalez, F.; Sharma, S.; Svensson, L. The Lewis A phenotype is a restriction factor for Rotateq and Rotarix vaccine-take in Nicaraguan children. Sci. Rep. 2018, 8, 1502. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Hagbom, M.; Svensson, L.; Nordgren, J. The Impact of Human Genetic Polymorphisms on Rotavirus Susceptibility, Epidemiology, and Vaccine Take. Viruses 2020, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Peña-Gil, N.; Santiso-Bellón, C.; Gozalbo-Rovira, R.; Buesa, J.; Monedero, V.; Rodríguez-Díaz, J. The Role of Host Glycobiology and Gut Microbiota in Rotavirus and Norovirus Infection, an Update. Int. J. Mol. Sci. 2021, 22, 13473. [Google Scholar] [CrossRef] [PubMed]
- Armah, G.E.; Cortese, M.M.; Dennis, F.E.; Yu, Y.; Morrow, A.L.; McNeal, M.M.; Lewis, K.D.C.; A Awuni, D.; Armachie, J.; Parashar, U.D. Rotavirus Vaccine Take in Infants Is Associated with Secretor Status. J. Infect. Dis. 2019, 219, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Roier, S.; Mangala Prasad, V.; McNeal, M.M.; Lee, K.K.; Petsch, B.; Rauch, S. mRNA-based VP8* nanoparticle vaccines against rotavirus are highly immunogenic in rodents. NPJ Vaccines 2023, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Groome, M.J.; Fairlie, L.; Morrison, J.; Fix, A.; Koen, A.; Masenya, M.; Jose, L.; Madhi, S.A.; Page, N.; McNeal, M.; et al. Safety and immunogenicity of a parenteral trivalent P2-VP8 subunit rotavirus vaccine: A multisite, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2020, 20, 851–863. [Google Scholar] [CrossRef]
- Vila-Vicent, S.; Gozalbo-Rovira, R.; Rubio-Del-Campo, A.; Santiso-Bellón, C.; Navarro-Lleó, N.; Muñoz, C.; Jose, L.; Page, N.; McNeal, M.; Madhi, S.A.; et al. Sero-epidemiological study of the rotavirus VP8* protein from different P genotypes in Valencia, Spain. Sci. Rep. 2020, 10, 7753. [Google Scholar] [CrossRef]
- Groome, M.J.; Koen, A.; Fix, A.; Page, N.; Jose, L.; Madhi, S.A.; McNeal, M.; Dally, L.; Cho, I.; Power, M.; et al. Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2017, 17, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, D.E.; Jiang, B. Evolution of P[8], P[4], and P[6] VP8* genes of human rotaviruses globally reported during 1974 and 2017: Possible implications for rotavirus vaccines in development. Hum. Vaccines Immunother. 2019, 15, 3003–3008. [Google Scholar] [CrossRef] [PubMed]
- Zeller, M.; Patton, J.T.; Heylen, E.; De Coster, S.; Ciarlet, M.; Van Ranst, M.; Matthijnssens, J. Genetic analyses reveal differences in the VP7 and VP4 antigenic epitopes between human rotaviruses circulating in Belgium and rotaviruses in rotarix and RotaTeq. J. Clin. Microbiol. 2012, 50, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.; Arora, R.; Arora, R.; Chitambar, S. Sequence analysis of VP7 and VP4 genes of G1P[8] rotaviruses circulating among diarrhoeic children in Pune, India: A comparison with Rotarix and RotaTeq vaccine strains. Vaccine 2014, 32 (Suppl. 1), A75–A83. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.V.; Sashina, T.A.; Fomina, S.G.; Novikova, N.A. Comparative characteristics of the VP7 and VP4 antigenic epitopes of the rotaviruses circulating in Russia (Nizhny novgorod) and the Rotarix and RotaTeq vaccines. Arch. Virol. 2015, 160, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Cao, D.; Jones, R.W.; Li, J.; Szu, S.; Hoshino, Y. Construction and characterization of human rotavirus recombinant VP8* subunit parenteral vaccine candidates. Vaccine 2012, 30, 6121–6126. [Google Scholar] [CrossRef]
- Piekarska, A.; Kacerka, A.; Majda-Stanisławska, E.; Jóźwiak, B.; Sidorkiewicz, M. Predominance of genotype P[9]G3 in rotavirus gastroenteritis in Polish children. Arch. Med. Sci. 2015, 11, 577–583. [Google Scholar] [CrossRef]
- Quaye, O.; Roy, S.; Rungsrisuriyachai, K.; Esona, M.D.; Xu, Z.; Tam, K.I.; Banegas, D.J.C.; Rey-Benito, G.; Bowen, M.D. Characterisation of a rare, reassortant human G10P[14] rotavirus strain detected in Honduras. Mem. Inst. Oswaldo Cruz. 2018, 113, 9–16. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Potgieter, C.A.; Ciarlet, M.; Parreño, V.; Martella, V.; Bányai, K.; Garaicoechea, L.; Palombo, E.A.; Novo, L.; Zeller, M.; et al. Are human P[14] rotavirus strains the result of interspecies transmissions from sheep or other ungulates that belong to the mammalian order Artiodactyla? J. Virol. 2009, 83, 2917–2929. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárcamo-Calvo, R.; Boscá-Sánchez, I.; López-Navarro, S.; Navarro-Lleó, N.; Peña-Gil, N.; Santiso-Bellón, C.; Buesa, J.; Gozalbo-Rovira, R.; Rodríguez-Díaz, J. Immunogenicity of a Rotavirus VP8* Multivalent Subunit Vaccine in Mice. Viruses 2024, 16, 1135. https://doi.org/10.3390/v16071135
Cárcamo-Calvo R, Boscá-Sánchez I, López-Navarro S, Navarro-Lleó N, Peña-Gil N, Santiso-Bellón C, Buesa J, Gozalbo-Rovira R, Rodríguez-Díaz J. Immunogenicity of a Rotavirus VP8* Multivalent Subunit Vaccine in Mice. Viruses. 2024; 16(7):1135. https://doi.org/10.3390/v16071135
Chicago/Turabian StyleCárcamo-Calvo, Roberto, Irene Boscá-Sánchez, Sergi López-Navarro, Noemi Navarro-Lleó, Nazaret Peña-Gil, Cristina Santiso-Bellón, Javier Buesa, Roberto Gozalbo-Rovira, and Jesús Rodríguez-Díaz. 2024. "Immunogenicity of a Rotavirus VP8* Multivalent Subunit Vaccine in Mice" Viruses 16, no. 7: 1135. https://doi.org/10.3390/v16071135






