The Transmission of SARS-CoV-2 from COVID-19-Diagnosed People to Their Pet Dogs and Cats in a Multi-Year Surveillance Project
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey
2.2. Sample Collection
2.3. ELISA
2.4. Statistical Analysis
3. Results
3.1. ELISA
3.2. Lifestyle Factors
3.3. Clinical Signs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garrity, T.F.; Stallones, L.F.; Marx, M.B.; Johnson, T.P. Pet ownership and attachment as supportive factors in the health of the elderly. Anthrozoös 1989, 3, 35–44. [Google Scholar] [CrossRef]
- Gee, N.R.; Mueller, M.K.; Curl, A.L. Human-animal interaction and older adults: An overview. Front. Psychol. 2017, 8, 1416. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, P.J. Emerging Cardiovascular Risk Research: Impact of Pets on Cardiovascular Risk Prevention. Curr. Cardiovasc. Risk Rep. 2016, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Wegienka, G.; Johnson, C.C.; Havstad, S.; Ownby, D.R.; Nicholas, C.; Zoratti, E.M. Lifetime dog and cat exposure and dog- and cat-specific sensitization at age 18 years. Clin. Exp. Allergy 2011, 41, 979–986. [Google Scholar] [CrossRef] [PubMed]
- American Pet Products Association. Pet Industry Market Size, Trends & Ownership Statistics. Available online: https://www.americanpetproducts.org/press_industrytrends.asp (accessed on 15 May 2023).
- Chomel, B.B.; Sun, B. Zoonoses in the bedroom. Emerg. Infect. Dis. 2011, 17, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Meisner, J.; Baszler, T.V.; Kuehl, K.E.; Ramirez, V.; Baines, A.; Frisbie, L.A.; Lofgren, E.T.; de Avila, D.M.; Wolking, R.M.; Bradway, D.S.; et al. Household Transmission of SARS-CoV-2 from Humans to Pets, Washington and Idaho, USA. Emerg. Infect. Dis. 2022, 28, 2425–2434. [Google Scholar] [CrossRef] [PubMed]
- Human Animal Bond Research Institute. Survey of U.S. Pet Owners. Available online: https://habri.org/pet-owners-survey/#2021-survey (accessed on 15 May 2023).
- Cai, H.; Duan, W. Changing Perceptions and Uses of “Companion Animal” Public and Pseudo-Public Spaces in Cities during COVID-19 Pandemic: The Case of Beijing. Land 2022, 11, 1475. [Google Scholar] [CrossRef]
- Tissot, S. Of Dogs and Men: The Making of Spatial Boundaries in a Gentrifying Neighborhood. City Community 2011, 10, 265–284. [Google Scholar] [CrossRef]
- Wilkin, C.L.; Fairlie, P.; Ezzedeen, S.R. Who let the dogs in? A look at pet-friendly workplaces. Int. J. Workplace Health Manag. 2016, 9, 96–109. [Google Scholar] [CrossRef]
- Stull, J.W.; Brophy, J.; Weese, J.S. Reducing the risk of pet-associated zoonotic infections. CMAJ 2015, 187, 736–743. [Google Scholar] [CrossRef]
- Dórea, F.C.; Sanchez, J.; Revie, C.W. Veterinary syndromic surveillance: Current initiatives and potential for development. Prev. Vet. Med. 2011, 101, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.E.; Lund, E. Disease Reporting and Surveillance: Where Do Companion Animal Diseases Fit in? Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Stärk, K.D.C.; Regula, G.; Hernandez, J.; Knopf, L.; Fuchs, K.; Morris, R.S.; Davies, P. Concepts for risk-based surveillance in the field of veterinary medicine and veterinary public health: Review of current approaches. BMC Health Serv. Res. 2006, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, P.C.; Van Buren, J.W.; Neterer, M.; Zhou, C. Disease surveillance and referral bias in the veterinary medical database. Prev. Vet. Med. 2010, 94, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Kass, P.H.; Weng, H.Y.; Gaona, M.A.; Hille, A.; Sydow, M.H.; Lund, E.M.; Markwell, P.J. Syndromic surveillance in companion animals utilizing electronic medical records data: Development and proof of concept. PeerJ 2016, 4, e1940. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.Y.; Gaona, M.A.L.; Kass, P.H. Evaluation of a novel syndromic surveillance system for the detection of the 2007 melamine-related nephrotoxicosis foodborne outbreak in dogs and cats in the United States. PeerJ 2020, 8, e9093. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Singleton, D.A.; Noble, P.J.M.; Pinchbeck, G.L.; Smith, S.; Brant, B.; Smyth, S.; Radford, A.D.; Appleton, C.; Jewell, C.; et al. Small animal disease surveillance 2020/21: SARS-CoV-2, syndromic surveillance and an outbreak of acute vomiting in UK dogs. Vet. Rec. 2021, 188, 304. [Google Scholar] [CrossRef]
- Bienzle, D.; Rousseau, J.; Marom, D.; MacNicol, J.; Jacobson, L.; Sparling, S.; Prystajecky, N.; Fraser, E.; Weese, J.S. Risk Factors for SARS-CoV-2 Infection and Illness in Cats and Dogs. Emerg. Infect. Dis. 2022, 28, 1154–1162. [Google Scholar] [CrossRef]
- Fritz, M.; Rosolen, B.; Krafft, E.; Becquart, P.; Elguero, E.; Vratskikh, O.; Denolly, S.; Boson, B.; Vanhomwegen, J.; Gouilh, M.A.; et al. High prevalence of SARS-CoV-2 antibodies in pets from COVID-19+ households. One Health 2021, 11, 100192. [Google Scholar] [CrossRef]
- Goryoka, G.W.; Cossaboom, C.M.; Gharpure, R.; Dawson, P.; Tansey, C.; Rossow, J.; Mrotz, V.; Rooney, J.; Torchetti, M.; Loiacono, C.M.; et al. One Health Investigation of SARS-CoV-2 Infection and Seropositivity among Pets in Households with Confirmed Human COVID-19 Cases-Utah and Wisconsin, 2020. Viruses 2021, 13, 1813. [Google Scholar] [CrossRef]
- Hamer, S.A.; Pauvolid-Correa, A.; Zecca, I.B.; Davila, E.; Auckland, L.D.; Roundy, C.M.; Tang, W.; Torchetti, M.K.; Killian, M.L.; Jenkins-Moore, M.; et al. SARS-CoV-2 Infections and Viral Isolations among Serially Tested Cats and Dogs in Households with Infected Owners in Texas, USA. Viruses 2021, 13, 938. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Hoque, M.; Adekanmbi, F.; Kelly, P.; Jenkins-Moore, M.; Torchetti, M.K.; Chenoweth, K.; Wood, T.; Wang, C. Antibodies to SARS-CoV-2 in dogs and cats, USA. Emerg. Microbes Infect. 2021, 10, 1669–1674. [Google Scholar] [CrossRef]
- Dileepan, M.; Di, D.; Huang, Q.; Ahmed, S.; Heinrich, D.; Ly, H.; Liang, Y. Seroprevalence of SARS-CoV-2 (COVID-19) exposure in pet cats and dogs in Minnesota, USA. Virulence 2021, 12, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Michael, H.T.; Waterhouse, T.; Estrada, M.; Seguin, M.A. Frequency of respiratory pathogens and SARS-CoV-2 in canine and feline samples submitted for respiratory testing in early 2020. J. Small Anim. Pract. 2021, 62, 336–342. [Google Scholar] [CrossRef]
- Gregg, M.; Datta, S.; Lorenz, D. Variance estimation in tests of clustered categorical data with informative cluster size. Stat. Methods Med. Res. 2020, 29, 3396–3408. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; et al. Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 29 August 2022).
- United States Census Bureau. Census Regions and Divisions of the United States. Available online: https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf (accessed on 15 May 2023).
- Temmam, S.; Barbarino, A.; Maso, D.; Behillil, S.; Enouf, V.; Huon, C.; Jaraud, A.; Chevallier, L.; Backovic, M.; Perot, P.; et al. Absence of SARS-CoV-2 infection in cats and dogs in close contact with a cluster of COVID-19 patients in a veterinary campus. One Health 2020, 10, 100164. [Google Scholar] [CrossRef] [PubMed]
- Yaglom, H.D.; Hecht, G.; Goedderz, A.; Jasso-Selles, D.; Ely, J.L.; Ruberto, I.; Bowers, J.R.; Engelthaler, D.M.; Venkat, H. Genomic investigation of a household SARS-CoV-2 disease cluster in Arizona involving a cat, dog, and pet owner. One Health 2021, 13, 100333. [Google Scholar] [CrossRef]
- Venkat, H.; Yaglom, H.D.; Hecht, G.; Goedderz, A.; Ely, J.L.; Sprenkle, M.; Martins, T.; Jasso-Selles, D.; Lemmer, D.; Gesimondo, J.; et al. Investigation of SARS-CoV-2 Infection among Companion Animals in Households with Confirmed Human COVID-19 Cases. Pathogens 2024, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Lambrou, A.S.; Shirk, P.; Steele, M.K.; Paul, P.; Paden, C.R.; Cadwell, B.; Reese, H.E.; Aoki, Y.; Hassell, N.; Zheng, X.Y.; et al. Genomic Surveillance for SARS-CoV-2 Variants: Predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) Variants—United States, June 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 206–211. [Google Scholar] [CrossRef]
- Madewell, Z.J.; Yang, Y.; Longini, I.M., Jr.; Halloran, M.E.; Dean, N.E. Household Secondary Attack Rates of SARS-CoV-2 by Variant and Vaccination Status: An Updated Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e229317. [Google Scholar] [CrossRef]
- Lyoo, K.S.; Lee, H.; Lee, S.G.; Yeom, M.; Lee, J.Y.; Kim, K.C.; Yang, J.S.; Song, D. Experimental Infection and Transmission of SARS-CoV-2 Delta and Omicron Variants among Beagle Dogs. Emerg. Infect. Dis. 2023, 29, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Arevalo, S.; Barneto, A.; Ramos, A.M.; Rivera, B.; Sanchez, R.; Sanchez-Morales, L.; Perez-Sancho, M.; Buendia, A.; Ferreras, E.; Ortiz-Menendez, J.C.; et al. Large-scale study on virological and serological prevalence of SARS-CoV-2 in cats and dogs in Spain. Transbound. Emerg. Dis. 2022, 69, e759–e774. [Google Scholar] [CrossRef] [PubMed]
- Kannekens-Jager, M.M.; de Rooij, M.M.T.; de Groot, Y.; Biesbroeck, E.; de Jong, M.K.; Pijnacker, T.; Smit, L.A.M.; Schuurman, N.; Broekhuizen-Stins, M.J.; Zhao, S.; et al. SARS-CoV-2 infection in dogs and cats is associated with contact to COVID-19-positive household members. Transbound. Emerg. Dis. 2022, 69, 4034–4040. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Arrondo, I.; Portillo, A.; Palomar, A.M.; Santibanez, S.; Santibanez, P.; Cervera, C.; Oteo, J.A. Detection of SARS-CoV-2 in pets living with COVID-19 owners diagnosed during the COVID-19 lockdown in Spain: A case of an asymptomatic cat with SARS-CoV-2 in Europe. Transbound. Emerg. Dis. 2021, 68, 973–976. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Forecasting Team. Past SARS-CoV-2 infection protection against re-infection: A systematic review and meta-analysis. Lancet 2023, 401, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Dela Cruz, C.S.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. 2020, 71, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, J.; Xu, X.; Liao, G.; Chen, Y.; Hu, C.H. Patterns of IgG and IgM antibody response in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Liew, A.Y.; Carpenter, A.; Moore, T.A.; Wallace, R.M.; Hamer, S.A.; Hamer, G.L.; Fischer, R.S.B.; Zecca, I.B.; Davila, E.; Auckland, L.D.; et al. Clinical and epidemiologic features of SARS-CoV-2 in dogs and cats compiled through national surveillance in the United States. J. Am. Vet. Med. Assoc. 2023, 261, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Maine, G.N.; Lao, K.M.; Krishnan, S.M.; Afolayan-Oloye, O.; Fatemi, S.; Kumar, S.; VanHorn, L.; Hurand, A.; Sykes, E.; Sun, Q. Longitudinal characterization of the IgM and IgG humoral response in symptomatic COVID-19 patients using the Abbott Architect. J. Clin. Virol. 2020, 133, 104663. [Google Scholar] [CrossRef]
- Bosco-Lauth, A.M.; Hartwig, A.E.; Porter, S.M.; Gordy, P.W.; Nehring, M.; Byas, A.D.; VandeWoude, S.; Ragan, I.K.; Maison, R.M.; Bowen, R.A. Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in cats. Proc. Natl. Acad. Sci. USA 2020, 117, 26382–26388. [Google Scholar] [CrossRef]
- Decaro, N.; Grassi, A.; Lorusso, E.; Patterson, E.I.; Lorusso, A.; Desario, C.; Anderson, E.R.; Vasinioti, V.; Wastika, C.E.; Hughes, G.L.; et al. Long-term persistence of neutralizing SARS-CoV-2 antibodies in pets. Transbound. Emerg. Dis. 2022, 69, 3073–3076. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, N.N.; Trujillo, J.D.; Carossino, M.; Meekins, D.A.; Morozov, I.; Madden, D.W.; Indran, S.V.; Bold, D.; Balaraman, V.; Kwon, T.; et al. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg. Microbes Infect. 2020, 9, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
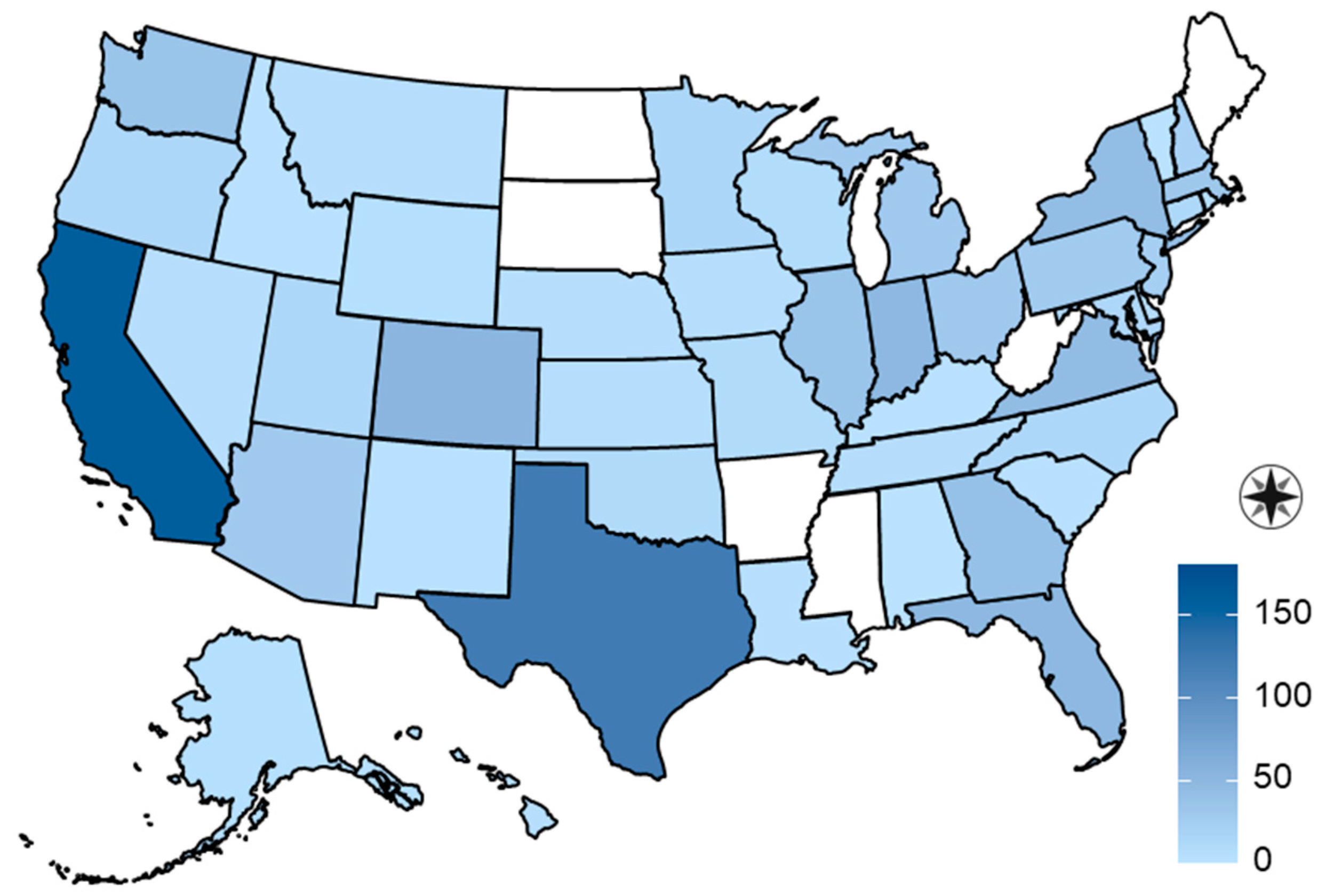
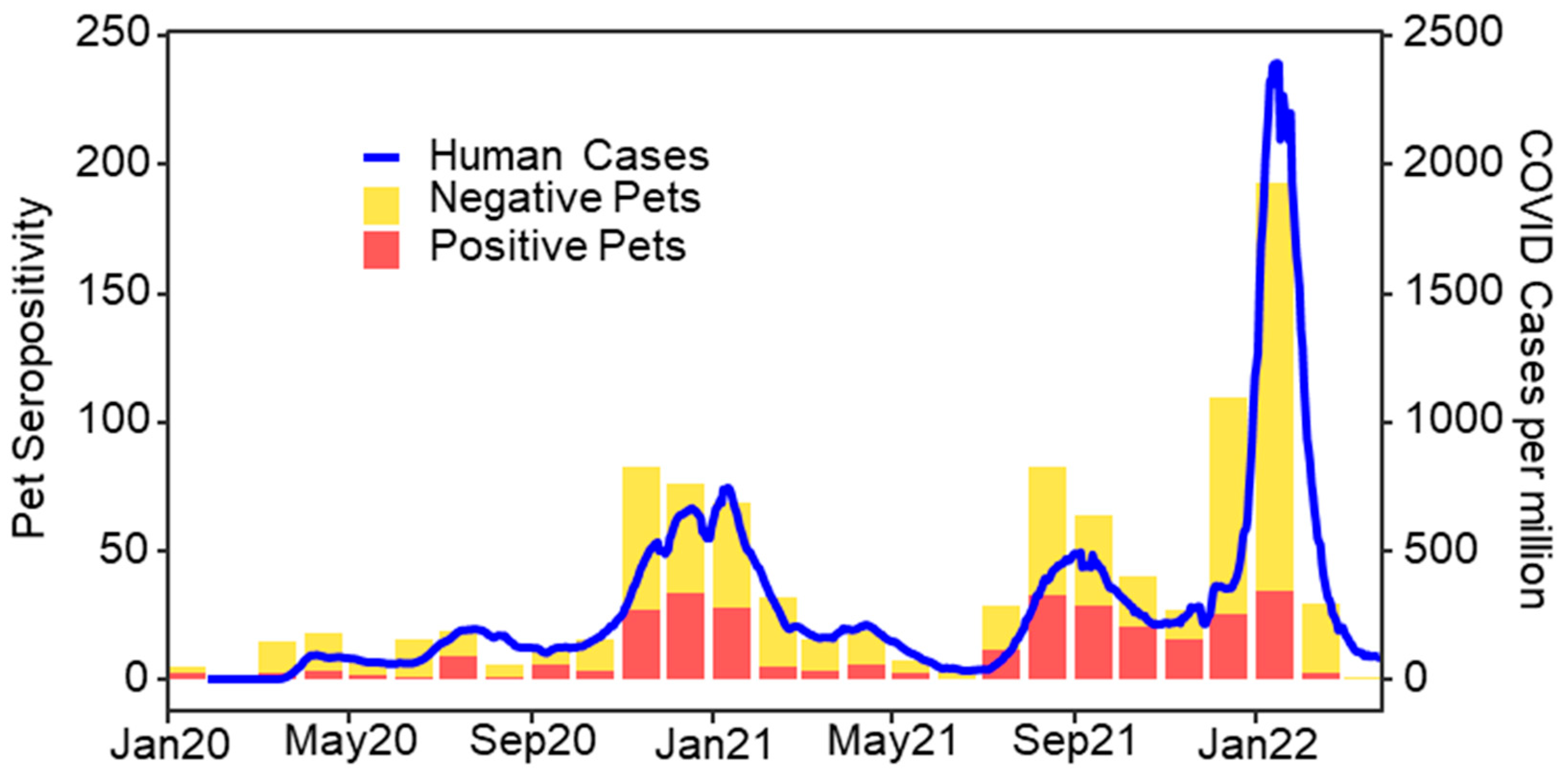

| Variable | β | SE | OR (95% CI) | p Value |
|---|---|---|---|---|
| Age (years) | 0.044 | 0.022 | 1.05 (1.01, 1.09) | 0.04 |
| <2 h in room with infected person | −1.425 | 0.501 | 0.24 (0.09, 0.64) | 0.004 |
| Slept in or on bed of infected person | 0.215 | 0.196 | 1.24 (0.85, 1.82) | 0.27 |
| Characteristic | Dogs | Cats | ||||
|---|---|---|---|---|---|---|
| Seronegative N = 500 | Seropositive N = 247 | p Value | Seronegative N = 185 | Seropositive N = 68 | p Value | |
| Age, y, median (range) | 6 (0.4, 18) | 7 (0.5, 16) | 0.03 | 6 (0.5, 20) | 5 (0.8, 16) | 0.58 |
| Feline lifestyle | 0.53 | |||||
| Indoor exclusively | 143 (77) | 57 (84) | ||||
| Mainly indoor with some outdoor access | 30 (16) | 8 (12) | ||||
| Large amounts of time indoors and outdoors | 12 (7) | 3 (4) | ||||
| Went on leashed walks | 253 (51) | 122 (49) | 0.77 | |||
| Went to an off-leash or dog park | 26 (5) | 13 (5) | 0.28 | |||
| Spent unsupervised time outdoors † | 219 (44) | 123 (50) | 0.16 | 19 (10) | 8 (12) | 0.75 |
| Visited a veterinary clinic | 56 (11) | 16 (6) | 0.06 | 3 (2) | 5 (7) | 0.05 |
| Went to a groomer or boarding facility | 28 (6) | 7 (3) | 0.11 | 0 (0) | 0 (0) | |
| Slept in or on the bed of an infected person | 341 (68) | 189 (77) | 0.03 | 161 (87) | 59 (87) | 0.89 |
| Licked the face or hands of an infected person | 423 (85) | 215 (87) | 0.43 | 93 (50) | 41 (60) | 0.20 |
| Kissed by an infected person | 350 (70) | 191 (77) | 0.05 | 122 (66) | 46 (68) | 0.83 |
| Sat on the lap of an infected person | 445 (89) | 229 (93) | 0.13 | 175 (95) | 66 (97) | 0.45 |
| Spent <2 h in the room with an infected person | 46 (9) | 5 (2) | 0.001 | 16 (9) | 5 (7) | 0.71 |
| Clinical Signs | Dogs | Cats | ||||
|---|---|---|---|---|---|---|
| Seronegative N = 500 | Seropositive N = 247 | p Value | Seronegative N = 185 | Seropositive N = 68 | p Value | |
| Any clinical sign | 67 (13) | 28 (11) | 0.49 | 42 (23) | 15 (22) | 0.87 |
| Respiratory signs † | 37 (7) | 16 (6) | 0.69 | 35 (19) | 10 (15) | 0.40 |
| Coughing | 25 (5) | 9 (4) | 0.45 | 14 (8) | 6 (9) | 0.74 |
| Sneezing | 27 (5) | 9 (4) | 0.32 | 30 (16) | 8 (12) | 0.33 |
| Difficulty breathing | 6 (1) | 0 (0) | 0.98 | 2 (1) | 0 (0) | 0.99 |
| Vomiting | 14 (3) | 3 (1) | 0.22 | 7 (4) | 1 (1) | 0.41 |
| Diarrhea | 13 (3) | 3 (1) | 0.26 | 1 (1) | 1 (1) | 0.50 |
| Decreased appetite | 15 (3) | 7 (3) | 0.93 | 8 (4) | 7 (10) | 0.10 |
| Decreased energy | 30 (6) | 10 (4) | 0.31 | 8 (4) | 7 (10) | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimmerlein, A.K.; McKee, T.S.; Bergman, P.J.; Sokolchik, I.; Leutenegger, C.M. The Transmission of SARS-CoV-2 from COVID-19-Diagnosed People to Their Pet Dogs and Cats in a Multi-Year Surveillance Project. Viruses 2024, 16, 1157. https://doi.org/10.3390/v16071157
Kimmerlein AK, McKee TS, Bergman PJ, Sokolchik I, Leutenegger CM. The Transmission of SARS-CoV-2 from COVID-19-Diagnosed People to Their Pet Dogs and Cats in a Multi-Year Surveillance Project. Viruses. 2024; 16(7):1157. https://doi.org/10.3390/v16071157
Chicago/Turabian StyleKimmerlein, Anne K., Talon S. McKee, Philip J. Bergman, Irina Sokolchik, and Christian M. Leutenegger. 2024. "The Transmission of SARS-CoV-2 from COVID-19-Diagnosed People to Their Pet Dogs and Cats in a Multi-Year Surveillance Project" Viruses 16, no. 7: 1157. https://doi.org/10.3390/v16071157
APA StyleKimmerlein, A. K., McKee, T. S., Bergman, P. J., Sokolchik, I., & Leutenegger, C. M. (2024). The Transmission of SARS-CoV-2 from COVID-19-Diagnosed People to Their Pet Dogs and Cats in a Multi-Year Surveillance Project. Viruses, 16(7), 1157. https://doi.org/10.3390/v16071157





