Development and Optimization of Oligonucleotide Ligation Assay (OLA) Probes for Detection of HIV-1 Resistance to Dolutegravir
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of HIV-1 Integrase Mutations to Include in the OLA-Simple Kit
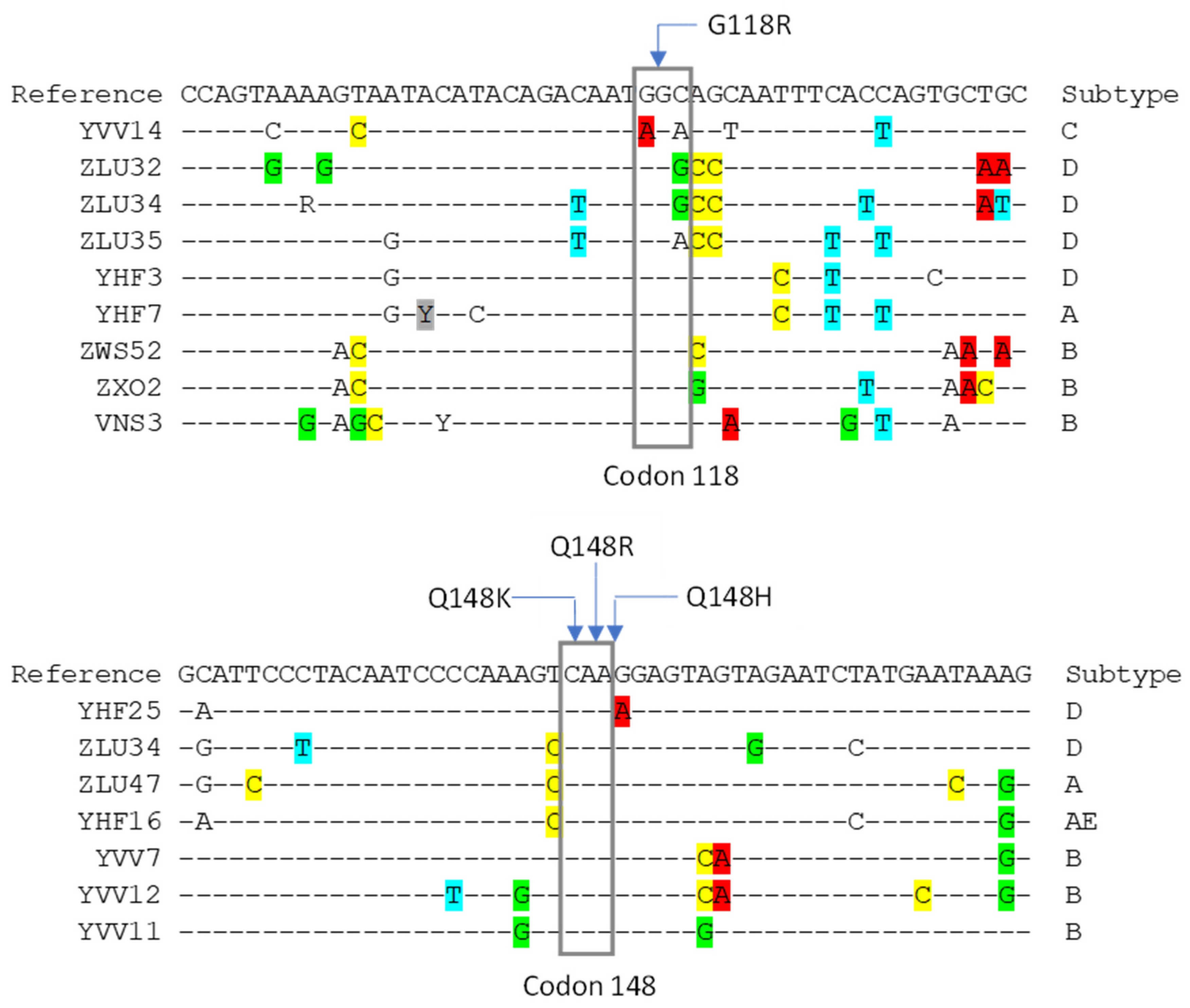
2.2. Design of OLA Probes
2.3. Laboratory-Based OLA
2.4. Assessment of OLA Probes in Clinical Specimens
3. Results
3.1. Selection of DTG-Resistance Mutations for Probe Design
3.2. Performance of OLA Probes to Detect DTG Resistance Mutations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Policy Brief: Update of Recommendations on First- and Second-Line Antiretroviral Regimens; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Cottrell, M.L.; Hadzic, T.; Kashuba, A.D. Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. Clin. Pharmacokinet. 2013, 52, 981–994. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Llibre, J.M.; Pulido, F.; Garcia, F.; Garcia Deltoro, M.; Blanco, J.L.; Delgado, R. Genetic barrier to resistance for dolutegravir. AIDS Rev. 2015, 17, 56–64. [Google Scholar] [PubMed]
- End resistance to dolutegravir roll-out. Lancet HIV 2020, 7, e593. [CrossRef] [PubMed]
- WHO. HIV Policy Adoption and Implementatin Status in Countries; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Group, N.A.S.; Kouanfack, C.; Mpoudi-Etame, M.; Omgba Bassega, P.; Eymard-Duvernay, S.; Leroy, S.; Boyer, S.; Peeters, M.; Calmy, A.; Delaporte, E. Dolutegravir-Based or Low-Dose Efavirenz-Based Regimen for the Treatment of HIV-1. N. Engl. J. Med. 2019, 381, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Aboud, M.; Kaplan, R.; Lombaard, J.; Zhang, F.; Hidalgo, J.A.; Mamedova, E.; Losso, M.H.; Chetchotisakd, P.; Brites, C.; Sievers, J.; et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): An open-label, non-inferiority, phase 3b trial. Lancet Infect. Dis. 2019, 19, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Paton, N.I.; Musaazi, J.; Kityo, C.; Walimbwa, S.; Hoppe, A.; Balyegisawa, A.; Kaimal, A.; Mirembe, G.; Tukamushabe, P.; Ategeka, G.; et al. Dolutegravir or Darunavir in Combination with Zidovudine or Tenofovir to Treat HIV. N. Engl. J. Med. 2021, 385, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Venter, W.D.F.; Moorhouse, M.; Sokhela, S.; Fairlie, L.; Mashabane, N.; Masenya, M.; Serenata, C.; Akpomiemie, G.; Qavi, A.; Chandiwana, N.; et al. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N. Engl. J. Med. 2019, 381, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Loosli, T.; Hossmann, S.; Ingle, S.M.; Okhai, H.; Kusejko, K.; Mouton, J.; Bellecave, P.; van Sighem, A.; Stecher, M.; d’Arminio Monforte, A.; et al. HIV-1 drug resistance in people on dolutegravir-based antiretroviral therapy: A collaborative cohort analysis. Lancet HIV 2023, 10, e733–e741. [Google Scholar] [CrossRef] [PubMed]
- Schramm, B.; Temfack, E.; Descamps, D.; Nicholas, S.; Peytavin, G.; Bitilinyu-Bangoh, J.E.; Storto, A.; Le, M.P.; Abdi, B.; Ousley, J.; et al. Viral suppression and HIV-1 drug resistance 1 year after pragmatic transitioning to dolutegravir first-line therapy in Malawi: A prospective cohort study. Lancet HIV 2022, 9, e544–e553. [Google Scholar] [CrossRef] [PubMed]
- Seatla, K.K.; Maruapula, D.; Choga, W.T.; Ntsipe, T.; Mathiba, N.; Mogwele, M.; Kapanda, M.; Nkomo, B.; Ramaabya, D.; Makhema, J.; et al. HIV-1 Subtype C Drug Resistance Mutations in Heavily Treated Patients Failing Integrase Strand Transfer Inhibitor-Based Regimens in Botswana. Viruses 2021, 13, 594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diaz, R.S.; Hunter, J.R.; Camargo, M.; Dias, D.; Galinskas, J.; Nassar, I.; de Lima, I.B.; Caldeira, D.B.; Sucupira, M.C.; Schechter, M. Dolutegravir-associated resistance mutations after first-line treatment failure in Brazil. BMC Infect. Dis. 2023, 23, 347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanise, H.; van Oosterhout, J.J.; Bisani, P.; Songo, J.; Matola, B.W.; Chipungu, C.; Simon, K.; Cox, C.; Hosseinipour, M.C.; Sagno, J.B.; et al. Virological Findings and Treatment Outcomes of Cases That Developed Dolutegravir Resistance in Malawi’s National HIV Treatment Program. Viruses 2023, 16, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- WHO. HIV Drug Resistance: Brief Report 2024; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Benning, L.; Mantsios, A.; Kerrigan, D.; Coleman, J.S.; Golub, E.; Blackstock, O.; Konkle-Parker, D.; Philbin, M.; Sheth, A.; Adimora, A.A.; et al. Examining adherence barriers among women with HIV to tailor outreach for long-acting injectable antiretroviral therapy. BMC Women’s Health 2020, 20, 152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cevik, M.; Orkin, C.; Sax, P.E. Emergent Resistance to Dolutegravir Among INSTI-Naive Patients on First-line or Second-line Antiretroviral Therapy: A Review of Published Cases. Open Forum Infect. Dis. 2020, 7, ofaa202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lubke, N.; Jensen, B.; Huttig, F.; Feldt, T.; Walker, A.; Thielen, A.; Daumer, M.; Obermeier, M.; Kaiser, R.; Knops, E.; et al. Failure of Dolutegravir First-Line ART with Selection of Virus Carrying R263K and G118R. N. Engl. J. Med. 2019, 381, 887–889. [Google Scholar] [CrossRef] [PubMed]
- van Oosterhout, J.J.; Chipungu, C.; Nkhoma, L.; Kanise, H.; Hosseinipour, M.C.; Sagno, J.B.; Simon, K.; Cox, C.; Hoffman, R.; Steegen, K.; et al. Dolutegravir Resistance in Malawi’s National HIV Treatment Program. Open Forum Infect. Dis. 2022, 9, ofac148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lessells, R.J.; Avalos, A.; de Oliveira, T. Implementing HIV-1 genotypic resistance testing in antiretroviral therapy programs in Africa: Needs, opportunities, and challenges. AIDS Rev. 2013, 15, 221–229. [Google Scholar] [PubMed] [PubMed Central]
- Panpradist, N.; Beck, I.A.; Chung, M.H.; Kiarie, J.N.; Frenkel, L.M.; Lutz, B.R. Simplified Paper Format for Detecting HIV Drug Resistance in Clinical Specimens by Oligonucleotide Ligation. PLoS ONE 2016, 11, e0145962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panpradist, N.; Beck, I.A.; Vrana, J.; Higa, N.; McIntyre, D.; Ruth, P.S.; So, I.; Kline, E.C.; Kanthula, R.; Wong-On-Wing, A.; et al. OLA-Simple: A software-guided HIV-1 drug resistance test for low-resource laboratories. EBioMedicine 2019, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Panpradist, N.; Beck, I.A.; Ruth, P.S.; Ávila-Ríos, S.; García-Morales, C.; Soto-Nava, M.; Tapia-Trejo, D.; Matías-Florentino, M.; Paz-Juarez, H.E.; del Arenal-Sanchez, S.; et al. Near point-of-care, point-mutation test to detect drug resistance in HIV-1: A validation study in a Mexican cohort. AIDS 2020, 34, 1331–1338. [Google Scholar] [PubMed]
- Panpradist, N.; Beck, I.A.; Miller, A.; Cash, A.M.; Campbell, J.; Stewart, S.W.; Ruth, P.S.; Chohan, B.; Owiti, P.; Akinyi, G.; et al. Diagnostic accuracy of a near point-of-care HIV drug resistance test OLA-Simple: A field validation study in Kenya. In Proceedings of the XXX International Workshop on HIV Drug Resistance and Treatment Strategies, Cape Town, South Africa, 20–22 September 2023. [Google Scholar]
- Vrana, J.D.; Panpradist, N.; Higa, N.; Ko, D.; Ruth, P.; Kanthula, R.; Lai, J.J.; Yang, Y.; Sakr, S.R.; Chohan, B. Implementation of an interactive mobile application to pilot a rapid assay to detect HIV drug resistance mutations in Kenya. PLOS Global Public Health 2022, 2, e0000185. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.Y.; Gonzales, M.J.; Kantor, R.; Betts, B.J.; Ravela, J.; Shafer, R.W. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res. 2003, 31, 298–303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shafer, R.W. Rationale and uses of a public HIV drug-resistance database. J. Infect. Dis. 2006, 194 (Suppl. S1), S51–S58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beck, I.A.; Deng, W.; Payant, R.; Hall, R.; Bumgarner, R.E.; Mullins, J.I.; Frenkel, L.M. Validation of an oligonucleotide ligation assay for quantification of human immunodeficiency virus type 1 drug-resistant mutants by use of massively parallel sequencing. J. Clin. Microbiol. 2014, 52, 2320–2327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, N.; Flavell, S.; Ferns, B.; Frampton, D.; Edwards, S.G.; Miller, R.F.; Grant, P.; Nastouli, E.; Gupta, R.K. Development of the R263K Mutation to Dolutegravir in an HIV-1 Subtype D Virus Harboring 3 Class-Drug Resistance. Open Forum Infect. Dis. 2019, 6, ofy329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Botha, J.C.; Steegen, K.; Edoo, M.; Nel, J.; van Zyl, G.U. Low-level viraemia despite emergence of dolutegravir-resistant variants. S. Afr. J. HIV Med. 2022, 23, 1398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Braun, D.L.; Scheier, T.; Ledermann, U.; Flepp, M.; Metzner, K.J.; Boni, J.; Gunthard, H.F. Emergence of Resistance to Integrase Strand Transfer Inhibitors during Dolutegravir Containing Triple-Therapy in a Treatment-Experienced Patient with Pre-Existing M184V/I Mutation. Viruses 2020, 12, 1330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cahn, P.; Pozniak, A.L.; Mingrone, H.; Shuldyakov, A.; Brites, C.; Andrade-Villanueva, J.F.; Richmond, G.; Buendia, C.B.; Fourie, J.; Ramgopal, M.; et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: Week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013, 382, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Cahn, P.; Sierra Madero, J.; Arribas, J.R.; Antinori, A.; Ortiz, R.; Clarke, A.E.; Hung, C.C.; Rockstroh, J.K.; Girard, P.M.; Sievers, J.; et al. Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy—Naive adults with HIV-1 infection. AIDS 2022, 36, 39–48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cardoso, M.; Baptista, T.; Diogo, I.; Aleixo, M.J.; Marques, N.; Mansinho, K.; Gomes, P. Two cases of dolutegravir failure with R263K mutation. AIDS 2018, 32, 2639–2640. [Google Scholar] [CrossRef] [PubMed]
- Chinula, L.; Ziemba, L.; Brummel, S.; McCarthy, K.; Coletti, A.; Krotje, C.; Johnston, B.; Knowles, K.; Moyo, S.; Stranix-Chibanda, L.; et al. Efficacy and safety of three antiretroviral therapy regimens started in pregnancy up to 50 weeks post partum: A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet HIV 2023, 10, e363–e374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cochrane, S.; Daniel, J.; Forsyth, S.; Smit, E. First reported case of integrase (R263K, G163R) and reverse transcriptase (M184V)-transmitted drug resistance from a drug-naive patient failing Triumeq. AIDS 2018, 32, 1905–1907. [Google Scholar] [CrossRef] [PubMed]
- Frange, P.; Blanche, S.; Veber, F.; Avettand-Fenoel, V. Dolutegravir in the long term in children and adolescents: Frequent virological failure but rare acquired genotypic resistance. HIV Med. 2021, 22, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, J.A.; Du, Y.; Zhang, T.H.; Sun, R.; Landovitz, R.J. Emergence of Integrase Resistance Mutations During Initial Therapy Containing Dolutegravir. Clin. Infect. Dis. 2018, 67, 791–794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gil, H.; Delgado, E.; Benito, S.; Moreno-Lorenzo, M.; Thomson, M.M.; Spanish Group for the Study of Antiretroviral Drug Resistance. Factors associated with HIV-1 resistance to integrase strand transfer inhibitors in Spain: Implications for dolutegravir-containing regimens. Front. Microbiol. 2022, 13, 1051096. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lepik, K.J.; Harrigan, P.R.; Yip, B.; Wang, L.; Robbins, M.A.; Zhang, W.W.; Toy, J.; Akagi, L.; Lima, V.D.; Guillemi, S.; et al. Emergent drug resistance with integrase strand transfer inhibitor-based regimens. AIDS 2017, 31, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Mahomed, K.; Wallis, C.L.; Dunn, L.; Maharaj, S.; Maartens, G.; Meintjes, G. Case report: Emergence of dolutegravir resistance in a patient on second-line antiretroviral therapy. S. Afr. J. HIV Med. 2020, 21, 1062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malinga, S.; Khan, A.; Archary, M. Breaking the unbreakable: A paediatric case of dolutegravir resistance from KwaZulu-Natal. S. Afr. J. HIV Med. 2023, 24, 1458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mandikiyana Chirimuta, L.A.; Pascoe, M.J.; Lowe, S. Emergent dolutegravir resistance in integrase-naive, treatment experienced patients from Zimbabwe. S. Afr. J. HIV Med. 2022, 23, 1435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paton, N.I.; Musaazi, J.; Kityo, C.; Walimbwa, S.; Hoppe, A.; Balyegisawa, A.; Asienzo, J.; Kaimal, A.; Mirembe, G.; Lugemwa, A.; et al. Efficacy and safety of dolutegravir or darunavir in combination with lamivudine plus either zidovudine or tenofovir for second-line treatment of HIV infection (NADIA): Week 96 results from a prospective, multicentre, open-label, factorial, randomised, non-inferiority trial. Lancet HIV 2022, 9, e381–e393. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.J.; Chueca, N.; D’Avolio, A.; Zarzalejos, J.M.; Garcia, F. Virological Failure in HIV to Triple Therapy With Dolutegravir-Based Firstline Treatment: Rare but Possible. Open Forum Infect. Dis. 2019, 6, ofy332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Revollo, B.; Vinuela, L.; de la Mora, L.; Garcia, F.; Noguera-Julian, M.; Parera, M.; Paredes, R.; Llibre, J.M. Integrase resistance emergence with dolutegravir/lamivudine with prior HIV-1 suppression. J. Antimicrob. Chemother. 2022, 77, 1738–1740. [Google Scholar] [CrossRef] [PubMed]
- Steegen, K.; van Zyl, G.; Letsoalo, E.; Claassen, M.; Hans, L.; Carmona, S. Resistance in patients failing integrase strand transfer inhibitors: A call to replace raltegravir with dolutegravir in third-line treatment in South Africa. Open Forum Infect. Dis. 2019, 6, ofz377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taiwo, B.O.; Zheng, L.; Stefanescu, A.; Nyaku, A.; Bezins, B.; Wallis, C.L.; Godfrey, C.; Sax, P.E.; Acosta, E.; Haas, D.; et al. ACTG A5353: A Pilot Study of Dolutegravir Plus Lamivudine for Initial Treatment of Human Immunodeficiency Virus-1 (HIV-1)-infected Participants With HIV-1 RNA <500,000 Copies/mL. Clin. Infect. Dis. 2018, 66, 1689–1697. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turkova, A.; White, E.; Mujuru, H.A.; Kekitiinwa, A.R.; Kityo, C.M.; Violari, A.; Lugemwa, A.; Cressey, T.R.; Musoke, P.; Variava, E.; et al. Dolutegravir as First- or Second-Line Treatment for HIV-1 Infection in Children. N. Engl. J. Med. 2021, 385, 2531–2543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Underwood, M.; Horton, J.; Nangle, K.; Hopking, J.; Smith, K.; Aboud, M.; Wynne, B.; Sievers, J.; Stewart, E.L.; Wang, R. Integrase Inhibitor Resistance Mechanisms and Structural Characteristics in Antiretroviral Therapy-Experienced, Integrase Inhibitor-Naive Adults with HIV-1 Infection Treated with Dolutegravir plus Two Nucleoside Reverse Transcriptase Inhibitors in the DAWNING Study. Antimicrob. Agents Chemother. 2022, 66, e0164321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Kampen, J.J.A.; Pham, H.T.; Yoo, S.; Overmars, R.J.; Lungu, C.; Mahmud, R.; Schurink, C.A.M.; van Boheemen, S.; Gruters, R.A.; Fraaij, P.L.A.; et al. HIV-1 resistance against dolutegravir fluctuates rapidly alongside erratic treatment adherence: A case report. J. Glob. Antimicrob. Resist. 2022, 31, 323–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vavro, C.; Ruel, T.; Wiznia, A.; Montanez, N.; Nangle, K.; Horton, J.; Buchanan, A.M.; Stewart, E.L.; Palumbo, P. Emergence of Resistance in HIV-1 Integrase with Dolutegravir Treatment in a Pediatric Population from the IMPAACT P1093 Study. Antimicrob. Agents Chemother. 2022, 66, e0164521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamori, D.; Barabona, G.; Rugemalila, J.; Maokola, W.; Masoud, S.S.; Mizinduko, M.; Sabasaba, A.; Ruhago, G.; Sambu, V.; Mushi, J.; et al. Emerging integrase strand transfer inhibitor drug resistance mutations among children and adults on ART in Tanzania: Findings from a national representative HIV drug resistance survey. J. Antimicrob. Chemother. 2023, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Tao, K.; Kouamou, V.; Avalos, A.; Scott, J.; Grant, P.M.; Rhee, S.Y.; McCluskey, S.M.; Jordan, M.R.; Morgan, R.L.; et al. Prevalence of Emergent Dolutegravir Resistance Mutations in People Living with HIV: A Rapid Scoping Review. Viruses 2024, 16, 399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tao, K.; Rhee, S.Y.; Chu, C.; Avalos, A.; Ahluwalia, A.K.; Gupta, R.K.; Jordan, M.R.; Shafer, R.W. Treatment Emergent Dolutegravir Resistance Mutations in Individuals Naive to HIV-1 Integrase Inhibitors: A Rapid Scoping Review. Viruses 2023, 15, 1932. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beck, I.A.; Mahalanabis, M.; Pepper, G.; Wright, A.; Hamilton, S.; Langston, E.; Frenkel, L.M. Rapid and sensitive oligonucleotide ligation assay for detection of mutations in human immunodeficiency virus type 1 associated with high-level resistance to protease inhibitors. J. Clin. Microbiol. 2002, 40, 1413–1419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Landegren, U.; Kaiser, R.; Sanders, J.; Hood, L. A ligase-mediated gene detection technique. Science 1988, 241, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Beck, I.A.; Crowell, C.; Kittoe, R.; Bredell, H.; Machaba, M.; Willamson, C.; Janssens, W.; Jallow, S.; van der Groen, G.; Shao, Y.; et al. Optimization of the oligonucleotide ligation assay, a rapid and inexpensive test for detection of HIV-1 drug resistance mutations, for non-North American variants. J. Acquir. Immune Defic. Syndr. 2008, 48, 418–427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Edelstein, R.E.; Nickerson, D.A.; Tobe, V.O.; Manns-Arcuino, L.A.; Frenkel, L.M. Oligonucleotide ligation assay for detecting mutations in the human immunodeficiency virus type 1 pol gene that are associated with resistance to zidovudine, didanosine, and lamivudine. J. Clin. Microbiol. 1998, 36, 569–572. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, D.M.; Lin, P.K. Synthesis and duplex stability of oligonucleotides containing adenine-guanine analogues. Carbohydr. Res. 1991, 216, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.K.; Brown, D.M. Synthesis and duplex stability of oligonucleotides containing cytosine-thymine analogues. Nucleic Acids Res. 1989, 17, 10373–10383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, P.K.; Brown, D.M. Synthesis of oligodeoxyribonucleotides containing degenerate bases and their use as primers in the polymerase chain reaction. Nucleic Acids Res. 1992, 20, 5149–5152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, H.; Nichols, R. PCR amplification using deoxyinosine to replace an entire codon and at ambiguous positions. Biotechniques 1994, 16, 24–26. [Google Scholar] [PubMed]
- Loakes, D.; Brown, D.M. 5-Nitroindole as an universal base analogue. Nucleic Acids Res. 1994, 22, 4039–4043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arimide, D.A.; Szojka, Z.I.; Zealiyas, K.; Gebreegziabxier, A.; Adugna, F.; Sasinovich, S.; Bjorkman, P.; Medstrand, P. Pre-Treatment Integrase Inhibitor Resistance and Natural Polymorphisms among HIV-1 Subtype C Infected Patients in Ethiopia. Viruses 2022, 14, 729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quashie, P.K.; Oliviera, M.; Veres, T.; Osman, N.; Han, Y.S.; Hassounah, S.; Lie, Y.; Huang, W.; Mesplede, T.; Wainberg, M.A. Differential effects of the G118R, H51Y, and E138K resistance substitutions in different subtypes of HIV integrase. J. Virol. 2015, 89, 3163–3175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dekker, J.G.; Klaver, B.; Berkhout, B.; Das, A.T. HIV-1 3′-Polypurine Tract Mutations Confer Dolutegravir Resistance by Switching to an Integration-Independent Replication Mechanism via 1-LTR Circles. J. Virol. 2023, 97, e0036123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hikichi, Y.; Van Duyne, R.; Pham, P.; Groebner, J.L.; Wiegand, A.; Mellors, J.W.; Kearney, M.F.; Freed, E.O. Mechanistic Analysis of the Broad Antiretroviral Resistance Conferred by HIV-1 Envelope Glycoprotein Mutations. mBio 2021, 12, e03134-20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hikichi, Y.; Grover, J.R.; Schafer, A.; Mothes, W.; Freed, E.O. Epistatic pathways can drive HIV-1 escape from integrase strand transfer inhibitors. Sci. Adv. 2024, 10, eadn0042. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Detection Rate | Major Integrase Inhibitor (INSTI) Resistance Mutations (Stanford HIV Drug Resistance Database 1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T66IK | E92Q | G118R | E138KAT | G140SAC | Y143RCH | S147G | Q148HRK | N155H | R263K | |
| Frequency (%) | 13 (17.1) | 2 (2.6) | 26 (34.2) | 24 (31.6) | 5 (6.6) | 1 (1.3) | 6 (7.9) | 11 (14.5) | 8 (10.5) | 37 (48.7) |
| Number (%) detected as single mutations | 1 (1.3) | 0 | 7 (9.2) | 0 | 0 | 0 | 1 (1.3) | 3 (3.9) | 3 (3.9) | 29 (38.2) |
| Codon | Mutation | Probe Type | Sequence (5′ →3′) b |
|---|---|---|---|
| A. Original probes | |||
| 118 | G118R | Subtype A/AE wildtype | dig-CCAGTAAAARTARTACACACAGACAAYG |
| Subtype C/D wildtype | dig-CCAGTMAAARTARTACATACAGACAATG | ||
| Subtype A/AE mutant | f-CCAGTAAAARTARTACACACAGACAAYC | ||
| Subtype C/D mutant | f-CCAGTMAAARTARTACATACAGACAATC | ||
| Common | p-GHAGCAATTTCACCAGYRCTGC-bio | ||
| Subtype C common | p-GHAGTAATTTCACCAGTRCTGC-bio | ||
| 148 | Q148K | Wildtype | dig-GRATTCCCTACAATCCCCAAAGYC |
| Mutant | f-GRATTCCCTACAATCCCCAAAGYA | ||
| Common | p-ARGGAGTAGTAGAATCYATGAATAA-bio | ||
| Q148R | Wildtype | dig-ATTCCCTACAATCCCCAAAGTCA | |
| Mutant | f-ATTCCCTACAATCCCCAAAGTCG | ||
| Common | p-RGGAGTAGTAGAATCYATGAATAAAG-bio | ||
| Q148H | Wildtype | dig-TTCCCTACAATCCCCAAAGTCAR | |
| Mutant | f-TTCCCTACAATCCCCAAAGTCAC | ||
| Common | p-GGAGTAGTAGAATCYATGAATAAAG-bio | ||
| 155 | N155H | Wildtype | dig-GTCAAGGAGTAGTAGAATCYATRA |
| Mutant | f-GTCAAGGAGTAGTAGAATCYATRC | ||
| Common | p-AYAAAGAATTAAAGAAAATYATAGGRC-bio | ||
| 263 | R263K | Wildtype | dig-TGACATAAARGTAGTRCCAAGRAG |
| Mutant | f-TGACATAAARGTAGTRCCAAGRAA | ||
| Common | p-RAAAGCAAARATCATTAGGGATTAT-bio | ||
| Subtype C common | p-RAAAGYAAAAATCATTAAGGACTATG-bio | ||
| B. Modified probes c | |||
| 118 | G118R | Alt mutant | f-CCAGTAAAAGTARTACAYACAGACAAYA |
| Mod common | p-GHAGCAATTTYACCAGYGCTGC-bio | ||
| Alt common | p-GNCCYAATTTCACCAGTRCTGC-bio | ||
| 148 | Q148R | Mod wildtype | dig-ATTCCCTACAATCCCCAAAGYCA |
| Mod mutant | f-ATTCCCTACAATCCCCAAAGYCG | ||
| Mutation | OLA n = 244 | PacBio/Sanger n = 244 | |||
|---|---|---|---|---|---|
| Mutant | Wildtype | Indeterminate | Mutant | Wildtype | |
| G118R | 6 | 46 | 9 | 8 | 53 |
| Q148K/R/H * | 7 | 47 | 7 | 7 | 54 |
| N155H | 7 | 54 | 0 | 7 | 54 |
| R263K | 7 | 54 | 0 | 7 | 54 |
| Total | 27 | 201 | 16 | 29 | 215 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, I.A.; Boyce, C.L.; Bishop, M.D.; Vu, Y.L.; Fung, A.; Styrchak, S.; Panpradist, N.; Lutz, B.R.; Frenkel, L.M. Development and Optimization of Oligonucleotide Ligation Assay (OLA) Probes for Detection of HIV-1 Resistance to Dolutegravir. Viruses 2024, 16, 1162. https://doi.org/10.3390/v16071162
Beck IA, Boyce CL, Bishop MD, Vu YL, Fung A, Styrchak S, Panpradist N, Lutz BR, Frenkel LM. Development and Optimization of Oligonucleotide Ligation Assay (OLA) Probes for Detection of HIV-1 Resistance to Dolutegravir. Viruses. 2024; 16(7):1162. https://doi.org/10.3390/v16071162
Chicago/Turabian StyleBeck, Ingrid A., Ceejay L. Boyce, Marley D. Bishop, Yen L. Vu, Amanda Fung, Sheila Styrchak, Nuttada Panpradist, Barry R. Lutz, and Lisa M. Frenkel. 2024. "Development and Optimization of Oligonucleotide Ligation Assay (OLA) Probes for Detection of HIV-1 Resistance to Dolutegravir" Viruses 16, no. 7: 1162. https://doi.org/10.3390/v16071162






