The Generation and Characterization of Monoclonal Antibodies against the MPXV A29L Protein
Abstract
:1. Introduction
2. Methods
2.1. Viruses, Cells, and Animals
2.2. The Expression and Purification of the MPXV A29L Protein
2.3. The Generation and Screening of mAbs against the MPXV A29L Protein
2.4. ELISA
2.5. Indirect Immunofluorescence Assay
2.6. Western Blot Identification of mAbs
2.7. Identification of mAbs by Immunoprecipitation
2.8. Investigating A27L Protein Expression Using mAbs
2.9. Expression of A29L on HEK-293T Cells
3. Results
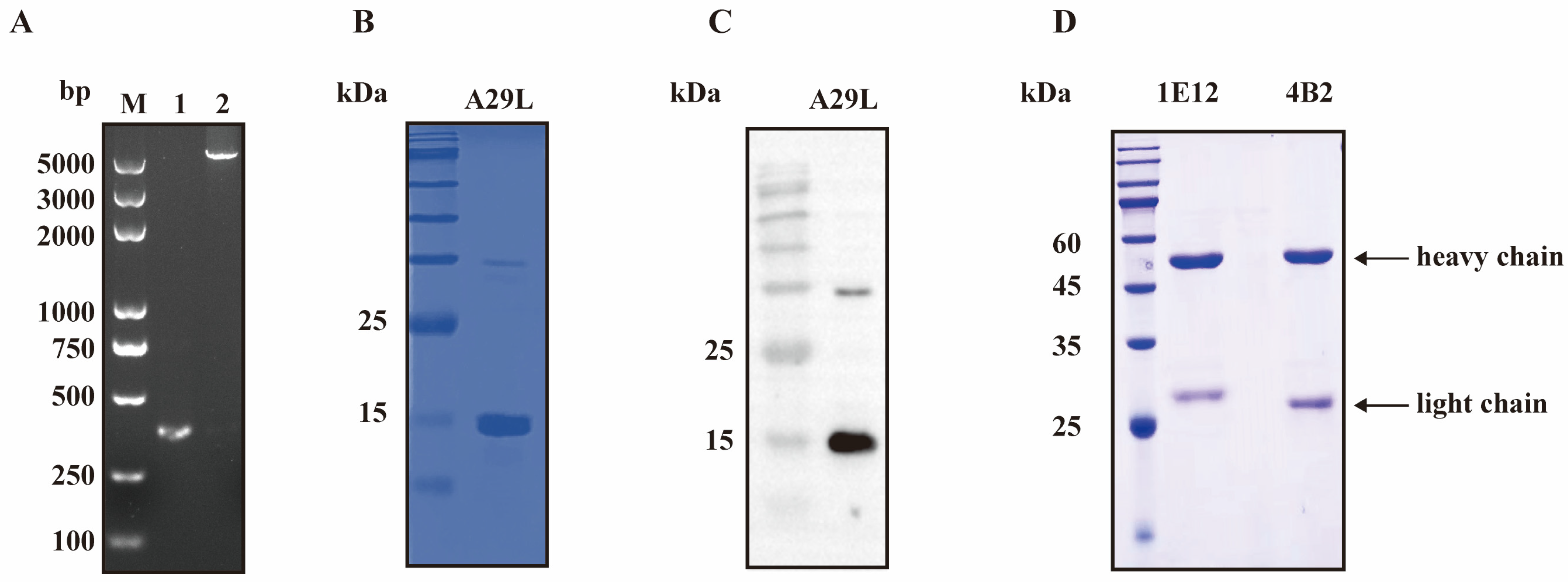
3.1. The Expression and Purification of the Recombinant MPXV A29L Protein
3.2. The Production and Characterization of the mAb against the MPXV A29L Protein
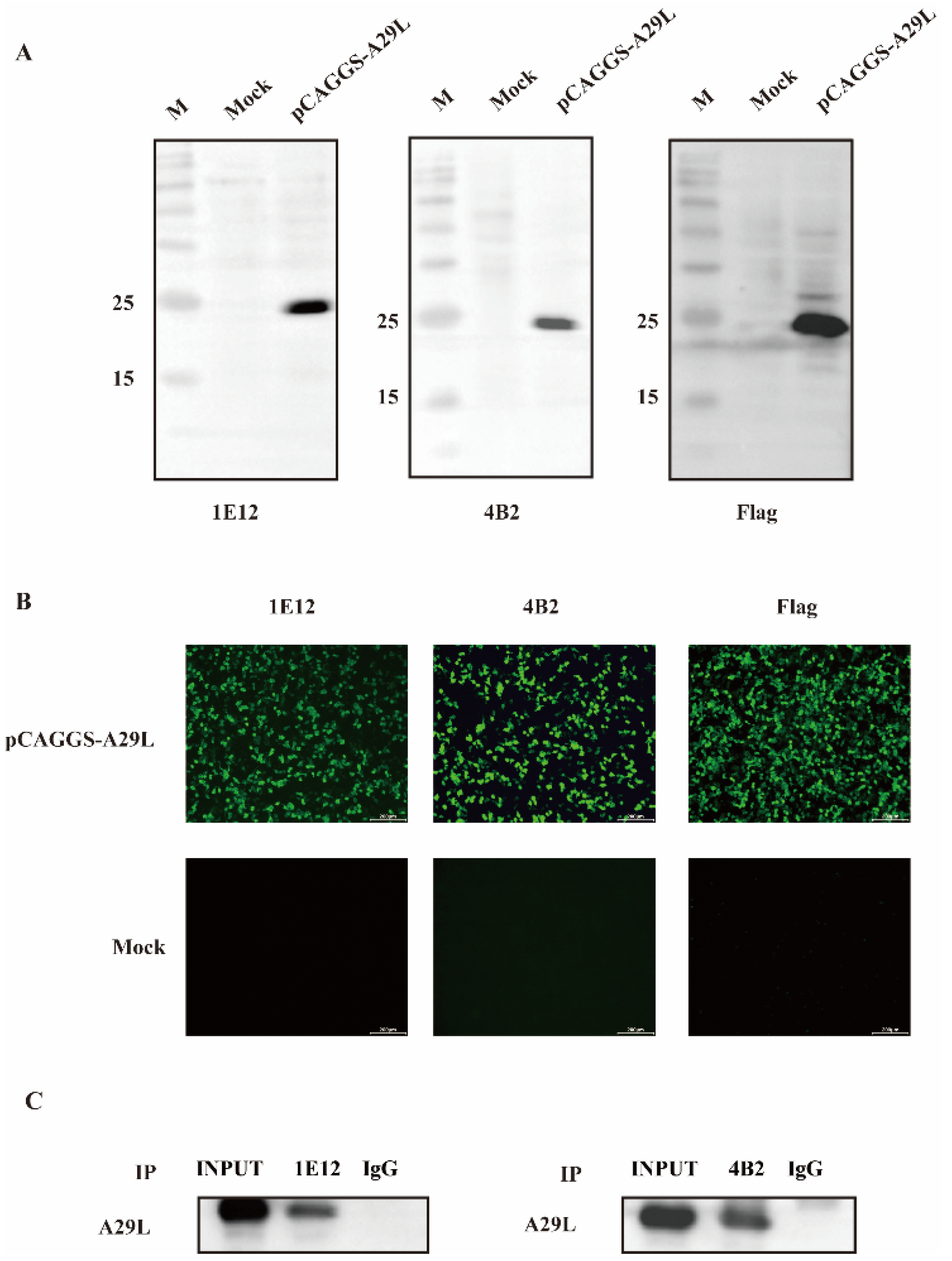
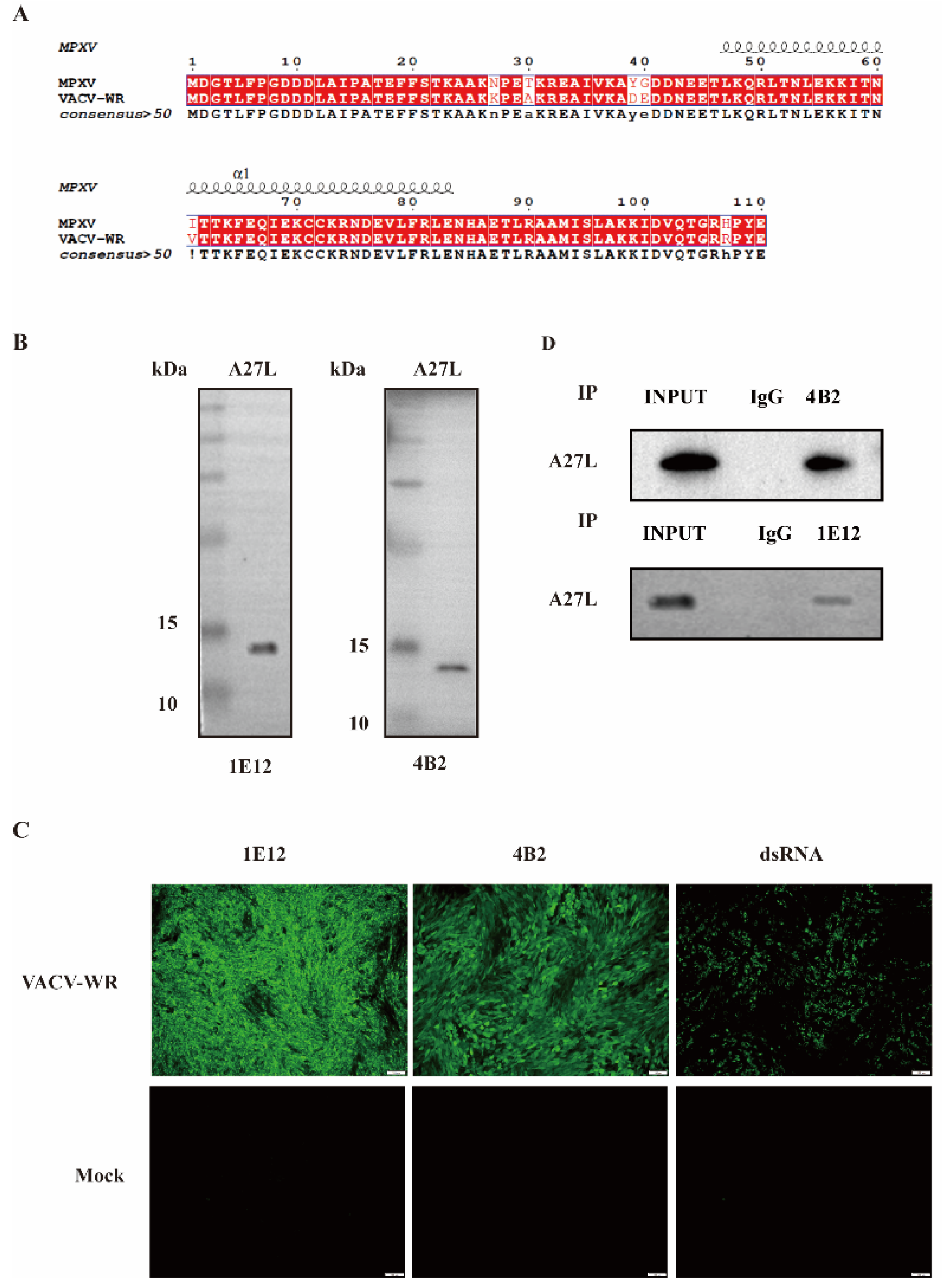
3.3. Immunogenicity and Specificity Assays of Monoclonal Antibodies
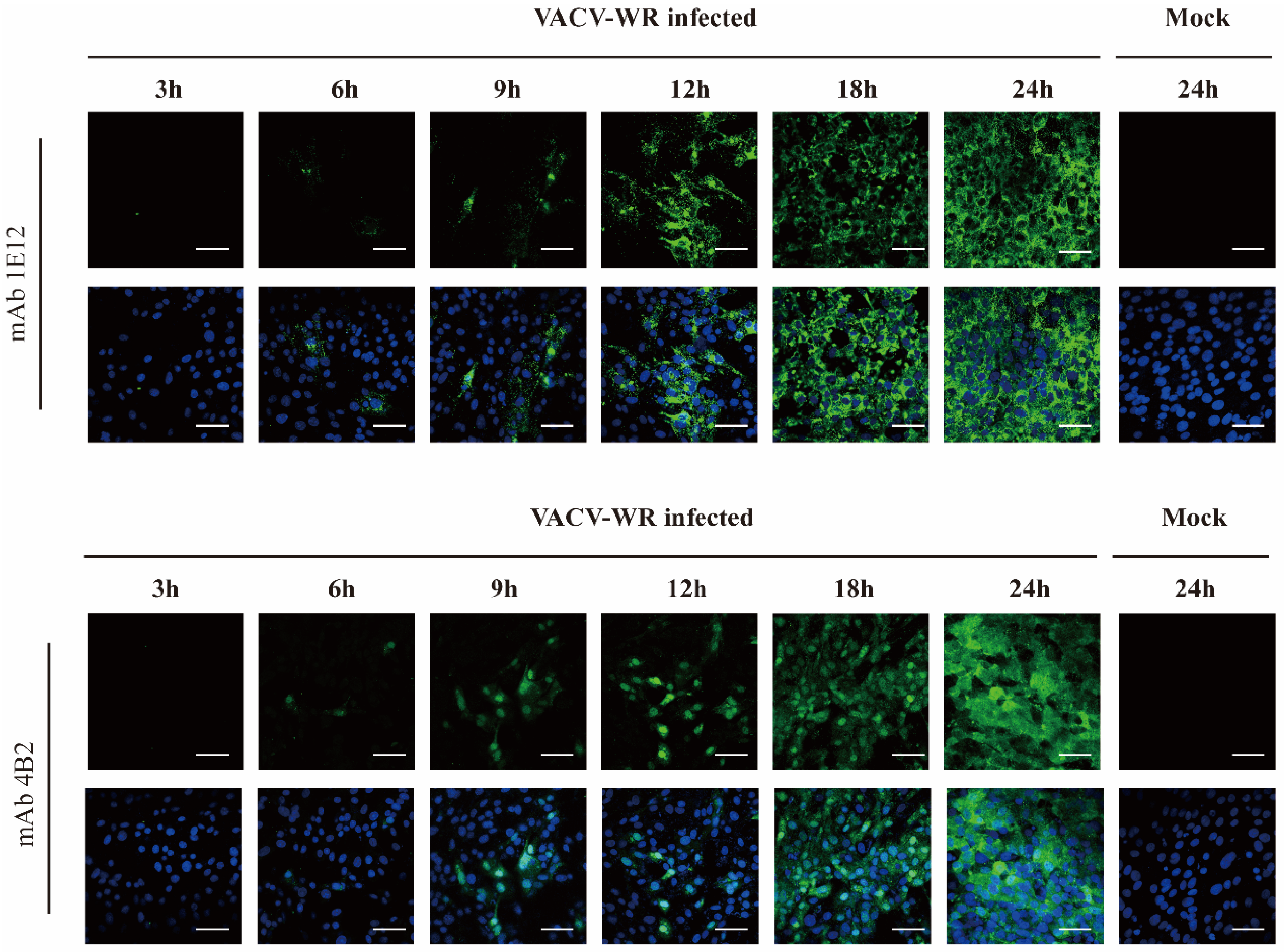
3.4. A27L Protein Expression in VACV WR-Infected Cells at Different Time Points
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, Q.; Wang, C.; Chuai, X.; Chiu, S. Monkeypox virus: A re-emergent threat to humans. Virol. Sin. 2022, 37, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Sale, T.A.; Melski, J.W.; Stratman, E.J. Monkeypox: An epidemiologic and clinical comparison of African and US disease. J. Am. Acad. Dermatol. 2006, 55, 478–481. [Google Scholar] [CrossRef]
- Cohen, J. GLOBAL HEALTH Monkeypox outbreak questions intensify as cases soar. Science 2022, 376, 902–903. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, X.; Zhu, Z.; Sun, L.; Lu, H. The Novel Monkeypox Outbreak: What Should We Know and Reflect On? Zoonoses 2022, 2, 1. [Google Scholar] [CrossRef]
- Foster, S.O.; Brink, E.W.; Hutchins, D.L.; Pifer, J.M.; Lourie, B.; Moser, C.R.; Cummings, E.C.; Kuteyi, O.E.; Eke, R.E.; Titus, J.B.; et al. Human monkeypox. Bull. World Health Organ. 1972, 46, 569–576. [Google Scholar] [PubMed]
- Breman, J.G.; Kalisaruti; Steniowski, M.V.; Zanotto, E.; Gromyko, A.I.; Arita, I. Human Monkeypox, 1970–1979. Bull. World Health Organ. 1980, 58, 165–182. [Google Scholar] [PubMed]
- Sejvar, J.J.; Chowdary, Y.; Schomogyi, M.; Stevens, J.; Patel, J.; Karem, K.; Fischer, M.; Kuehnert, M.J.; Zaki, S.R.; Paddock, C.D.; et al. Human monkeypox infection: A family cluster in the Midwestern United States. J. Infect. Dis. 2004, 190, 1833–1840. [Google Scholar] [CrossRef]
- Vaughan, A.M.; Cenciarelli, O.; Colombe, S.; de Sousa, L.A.; Fischer, N.; Gossner, C.M.; Pires, J.; Scardina, G.; Aspelund, G.; Avercenko, M.; et al. A large multicountry outbreak of monkeypox across 41 countries in the WHO European Region, 7 March to 23 August 2022. Eurosurveillance 2022, 27, 2200620. [Google Scholar] [CrossRef]
- Saxena, S.K.; Ansari, S.; Maurya, V.K.; Kumar, S.; Jain, A.; Paweska, J.T.; Tripathi, A.K.; Abdel-Moneim, A.S. Re-emerging human monkeypox: A major public-health debacle. J. Med. Virol. 2023, 95, e27902. [Google Scholar] [CrossRef]
- Iizuka, I.; Ami, Y.; Suzaki, Y.; Nagata, N.; Fukushi, S.; Ogata, M.; Morikawa, S.; Hasegawa, H.; Mizuguchi, M.; Kurane, I.; et al. A Single Vaccination of Nonhuman Primates with Highly Attenuated Smallpox Vaccine, LC16m8, Provides Long-term Protection against Monkeypox. Jpn. J. Infect. Dis. 2017, 70, 408–415. [Google Scholar] [CrossRef]
- Moss, B. Smallpox vaccines: Targets of protective immunity. Immunol. Rev. 2011, 239, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Murtadha, M.; Schnell, M.; Eisenlohr, L.C.; Hooper, J.; Flomenberg, P. Human T-cell responses to vaccinia virus envelope proteins. J. Virol. 2006, 80, 10010–10020. [Google Scholar] [CrossRef]
- Manes, N.P.; Estep, R.D.; Mottaz, H.M.; Moore, R.J.; Clauss, T.R.W.; Monroe, M.E.; Du, X.; Adkins, J.N.; Wong, S.W.; Smith, R.D. Comparative proteomics of human monkeypox and vaccinia intracellular mature and extracellular enveloped virions. J. Proteome Res. 2008, 7, 960–968. [Google Scholar] [CrossRef]
- Hughes, L.J.; Goldstein, J.; Pohl, J.; Hooper, J.W.; Pitts, R.L.; Townsend, M.B.; Bagarozzi, D.; Damon, I.K.; Karem, K.L. A highly specific monoclonal antibody against monkeypox virus detects the heparin binding domain of A27. Virology 2014, 464, 264–273. [Google Scholar] [CrossRef]
- Li, P.F.; Pachis, S.T.; Xu, G.G.; Schraauwen, R.; Incitti, R.; de Vries, A.C.; Bruno, M.J.; Peppelenbosch, M.P.; Alam, I.; Raymond, K.; et al. Mpox virus infection and drug treatment modelled in human skin organoids. Nat. Microbiol. 2023, 8, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar] [PubMed]
- Forni, D.; Cagliani, R.; Molteni, C.; Clerici, M.; Sironi, M. Monkeypox virus: The changing facets of a zoonotic pathogen. Infect. Genet. Evol. 2022, 105. [Google Scholar] [CrossRef]
- Hendrickson, R.C.; Wang, C.L.; Hatcher, E.L.; Lefkowitz, E.J. Orthopoxvirus Genome Evolution: The Role of Gene Loss. Viruses 2010, 2, 1933–1967. [Google Scholar] [CrossRef]
- Li, M.J.; Ren, Z.N.; Wang, Y.L.; Jiang, Y.S.; Yang, M.H.; Li, D.L.; Chen, J.Y.; Liang, Z.X.; Lin, Y.H.; Zeng, Z.J.; et al. Three neutralizing mAbs induced by MPXV A29L protein recognizing different epitopes act synergistically against orthopoxvirus. Emerg. Microbes Infect. 2023, 12, 2223669. [Google Scholar] [CrossRef]
- Orba, Y.; Sasaki, M.; Yamaguchi, H.; Ishii, A.; Thomas, Y.; Ogawa, H.; Hang’ombe, B.M.; Mweene, A.S.; Morikawa, S.; Saijo, M.; et al. Orthopoxvirus infection among wildlife in Zambia. J. Gen. Virol. 2015, 96, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, I.; Saijo, M.; Shiota, T.; Ami, Y.; Suzaki, Y.; Nagata, N.; Hasegawa, H.; Sakai, K.; Fukushi, S.; Mizutani, T.; et al. Loop-Mediated Isothermal Amplification-Based Diagnostic Assay for Monkeypox Virus Infections. J. Med. Virol. 2009, 81, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Zhang, M.; Zhang, M.; Jing, R.; Zhou, J.; Cao, H.; Liu, C.; Zhu, H.; Ghonaim, A.H.; Rouby, S.R.; et al. The Generation and Characterization of Monoclonal Antibodies against the MPXV A29L Protein. Viruses 2024, 16, 1184. https://doi.org/10.3390/v16081184
Zhu W, Zhang M, Zhang M, Jing R, Zhou J, Cao H, Liu C, Zhu H, Ghonaim AH, Rouby SR, et al. The Generation and Characterization of Monoclonal Antibodies against the MPXV A29L Protein. Viruses. 2024; 16(8):1184. https://doi.org/10.3390/v16081184
Chicago/Turabian StyleZhu, Wenlong, Mengjia Zhang, Mengdi Zhang, Ran Jing, Jiaru Zhou, Hua Cao, Changcheng Liu, Hongmei Zhu, Ahmed H. Ghonaim, Sherin R. Rouby, and et al. 2024. "The Generation and Characterization of Monoclonal Antibodies against the MPXV A29L Protein" Viruses 16, no. 8: 1184. https://doi.org/10.3390/v16081184





