The Altered Neonatal CD8+ T Cell Immunodominance Hierarchy during Influenza Virus Infection Impacts Peptide Vaccination
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice, Viral Stocks, and Influenza Virus Peptides
2.2. Infections
2.3. Cell Isolation
2.4. Flow Cytometry
2.5. Antigen Presentation Assay
2.6. Adoptive Transfer of PR8-Infected or Peptide-Pulsed BMDCs
2.7. Determination of Viral Titers
2.8. Statistics
3. Results
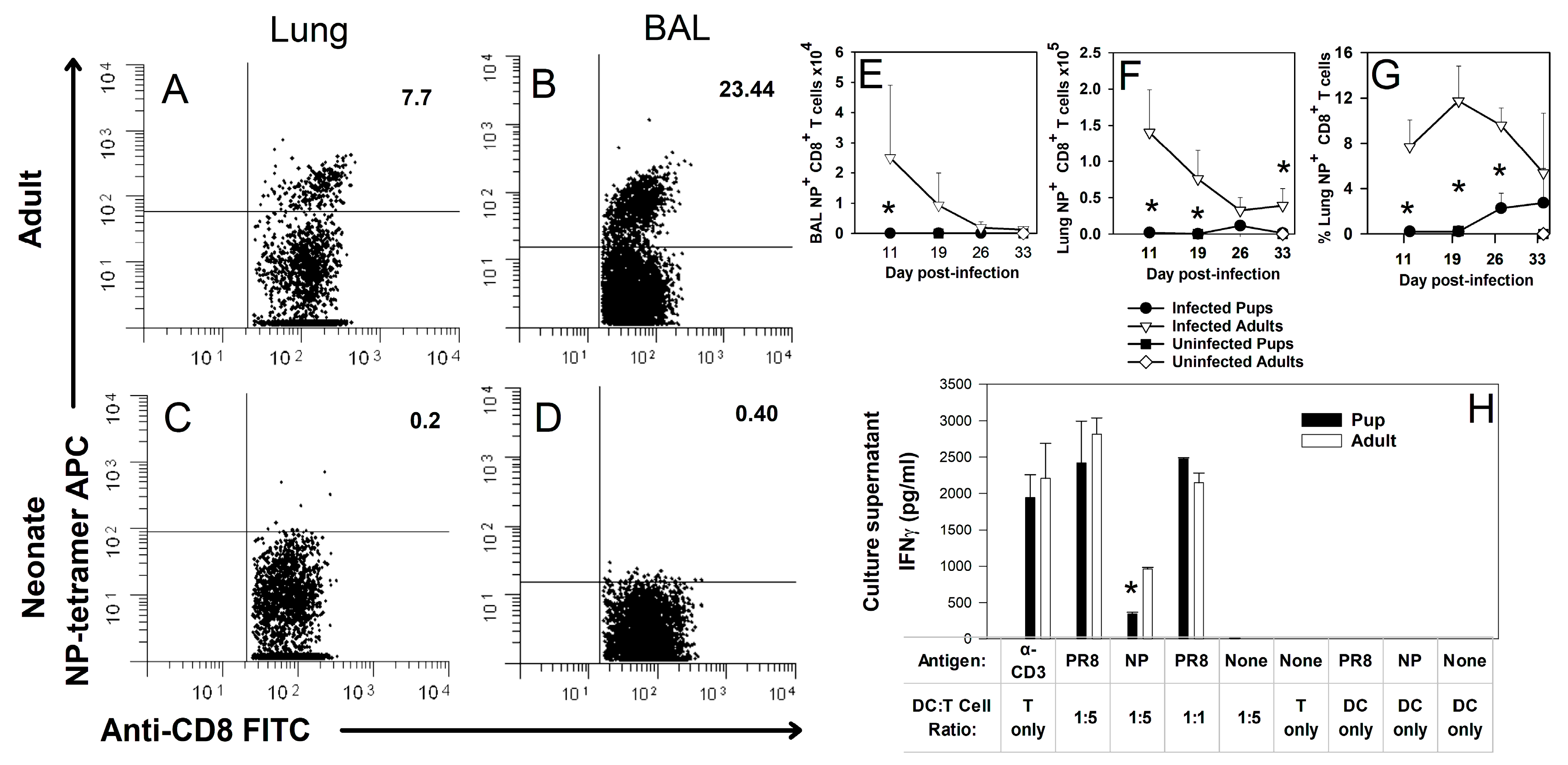
3.1. Neonates Do Not Produce CD8+ T Cells Specific for NP (366–374) during Primary Influenza Virus Infection
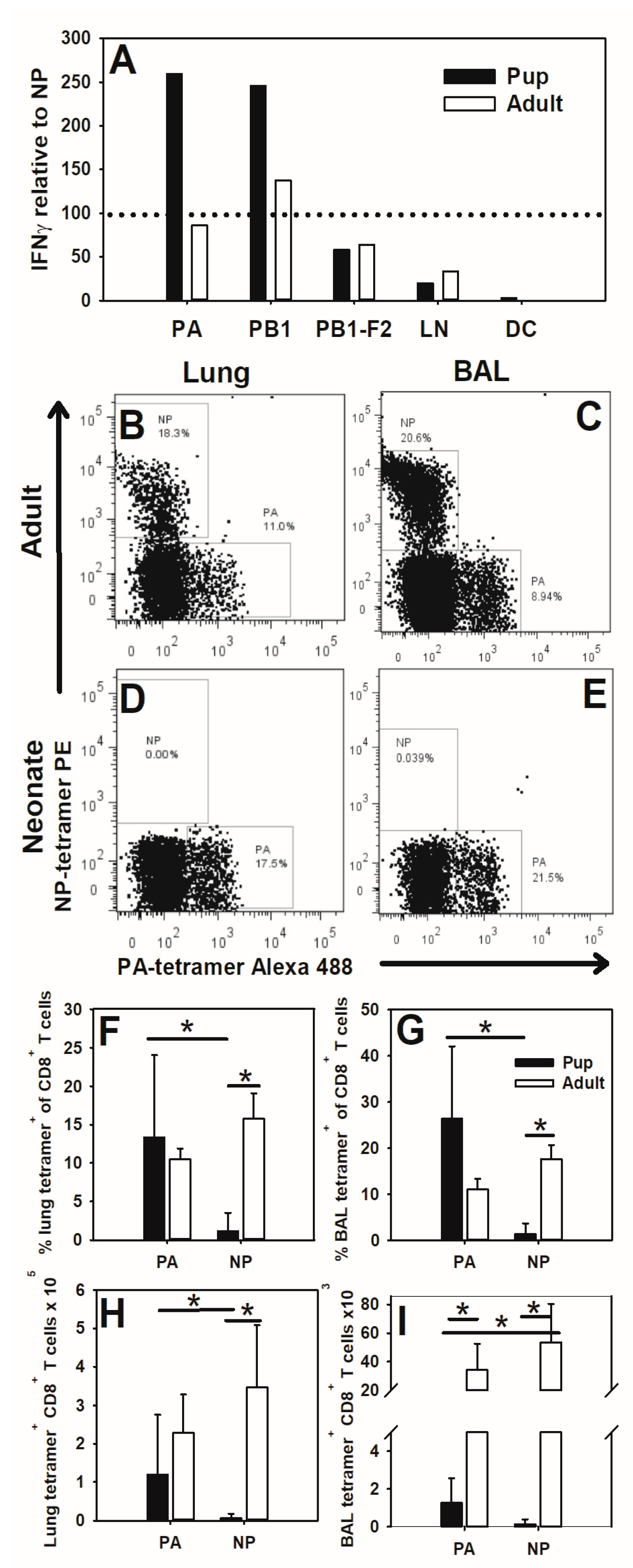
3.2. Neonates Respond to PA (224–233) during Influenza Virus Infection
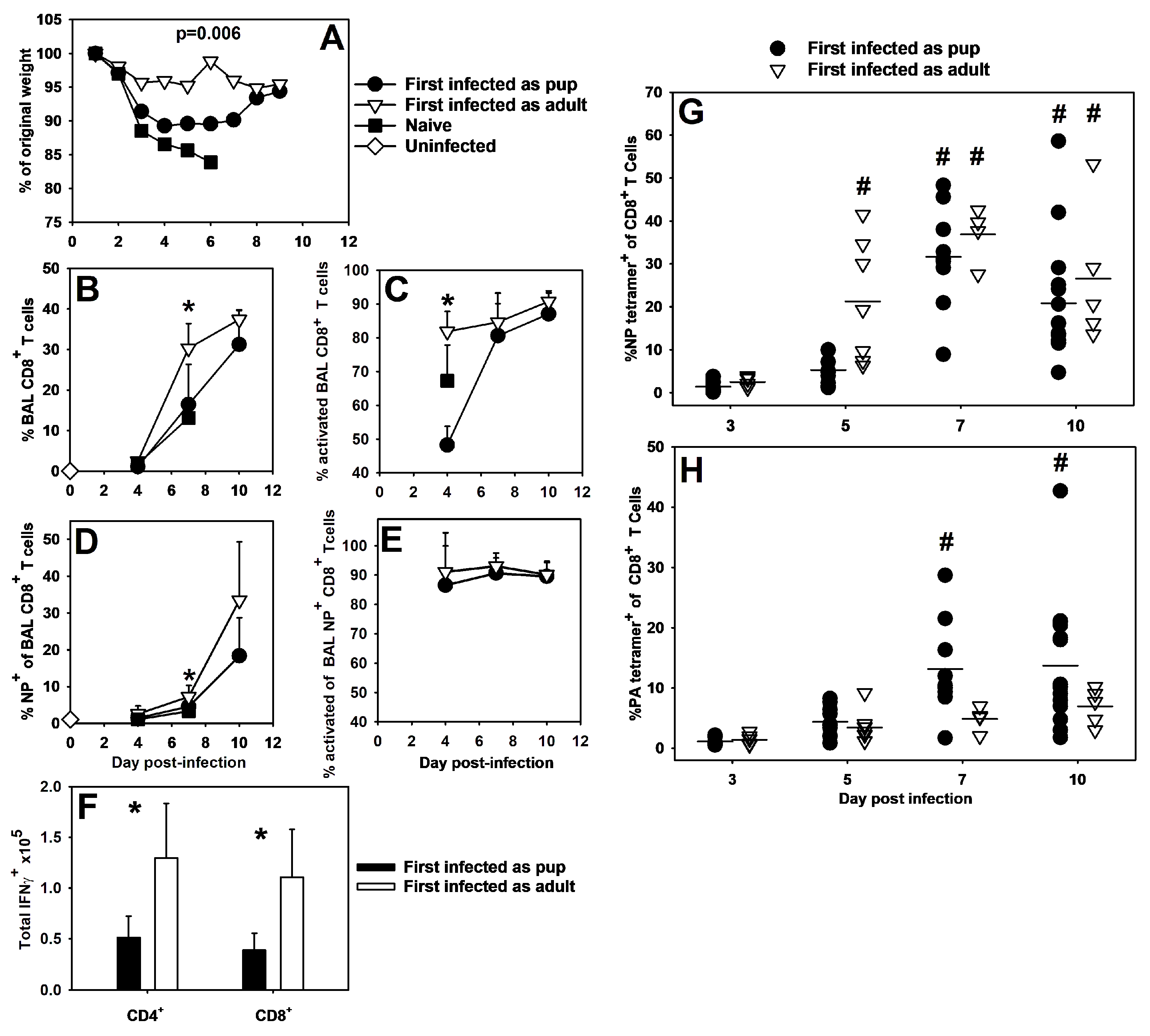
3.3. Neonatal Influenza Virus Infection Generates Weak T Cell Memory and Results in Altered CD8+ Specificity during Secondary Infection
3.4. Adoptive Transfer of Bone-Marrow-Derived Dendritic Cells Alters CD8+ T Cell Immunodominance during Influenza Virus Infection and Improves Influenza Virus Clearance in Pups
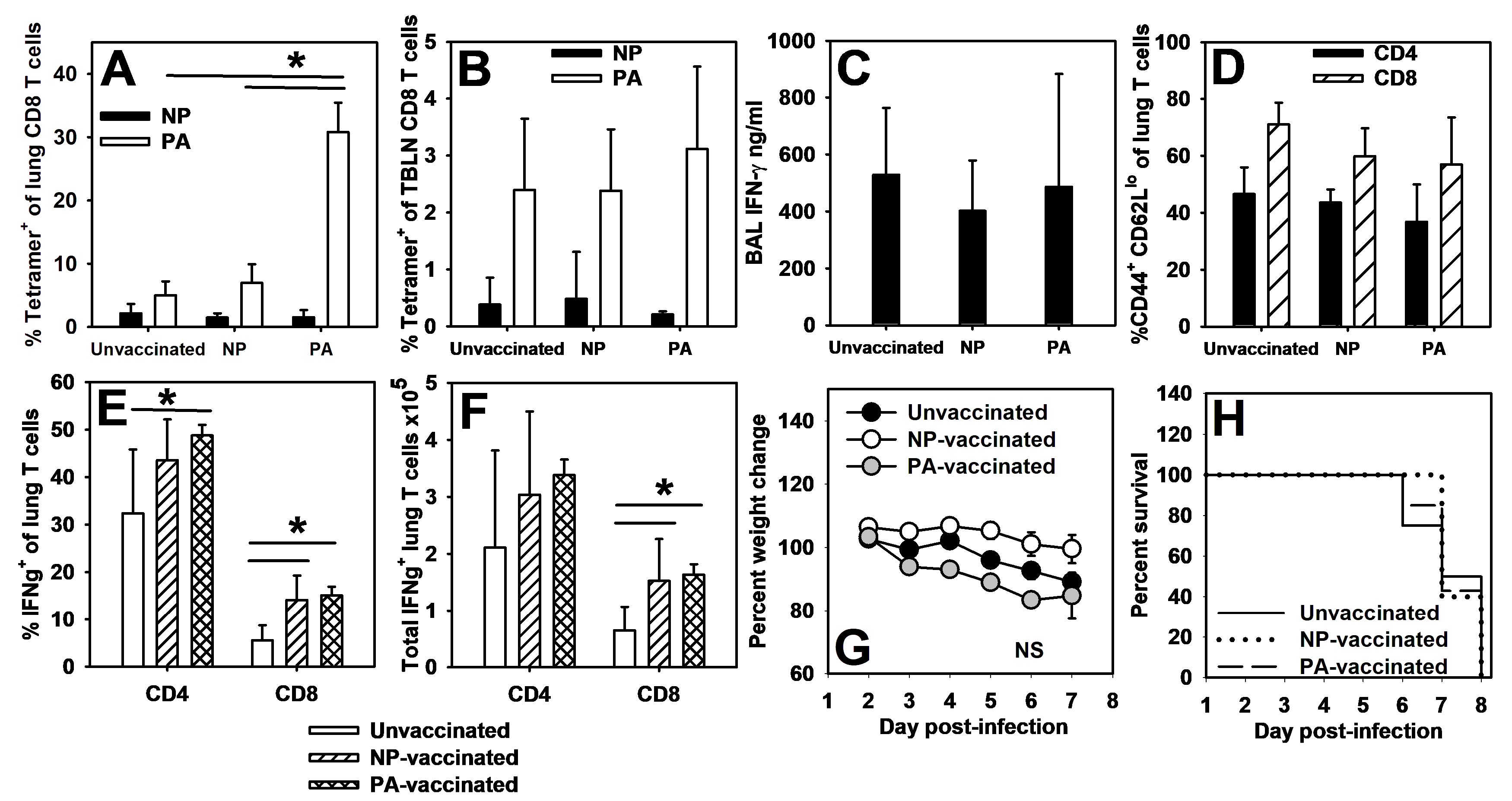
3.5. Pups Vaccinated with Peptide-Loaded BMDCs Display Memory Responses to PA (224–233), but Not NP (366–374)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, E.A.S.; Ip, M.; Tam, J.S.; Mounts, A.W.; Chau, S.L.; Law, S.K.; Goggins, W.; Simpson, L.A.; Chan, P.K.S. Burden of influenza infection in hospitalised children below 6 months of age and above in Hong Kong from 2005 to 2011. Vaccine 2014, 32, 6692–6698. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.; Chaves, S.S.; Daily Kirley, P.; Emerson, R.; Aragon, D.; Hancock, E.B.; Butler, L.; Baumbach, J.; Hollick, G.; Bennett, N.M.; et al. Estimating Influenza Disease Burden from Population-Based Surveillance Data in the United States. PLoS ONE 2015, 10, e0118369. [Google Scholar] [CrossRef]
- Antonova, E.N.; Rycroft, C.E.; Ambrose, C.S.; Heikkinen, T.; Principi, N. Burden of paediatric influenza in Western Europe: A systematic review. BMC Public Health 2012, 12, 968. [Google Scholar] [CrossRef]
- Neuzil, K.M.; Zhu, Y.; Griffin, M.R.; Edwards, K.M.; Thompson, J.M.; Tollefson, S.J.; Wright, P.F. Burden of interpandemic influenza in children younger than 5 years: A 25-year prosepctive study. J. Infect. Dis. 2002, 185, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Thommes, E.W.; Kruse, M.; Kohli, M.; Sharma, R.; Noorduyn, S.G. Review of seasonal influenza in Canada: Burden of disease and the cost-effectiveness of quadrivalent inactivated influenza vaccines. Hum. Vaccines Immunother. 2017, 13, 867–876. [Google Scholar] [CrossRef][Green Version]
- Sridhar, S.; Brokstad, K.A.; Cox, R.J. Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines. Vaccines 2015, 3, 373–389. [Google Scholar] [CrossRef]
- Basha, S.; Surendran, N.; Pichichero, M. Immune responses in neonates. Expert. Rev. Clin. Immunol. 2014, 10, 1171–1184. [Google Scholar] [CrossRef]
- Adkins, B.; Bu, Y.; Guevara, P. Murine Neonatal CD4+ Lymph Node Cells Are Highly Deficient in the Development of Antigen-Specific Th1 Function in Adoptive Adult Hosts. J. Immunol. 2002, 169, 4998–5004. [Google Scholar] [CrossRef] [PubMed]
- Ruckwardt, T.J.; Malloy, A.M.W.; Morabito, K.M.; Graham, B.S. Quantitative and Qualitative Deficits in Neonatal Lung-Migratory Dendritic Cells Impact the Generation of the CD8+ T Cell Response. PLoS Pathog. 2014, 10, e1003934. [Google Scholar] [CrossRef] [PubMed]
- Adkins, B.; Du, R.-Q. Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses. J. Immunol. 1998, 160, 4217–4224. [Google Scholar] [CrossRef]
- Lines, J.L.; Hoskins, S.; Hollifield, M.; Cauley, L.S.; Garvy, B.A. The Migration of T Cells in Response to Influenza Virus Is Altered in Neonatal Mice. J. Immunol. 2010, 185, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.H.; Cook-Mills, J.; Doherty, D.E.; Garvy, B.A. TNF-a-Dependent ICAM-1- and VCAM-1-Mediated Inflammatory Responses Are Delayed in Neonatal Mice Infected with Pneumocystis carinii. J. Immunol. 2003, 171, 4700–4707. [Google Scholar] [CrossRef]
- Welliver, T.P.; Garofalo, R.P.; Hosakote, Y.; Hintz, K.H.; Avendano, L.; Sanchez, K.; Velozo, L.; Jafri, H.; Chavez-Bueno, S.; Ogra, P.L.; et al. Severe Human Lower Respiratory Tract Illness Caused by Respiratory Syncytial Virus and Influenza Virus Is Characterized by the Absence of Pulmonary Cytotoxic Lymphocyte Responses. J. Infect. Dis. 2007, 195, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Welliver, T.P.; Reed, J.L.; Welliver, R.C., Sr. Respiratory syncytial virus and influenza virus infections: Observations from tissues of fatal infant cases. Pediatr. Infect. Dis. J. 2008, 27, S92–S96. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Ripple, M.; Balakrishna, S.; Troxclair, D.; Sandquist, D.; Ding, L.; Ahlert, T.A.; Cormier, S.A. Inchoate CD8+ T Cell Responses in Neonatal Mice Permit Influenza-Induced Persistent Pulmonary Dysfunction. J. Immunol. 2008, 181, 3486–3494. [Google Scholar] [CrossRef]
- Fadel, S.A.; Cowell, L.G.; Cao, S.; Ozaki, D.A.; Kepler, T.B.; Steeber, D.A.; Sarzotti, M. Neonate-primed CD8+ memory cells rival adult-primed memory cells in antigen-driven expansion and anti-viral protection. Int. Immunol. 2006, 18, 249–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, N.L.; Wissink, E.; Wang, J.; Pinello, J.F.; Davenport, M.P.; Grimson, A.; Rudd, B.D. Rapid proliferation and differentiation impairs the development of memory CD8+ T cells in early life. J. Immunol. 2014, 193, 177–184. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Knudson, C.J.; Hartwig, S.M.; Pewe, L.L.; Meyerholz, D.K.; Langlois, R.A.; Harty, J.T.; Varga, S.M. Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection. PLoS Pathog. 2018, 14, e1006810. [Google Scholar] [CrossRef] [PubMed]
- Estrada, L.D.; Schultz-Cherry, S. Development of a Universal Influenza Vaccine. J. Immunol. 2019, 202, 392–398. [Google Scholar] [CrossRef]
- van Doorn, E.; Pleguezuelos, O.; Liu, H.; Fernandez, A.; Bannister, R.; Stoloff, G.; Oftung, F.; Norley, S.; Huckriede, A.; Frijlink, H.W.; et al. Evaluation of the immunogenicity and safety of different doses and formulations of a broad spectrum influenza vaccine (FLU-v) developed by SEEK: Study protocol for a single-center, randomized, double-blind and placebo-controlled clinical phase IIb trial. BMC Infect. Dis. 2017, 17, 241. [Google Scholar] [CrossRef]
- Xiang, K.; Ying, G.; Yan, Z.; Shanshan, Y.; Lei, Z.; Hongjun, L.; Maosheng, S. Progress on adenovirus-vectored universal influenza vaccines. Hum. Vaccin. Immunother. 2015, 11, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Palese, P. Universal Influenza Virus Vaccines That Target the Conserved Hemagglutinin Stalk and Conserved Sites in the Head Domain. J. Infect. Dis. 2019, 219, S62–S67. [Google Scholar] [CrossRef] [PubMed]
- Portela, A.; Digard, P. The influenza virus nucleoprotein: A multifunctional RNA-binding protein pivotal to virus replication. J. Gen. Virol. 2002, 83, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Kosik, I.; Angeletti, D.; Gibbs, J.S.; Angel, M.; Takeda, K.; Kosikova, M.; Nair, V.; Hickman, H.D.; Xie, H.; Brooke, C.B.; et al. Neuraminidase inhibition contributes to influenza A virus neutralization by anti-hemagglutinin stem antibodies. J. Exp. Med. 2019, 216, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, B.J.; Decman, V.; Ali, M.A.; Abt, M.C.; Wolf, A.I.; Monticelli, L.A.; Mozdzanowska, K.; Angelosanto, J.M.; Artis, D.; Erikson, J.; et al. Cooperativity between CD8+ T cells, non-neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity. PLoS Pathog. 2013, 9, e1003207. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kang, J.O.; Chang, J. Nucleoprotein vaccine induces cross-protective cytotoxic T lymphocytes against both lineages of influenza B virus. Clin. Exp. Vaccine Res. 2019, 8, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Begom, S.; Bermingham, A.; Hoschler, K.; Adamson, W.; Carman, W.; Bean, T.; Barclay, W.; Deeks, J.J.; Lalvani, A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013, 19, 1305–1312. [Google Scholar] [CrossRef]
- Attaf, M.; Legut, M.; Cole, D.K.; Sewell, A.K. The T cell antigen receptor: The Swiss army knife of the immune system. Clin. Exp. Immunol. 2015, 181, 1–18. [Google Scholar] [CrossRef]
- Chen, W.; Anton, L.C.; Bennink, J.R.; Yewdell, J.W. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity 2000, 12, 83–93. [Google Scholar] [CrossRef]
- Crowe, S.R.; Turner, S.J.; Miller, S.C.; Roberts, A.D.; Rappolo, R.A.; Doherty, P.C.; Ely, K.H.; Woodland, D.L. Differential Antigen Presentation Regulates the Changing Patterns of CD8+ T Cell Immunodominance in Primary and Secondary Influenza Virus Infections. J. Exp. Med. 2003, 198, 399–410. [Google Scholar] [CrossRef]
- La Gruta, N.L.; Rothwell, W.T.; Cukalac, T.; Swan, N.G.; Valkenburg, S.A.; Kedzierska, K.; Thomas, P.G.; Doherty, P.C.; Turner, S.J. Primary CTL response magnitude in mice is determined by the extent of naive T cell recruitment and subsequent clonal expansion. J. Clin. Investig. 2010, 120, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.J.; Gracias, D.T.; Thayer, J.L.; Boesteanu, A.C.; Kumova, O.K.; Mueller, Y.M.; Hope, J.L.; Fraietta, J.A.; van Zessen, D.B.H.; Katsikis, P.D. Rapid Evolution of the CD8+ TCR Repertoire in Neonatal Mice. J. Immunol. 2016, 196, 2602–2613. [Google Scholar] [CrossRef] [PubMed]
- Ruckwardt, T.J.; Malloy, A.M.; Gostick, E.; Price, D.A.; Dash, P.; McClaren, J.L.; Thomas, P.G.; Graham, B.S. Neonatal CD8 T-cell hierarchy is distinct from adults and is influenced by intrinsic T cell properties in respiratory syncytial virus infected mice. PLoS Pathog. 2011, 7, e1002377. [Google Scholar] [CrossRef] [PubMed]
- Cottey, R.; Rowe, C.A.; Bender, B.S. Influenza Virus. Curr. Protoc. Immunol. 2001, 42, 19.11.11–19.11.32. [Google Scholar] [CrossRef] [PubMed]
- Uddback, I.E.; Steffensen, M.A.; Pedersen, S.R.; Nazerai, L.; Thomsen, A.R.; Christensen, J.P. PB1 as a potential target for increasing the breadth of T-cell mediated immunity to Influenza A. Sci. Rep. 2016, 6, 35033. [Google Scholar] [CrossRef] [PubMed]
- Belz, G.T.; Stevenson, P.G.; Doherty, P.C. Contemporary analysis of MHC-related immunodominance hierarchies in the CD8+ T cell response to influenza A viruses. J. Immunol. 2000, 165, 2404–2409. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.G.; Brown, S.A.; Keating, R.; Yue, W.; Morris, M.Y.; So, J.; Webby, R.J.; Doherty, P.C. Hidden epitopes emerge in secondary influenza virus-specific CD8+ T cell responses. J. Immunol. 2007, 178, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pang, K.; Masterman, K.A.; Kennedy, G.; Basta, S.; Dimopoulos, N.; Hornung, F.; Smyth, M.; Bennink, J.R.; Yewdell, J.W. Reversal in the immunodominance hierarchy in secondary CD8+ T cell responses to influenza A virus: Roles for cross-presentation and lysis-independent immunodomination. J. Immunol. 2004, 173, 5021–5027. [Google Scholar] [CrossRef] [PubMed]
- Kilbourn, E.D. Future influenza vaccines and use of genetic recombinants. Bull. World Health Organ. 1969, 41, 643–645. [Google Scholar]
- Ruckwardt, T.J.; Morabito, K.M.; Bar-Haim, E.; Nair, D.; Graham, B.S. Neonatal mice possess two phenotypically and functionally distinct lung-migratory CD103+ dendritic cell populations following respiratory infection. Mucosal Immunol. 2018, 11, 186–198. [Google Scholar] [CrossRef]
- Malloy, A.M.; Ruckwardt, T.J.; Morabito, K.M.; Lau-Kilby, A.W.; Graham, B.S. Pulmonary Dendritic Cell Subsets Shape the Respiratory Syncytial Virus-Specific CD8+ T Cell Immunodominance Hierarchy in Neonates. J. Immunol. 2017, 198, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Mozdzanowska, K.; Palladino, G.; Gerhard, W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J. Immunol. 1994, 152, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Moldoveanu, Z.; Novak, M.J.; van Ginkel, F.W.; Ban, E.; Kiyono, H.; McGhee, J.R.; Mestecky, J. Heterosubtypic immunity to lethal influenza A virus infection is associated with virus-specific CD8(+) cytotoxic T lymphocyte responses induced in mucosa-associated tissues. Virology 1999, 254, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Zens, K.D.; Chen, J.K.; Guyer, R.S.; Wu, F.L.; Cvetkovski, F.; Miron, M.; Farber, D.L. Reduced generation of lung tissue-resident memory T cells during infancy. J. Exp. Med. 2017, 214, 2915–2932. [Google Scholar] [CrossRef] [PubMed]
- Rius, C.; Attaf, M.; Tungatt, K.; Bianchi, V.; Legut, M.; Bovay, A.; Donia, M.; Thor Straten, P.; Peakman, M.; Svane, I.M.; et al. Peptide-MHC Class I Tetramers Can Fail To Detect Relevant Functional T Cell Clonotypes and Underestimate Antigen-Reactive T Cell Populations. J. Immunol. 2018, 200, 2263–2279. [Google Scholar] [CrossRef]
- Rudd, B.D.; Venturi, V.; Smith, N.L.; Nzingha, K.; Goldberg, E.L.; Li, G.; Nikolich-Zugich, J.; Davenport, M.P. Acute neonatal infections ‘lock-in’ a suboptimal CD8+ T cell repertoire with impaired recall responses. PLoS Pathog. 2013, 9, e1003572. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heil, L.; Jewell, S.; Lines, J.L.; Garvy, B.A. The Altered Neonatal CD8+ T Cell Immunodominance Hierarchy during Influenza Virus Infection Impacts Peptide Vaccination. Viruses 2024, 16, 1271. https://doi.org/10.3390/v16081271
Heil L, Jewell S, Lines JL, Garvy BA. The Altered Neonatal CD8+ T Cell Immunodominance Hierarchy during Influenza Virus Infection Impacts Peptide Vaccination. Viruses. 2024; 16(8):1271. https://doi.org/10.3390/v16081271
Chicago/Turabian StyleHeil, Luke, Samantha Jewell, J. Louise Lines, and Beth A. Garvy. 2024. "The Altered Neonatal CD8+ T Cell Immunodominance Hierarchy during Influenza Virus Infection Impacts Peptide Vaccination" Viruses 16, no. 8: 1271. https://doi.org/10.3390/v16081271
APA StyleHeil, L., Jewell, S., Lines, J. L., & Garvy, B. A. (2024). The Altered Neonatal CD8+ T Cell Immunodominance Hierarchy during Influenza Virus Infection Impacts Peptide Vaccination. Viruses, 16(8), 1271. https://doi.org/10.3390/v16081271




